Application of Trap Cropping as Companion Plants for the Management of Agricultural Pests: A Review
Abstract
1. Introduction
2. Function of the Trap Cropping System in Agriculture
2.1. Description of the Trap Cropping System
2.2. Factors that Affect the Efficacy and Practicality of Trap Cropping Systems
3. Trap Cropping in Insect Pest Attraction and Repulsion
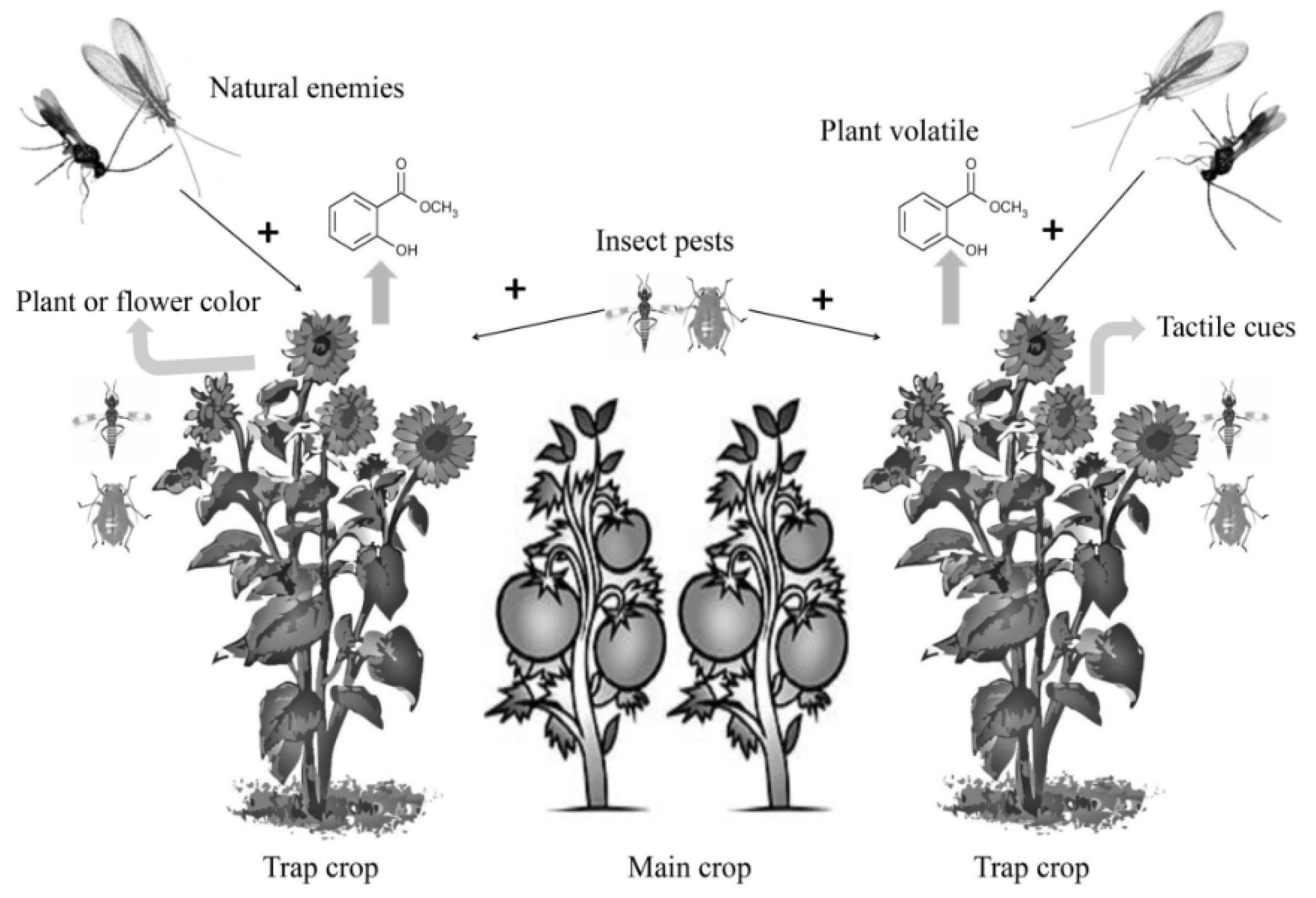
3.1. Trap Cropping in Insect Pest Management
3.2. Trap Cropping in Natural Enemy Attraction
3.3. Technological Tool for Trap Cropping to Improve Natural Enemy Attraction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adler, L.S.; Hazzard, R.V. Comparison of perimeter trap crop varieties: Effects on herbivory, pollination, and yield in butternut squash. Environ. Entomol. 2009, 38, 207–215. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, A.R. Resistance to insecticides in heliothine Lepidoptera: A global view. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998, 353, 1735–1750. [Google Scholar] [CrossRef]
- Heinz, K.M.; Van Driesche, R.G.; Parella, M.P. Biocontrol in Protected Culture; Ball Publishing: Batavia, IL, USA, 2004. [Google Scholar]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Hatt, S.; Boeraeve, F.; Artru, S.; Dufrêne, M.; Francis, F. Spatial diversification of agroecosystems to enhance biological control and other regulating services: An agroecological perspective. Sci. Total Environ. 2018, 621, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, H.M. Trap cropping in pest management. Annu. Rev. Entomol. 1991, 36, 119–138. [Google Scholar] [CrossRef]
- Shelton, A.M.; Badenes-Perez, F.R. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.H.; Ellner, S.P.; Lee, D.H.; Nyrop, J.P.; Sanderson, J.P. Designing an effective trap cropping strategy: The effects of attraction, retention and plant spatial distribution. J. Appl. Ecol. 2012, 49, 715–722. [Google Scholar]
- Parolin, P.; Bresch, C.; Desneux, N.; Brun, R.; Bout, A.; Boll, R.; Poncet, C. Secondary plants used in biological control: A review. Int. J. Pest Manag. 2012, 58, 91–100. [Google Scholar] [CrossRef]
- Parker, J.E.; Snyder, W.E.; Hamilton, G.C.; Rodriguez-Saona, C. Companion planting and insect pest control. In Weed and Pest Control Conventional and New Challenges; Soloneski, S., Larramendy, M., Eds.; InTech: Rijeka, Croatia, 2013; pp. 1–30. [Google Scholar]
- Naranjo, S.E.; Ellsworth, P.C.; Frisvold, G.B. Economic value of biological control in integrated pest management of managed plant systems. Annu. Rev. Entomol. 2015, 60, 621–645. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, G.; Gurr, G.M.; Kühne, S.; Wade, M.R.; Wratten, S.D.; Wyss, E. Arthropod pest management in organic crops. Annu. Rev. Entomol. 2007, 52, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Andow, D.A. Vegetational diversity and arthropod population response. Annu. Rev. Entomol. 1991, 36, 561–586. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Armbrecht, I.; Rivera, B.S.; Lerma, J.M.; Carmona, E.J.; Daza, M.C.; Escobar, S.; Galindo, V.; Gutiérrez, C.; López, S.D.; et al. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Root, R.B. Organization of a plant-arthropod association in simple and diverse habitats: The fauna of collards (Brassica oleracea). Ecol. Monogr. 1973, 43, 95–124. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Morrison, W.R.; Mathews, C.; Leskey, T.C.; Nielsen, A.L. Measuring host plant selection and retention of Halyomorpha halys by a trap crop. Entomol. Exp. Appl. 2017, 163, 197–208. [Google Scholar] [CrossRef]
- Tillman, P.G.; Northfield, T.D.; Mizell, R.F.; Riddle, T.C. Spatiotemporal patterns and dispersal of stink bugs (Heteroptera: Pentatomidae) in peanut-cotton farmscapes. Environ. Entomol. 2009, 38, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, P.D.; Martinson, H.M.; Bergmann, E.J.; Shrewsbury, P.M.; Raupp, M.J. Edge effects influence the abundance of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) in woody plant nurseries. Environ. Entomol. 2015, 44, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.K.; Larsen, L.M.; Sørensen, H. Host plant selection of the horseradish flea beetle Phyllotreta armoraciae (Coleoptera: Chrysomelidae): Identification of two flavonol glycosides stimulating feeding in combination with glucosinolates. Entomol. Exp. Appl. 1979, 26, 40–48. [Google Scholar] [CrossRef]
- Gao, Y.L.; Lei, Z.R.; Reitz, S.R. Western flower thrips resistance to insecticides: Detection, mechanisms, and management strategies. Pest Manag. Sci. 2012, 68, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Rea, J.H.; Wratten, S.D.; Sedcole, R.; Cameron, P.J.; Davis, S.I.; Chapman, R.B. Trap cropping to manage green vegetable bug Nezara viridula (L.) (Heteroptera: Pentatomidae) in sweet corn in New Zealand. Agric. Forest Entomol. 2002, 4, 101–107. [Google Scholar] [CrossRef]
- Dicke, M.; Hilker, M. Induced plant defences: From molecular biology to evolutionary ecology. Basic Appl. Ecol. 2003, 4, 3–14. [Google Scholar] [CrossRef]
- Reddy, G.V.P. Plant volatiles mediate orientation and plant preference by the predator Chrysoperla carnea Stephens (Neuroptera: Chrysopidae). Biol. Control 2002, 25, 49–55. [Google Scholar] [CrossRef]
- Zhu, J.; Cossé, A.A.; Obrycki, J.J.; Boo, K.S.; Baker, T.C. Olfactory reactions of the twelve-spotted lady beetle, Coleomegilla maculata and the green lacewing, Chrysoperla carnea to semiochemicals released from their prey and host plant: Electroantennogram and behavioral responses. J. Chem. Ecol. 1999, 25, 1163–1177. [Google Scholar] [CrossRef]
- Cook, S.M.; Rasmussen, H.B.; Birkett, M.A.; Murray, D.A.; Pye, B.J.; Watts, N.P.; Williams, I.H. Behavioural and chemical ecology underlying the success of turnip rape (Brassica rapa) trap crops in protecting oilseed rape (Brassica napus) from the pollen beetle (Meligethes aeneus). Arthropod Plant Interact. 2007, 1, 57. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Dively, G.; Pote, J.M.; Zinati, G.; Mathews, C. Identifying a potential trap crop for a novel insect pest, Halyomorpha halys (Hemiptera: Pentatomidae), in organic farms. Environ. Entomol. 2016, 45, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.; Bruce, T.; Pickett, J.; Hardie, J. Volatiles functioning as host cues in a blend become nonhost cues when presented alone to the black bean aphid. Anim. Behav. 2010, 79, 451–457. [Google Scholar] [CrossRef]
- Lambdon, P.W.; Hassall, M.; Boar, R.R.; Mithen, R. Asynchrony in the nitrogen and glucosinolate leaf-age profiles of Brassica: Is this a defensive strategy against generalist herbivores? Agric. Ecosyst. Environ. 2003, 97, 205–214. [Google Scholar] [CrossRef]
- Wallace, S.K.; Eigenbrode, S.D. Changes in the glucosinolate–myrosinase defense system in Brassica juncea cotyledons during seedling development. J. Chem. Ecol. 2002, 28, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Dogramaci, M.; Shrefler, J.W.; Roberts, B.W.; Pair, S.; Edelson, J.V. Comparison of management strategies for squash bugs (Hemiptera: Coreidae) in watermelon. J. Econ. Entomol. 2004, 97, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Pair, S.D. Evaluation of systemically treated squash trap plants and attracticidal baits for early-season control of striped and spotted cucumber beetles (Coleoptera: Chrysomelidae) and squash bug (Hemiptera: Coreidae) in cucurbit crops. J. Econ. Entomol. 1997, 90, 1307–1314. [Google Scholar] [CrossRef]
- Radin, A.M.; Drummond, F.A. Patterns of initial colonization of cucurbits, reproductive activity, and dispersion of striped cucumber beetle, Acalymma vittata(F.) (Coleoptera: Chrysomelidae). J. Agric. Entomol. 1994, 11, 115–123. [Google Scholar]
- Abate, T. Experiments with trap crops against African bollworm, Heliothis armigera, in Ethiopia. Entomol. Exp. Appl. 1988, 48, 135–140. [Google Scholar] [CrossRef]
- Potting, R.P.J.; Perry, J.N.; Powell, W. Insect behavioral ecology and other factors affecting the control efficacy of agro-ecosystem diversification strategies. Ecol. Model. 2005, 182, 199–216. [Google Scholar] [CrossRef]
- Yan, F.M. Chemical Ecology; China Science Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Schoonhoven, L.M.; Van Loon, J.J.; Dicke, M. Insect-Plant Biology, 2nd ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Eigenbrode, S.D.; Birch, A.N.; Lindzey, S.; Meadow, R.; Snyder, W.E. A mechanistic framework to improve understanding and applications of push-pull systems in pest management. J. Appl. Ecol. 2016, 53, 202–212. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wu, K.M.; Wyckhuys, K.A.; Guo, Y.Y. Potential of mungbean, Vigna radiatus as a trap crop for managing Apolygus lucorum (Hemiptera: Miridae) on Bt cotton. Crop. Prot. 2009, 28, 77–81. [Google Scholar] [CrossRef]
- Smyth, R.R.; Hoffmann, M.P.; Shelton, A.M. Effects of host plant phenology on oviposition preference of Crocidolomia pavonana (Lepidoptera: Pyralidae). Environ. Entomol. 2003, 32, 756–764. [Google Scholar] [CrossRef]
- Kumari, A.P.P.; Pasalu, I.C. Influence of planting pattern of trap crops on yellow stem borer, Scirpophaga incertulas (Walker), damage in rice. Ind. J. Plant Prot. 2003, 31, 78–83. [Google Scholar]
- Rhino, B.; Verchère, A.; Thibaut, C.; Ratnadass, A. Field evaluation of sweet corn varieties for their potential as a trap crop for Helicoverpa zea under tropical conditions. Int. J. Pest Manag. 2016, 62, 3–10. [Google Scholar] [CrossRef]
- Buhre, C.; Apfelbeck, R.; Hesse, F.; Van Look, M.; Mielke, C.; Ladewing, E. Survey on production technology-regional differences in sugar beet production in the field of plant protection. Sugar Ind. 2014, 139, 110–116. [Google Scholar]
- Hokkanen, H.M.T. Biological and agrotechnical control of the rape blossom beetle Meligethes aeneus (Coleoptera: Nitidulidae). Acta Entomol. Fenn. 1989, 53, 25–30. [Google Scholar]
- Khan, Z.R.; Pickett, J.A.; Wadhams, L.; Muyekho, F. Habitat management strategies for the control of cereal stemborers and striga in maize in Kenya. Insect Sci. Appl. 2001, 21, 375–380. [Google Scholar] [CrossRef]
- Buckland, K.R.; Alston, D.G.; Reeve, J.R.; Nischwitz, C.; Drost, D. Trap Crops in Onion to Reduce Onion Thrips and Iris Yellow Spot Virus. Southwest. Entomol. 2017, 42, 73–90. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Jones, V.P.; Nielsen, A.L. Utilizing immunomarking techniques to track Halyomorpha halys (Hemiptera: Pentatomidae) movement and distribution within a peach orchard. PeerJ 2016, 4, e1997. [Google Scholar] [CrossRef] [PubMed]
- Mathews, C.R.; Blaauw, B.; Dively, G.; Kotcon, J.; Moore, J.; Ogburn, E.; Pfeiffer, D.G.; Trope, T.; Walgenbach, J.F.; Welty, C.; et al. Evaluating a polyculture trap crop for organic management of Halyomorpha halys and native stink bugs in peppers. J. Pest Sci. 2017, 90, 1245–1255. [Google Scholar] [CrossRef]
- Srinivasan, K.; Moorthy, P.K. Indian mustard as a trap crop for management of major lepidopterous pests on cabbage. Int. J. Pest Manag. 1991, 37, 26–32. [Google Scholar] [CrossRef]
- Charleston, D.S.; Kfir, R. The possibility of using Indian mustard, Brassica juncea, as a trap crop for the diamondback moth, Plutella xylostella, in South Africa. Crop. Prot. 2000, 19, 455–460. [Google Scholar] [CrossRef]
- Kumar, S. Potential of Ethiopian mustard, Brassica carinata as a trap crop for large white butterfly, Pieris brassicae infesting Indian mustard, Brassica juncea. J. Pest Sci. 2017, 90, 129–137. [Google Scholar] [CrossRef]
- Trdan, S.; Valič, N.; Žnidarčič, D.; Vidrih, M.; Bergant, K.; Zlatič, E.; Milevoj, L. The role of Chinese cabbage as a trap crop for flea beetles (Coleoptera: Chrysomelidae) in production of white cabbage. Sci. Hort. 2005, 106, 12–24. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Ayers, G.S.; Grafius, E.J.; Stehr, F.W. Effects of neighboring nectar-producing plants on populations of pest Lepidoptera and their parasitoids in broccoli plantings. Great Lakes Entomol. 2017, 25, 3. [Google Scholar]
- Banks, J.E.; Ekbom, B. Modelling herbivore movement and colonization: Pest management potential of intercropping and trap cropping. Agric. Forest Entomol. 1999, 1, 165–170. [Google Scholar] [CrossRef]
- Price, P.W.; Bouton, C.E.; Gross, P.; McPheron, B.A.; Thompson, J.N.; Weis, A.E. Interactions among three trophic levels: Influence of plants on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst. 1980, 11, 41–65. [Google Scholar] [CrossRef]
- Castle, S.J. Concentration and management of Bemisia tabaci in cantaloupe as a trap crop for cotton. Crop. Prot. 2006, 25, 574–584. [Google Scholar] [CrossRef]
- Srinivasan, K.; Moorthy, P.K.; Raviprasad, T.N. African marigold as a trap crop for the management of the fruit borer Helicoverpa armigera on tomato. Int. J. Pest Manag. 2008, 40, 56–63. [Google Scholar] [CrossRef]
- Accinelli, G.; Lanzoni, A.; Ramilli, F.; Dradi, D.; Burgio, G. Trap crop: An agroecological approach to the management of Lygus rugulipennis on lettuce. Bull. Insectol. 2005, 58, 9–14. [Google Scholar]
- Swezey, S.L.; Nieto, D.J.; Pickett, C.H.; Hagler, J.R.; Bryer, J.A.; Machtley, S.A. Spatial density and movement of the Lygus spp. parasitoid Peristenus relictus (Hymenoptera: Braconidae) in organic strawberries with alfalfa trap crops. Environ. Entomol. 2014, 43, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, A.; Hazzard, R.; Adler, L.S.; Boucher, J. Using trap crops for control of Acalymma vittatum (Coleoptera: Chrysomelidae) reduces insecticide use in butternut squash. J. Econ. Entomol. 2009, 102, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.R.; Hu, G.; Johanowicz, D. Management of diamondback moth (Lepidoptera: Plutellidae) in cabbage using collard as a trap crop. HortScience 2000, 35, 875–879. [Google Scholar]
- Shelton, A.M.; Nault, B.A. Dead-end trap cropping: A technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 2004, 23, 497–503. [Google Scholar] [CrossRef]
- Smith, H.A.; Mcsorley, R. Potential of field corn as a barrier crop and eggplant as a trap crop for management of Bemisia argentifolii (Homoptera: Aleyrodidae) on common bean in North Florida. Fla. Entomol. 2000, 83, 145–158. [Google Scholar] [CrossRef]
- Kumar, V.; Mahla, M.K.; Lal, J.; Singh, B. Effect of abiotic factors on the seasonal incidence of fruit borer, Helicoverpa armigera (Hub.) on tomato with and without marigold as a trap crop. J. Entomol. Zool. Stud. 2017, 5, 803–807. [Google Scholar]
- Khan, Z.R.; James, D.G.; Midega, C.A.; Pickett, J.A. Chemical ecology and conservation biological control. Biol. Control 2008, 45, 210–224. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Márquez, B.P.; Petitpierre, E. Can flowering Barbarea spp. (Brassicaceae) be used simultaneously as a trap crop and in conservation biological control? J. Pest Sci. 2017, 90, 623–633. [Google Scholar] [CrossRef]
- Midega, C.A.; Khan, Z.R.; Pickett, J.A.; Nylin, S. Host plant selection behavior of Chilo partellus and its implication for effectiveness of a trap crop. Entomol. Exp. Appl. 2011, 138, 40–47. [Google Scholar] [CrossRef]
- Tillman, P.G. Sorghum as a trap crop for Nezara viridula L. (Heteroptera: Pentatomidae) in cotton in the southern United States. Environ. Entomol. 2006, 35, 771–783. [Google Scholar] [CrossRef]
- Smith, H.A.; Koenig, R.L.; McAuslane, H.J.; McSorley, R. Effect of silver reflective mulch and a summer squash trap crop on densities of immature Bemisia argentifolii (Homoptera: Aleyrodidae) on organic bean. J. Econ. Entomol. 2000, 93, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Badenes-Pérez, F.R.; Nault, B.A.; Shelton, A.M. Dynamics of diamondback moth oviposition in the presence of a highly preferred non-suitable host. Entomol. Exp. Appl. 2006, 120, 23–31. [Google Scholar] [CrossRef]
- Kovács, G.; Kaasik, R.; Metspalu, L.; Williams, I.H.; Luik, A.; Veromann, E. Could Brassica rapa, Brassica juncea and Sinapis alba facilitate the control of the cabbage seed weevil in oilseed rape crops? Biol. Control 2013, 65, 124–129. [Google Scholar] [CrossRef]
- Kaasik, R.; Kovács, G.; Kaart, T.; Metspalu, L.; Williams, I.H.; Veromann, E. Meligethes aeneus oviposition preferences, larval parasitism rate and species composition of parasitoids on Brassica nigra, Raphanus sativus and Eruca sativa compared with on Brassica napus. Biol. Control 2014, 69, 65–71. [Google Scholar] [CrossRef]
- Veromann, E.; Metspalu, L.; Williams, I.H.; Hiiesaar, K.; Mand, M.; Kaasik, R.; Kovacs, G.; Jogar, K.; Svilponis, E.; Kivimagi, I.; et al. Relative attractiveness of Brassica napus, Brassica nigra, Eruca sativa and Raphanus sativus for pollen beetle (Meligethes aeneus) and their potential for use in trap cropping. Arthropod Plant Interact. 2012, 6, 385–394. [Google Scholar] [CrossRef]
- Veromann, E.; Kaasik, R.; Kovács, G.; Metspalu, L.; Williams, I.H.; Mänd, M. Fatal attraction: Search for a dead-end trap crop for the pollen beetle (Meligethes aeneus). Arthropod Plant Interact. 2014, 8, 373–381. [Google Scholar] [CrossRef]
- Ehler, L.E.; Miller, J.C. Biological control in temporary agro ecosystems. Entomophaga 1978, 23, 207–212. [Google Scholar] [CrossRef]
- Wiedenmann, R.N.; Smith, J.W. Attributes of natural enemies in ephemeral crop habitats. Biol. Control 1997, 10, 16–22. [Google Scholar] [CrossRef]
- Wissinger, S.A. Cyclic colonization in predictably ephemeral habitats: A template for biological control in annual crop systems. Biol. Control 1997, 10, 4–15. [Google Scholar] [CrossRef]
- Kaplan, I. Attracting carnivorous arthropods with plant volatiles: The future of biocontrol or playing with fire? Biol. Control 2012, 60, 77–89. [Google Scholar] [CrossRef]
- Fujinuma, M.; Kainoh, Y.; Nemoto, H. Borago officinalis attracts the aphid parasitoid Aphidius colemani (Hymenoptera: Braconidae). Appl. Entomol. Zool. 2010, 45, 615–620. [Google Scholar] [CrossRef][Green Version]
- Hogg, B.N.; Bugg, R.L.; Daane, K.M. Attractiveness of common insectary and harvestable floral resources to beneficial insects. Biol. Control 2011, 56, 76–84. [Google Scholar] [CrossRef]
- Williams, L., III; Rodriguez-Saona, C.; Castle del Conte, S.C. Methyl jasmonate-induction of cotton: A field test of the “attract and reward” strategy of conservation biological control. AoB Plants 2017, 9, plx032. [Google Scholar] [CrossRef] [PubMed]
- Kovács, G.; Kaasik, R.; Kaart, T.; Metspalu, L.; Luik, A.; Veromann, E. In search of secondary plants to enhance the efficiency of cabbage seed weevil management. BioControl 2017, 62, 29–38. [Google Scholar] [CrossRef]
- Vinson, S.B. Behavioral chemicals in the augmentation of natural enemies. In Biological Control by Augmentation of Natural Enemies; Springer: New York, NY, USA, 1977; pp. 237–279. [Google Scholar]
- Gross, H.R. Employment of kairomones in the management of parasitoids. In Semiochemicals: Their Role in Pest Control; Nordlund, D.A., Jones, R.L., Lewis, W.J., Eds.; Wiley: New York, NY, USA, 1981; pp. 137–150. [Google Scholar]
- Nordlund, D.A.; Lewis, W.J.; Gross, H.R. Elucidation and employment of semiochemicals in the manipulation of entomophagous insects. In Management of Insect Pests with Semiochemicals; Springer: Boston, MA, USA, 1981; pp. 463–475. [Google Scholar]
- Powell, W. Enhancing parasitoid activity in crops. In Proceedings of the 13th Symposia of the Royal Entomological Society of London, London, UK, 18–19 September 1985; Royal Entomological Society: London, UK, 1986. [Google Scholar]
- Lewis, W.J.; Martin, W.R. Semiochemicals for use with parasitoids: Status and future. J. Chem. Ecol. 1990, 16, 3067–3089. [Google Scholar] [CrossRef] [PubMed]
- Bottrell, D.G.; Barbosa, P.; Gould, F. Manipulating natural enemies by plant variety selection and modification: A realistic strategy? Annu. Rev. Entomol. 1998, 43, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J.; Gershenzon, J.; Baldwin, I.T.; Kessler, A. Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotechnol. 2003, 14, 169–176. [Google Scholar] [CrossRef]
- Turlings, T.C.; Wäckers, F. Recruitment of predators and parasitoids by herbivore-injured plants. Adv. Insect Chem. Ecol. 2004, 2, 21–75. [Google Scholar]
- Pickett, J.A.; Bruce, T.J.; Chamberlain, K.E.; Hassanali, A.H.; Khan, Z.R.; Matthes, M.C.; Napier, J.A.; Smart, L.E.; Wadhams, L.J.; Woodcock, C.M. Plant volatiles yielding new ways to exploit plant defense. Chem. Ecol. Gene Ecosyst. 2006, 2, 161–173. [Google Scholar]
- Turlings, T.C.; Ton, J. Exploiting scents of distress: The prospect of manipulating herbivore-induced plant odours to enhance the control of agricultural pests. Curr. Opin. Plant Biol. 2006, 9, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Mumm, R.; Dicke, M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. The present review is one in the special series of reviews on animal-plant interactions. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]
- Hare, J.D. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu. Rev. Entomol. 2011, 56, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.; Gurr, G.M.; Simmons, A.T.; Wratten, S.D.; James, D.G.; Leeson, G.; Nicol, H.I. Insect attraction to synthetic herbivore-induced plant volatile-treated field crops. Agric. Forest Entomol. 2011, 13, 45–57. [Google Scholar] [CrossRef]
- James, D.G. Methyl salicylate is a field attractant for the golden eyed lacewing, Chrysopa oculata. Biocontrol. Sci. Technol. 2006, 16, 107–110. [Google Scholar] [CrossRef]
- James, D.G. Further field evaluation of synthetic herbivore-induced plan volatiles as attractants for beneficial insects. J. Chem. Ecol. 2005, 31, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Obrycki, J.J.; Ochieng, S.A.; Baker, T.C.; Pickett, J.A.; Smiley, D. Attraction of two lacewing species to volatiles produced by host plants and aphid prey. Naturwissenschaften 2005, 92, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.P.; Horton, D.R.; Mills, N.J.; Unruh, T.R.; Baker, C.C.; Melton, T.D.; Milickzy, E.; Steffan, S.A.; Shearer, P.W.; Amarasekare, K.G. Evaluating plant volatiles for monitoring natural enemies in apple, pear and walnut orchards. Biol. Control 2016, 102, 53–65. [Google Scholar] [CrossRef]
- Barloggio, G.; Tamm, L.; Nagel, P.; Luka, H. Selective flowers to attract and enhance Telenomus laeviceps (Hymenoptera: Scelionidae): A released biocontrol agent of Mamestra brassicae (Lepidoptera: Noctuidae). Bull. Entomol. Res. 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.E.; Potter, D.A. Potential for sugar sprays and flowering plants to increase parasitism of white grubs (Coleoptera: Scarabaeidae) by Tiphiid wasps (Hymenoptera: Tiphiidae). Environ. Entomol. 2004, 33, 619–626. [Google Scholar] [CrossRef]
- Evans, E.W.; Swallow, J.G. Numerical responses of natural enemies to artificial honeydew in Utah alfalfa. Environ. Entomol. 1993, 22, 1392–1401. [Google Scholar] [CrossRef]
- Jacob, H.S.; Evans, E.W. Effects of sugar spray and aphid honeydew on field populations of the parasitoid Bathyplectes curculionis (Hymenoptera: Ichneumonidae). Environ. Entomol. 1998, 27, 1563–1568. [Google Scholar] [CrossRef]
- Ewert, M.A.; Chiang, H.C. Dispersal of Three Species of Coccinellids in Corn Fields. Can. Entomol. 1966, 98, 999–1003. [Google Scholar] [CrossRef]
- Schiefelbein, J.W.; Chiang, H.C. Effects of spray of sucrose solution in a corn field on the populations of predatory insects and their prey. Entomophaga 1966, 11, 333–339. [Google Scholar] [CrossRef]
- Hagen, K.S. Ecosystem analysis: Plant cultivars (HPR), entomophagous species and food supplements. In Interactions of Plant Resistance and Parasitoids and Predators of Insects; Wiley: New York, NY, USA; Ellis Horwood Limited: Chichester, UK, 1986; pp. 151–197. [Google Scholar]
- Coll, M.; Guershon, M. Omnivory in terrestrial arthropods: Mixing plant and prey diets. Annu. Rev. Entomol. 2002, 47, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Wackers, F.L.; van Rijn, P.C. Food for protection: An introduction. In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Wackers, F.L., van Rijn, P.C.J., Bruin, J., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 1–14. [Google Scholar]
- Hogg, B.N.; Nelson, E.H.; Mills, N.J.; Daane, K.M. Floral resources enhance aphid suppression by a hoverfly. Entomol. Exp. Appl. 2011, 141, 138–144. [Google Scholar] [CrossRef]
- Salamanca, J.; Pareja, M.; Rodriguez-Saona, C.; Resende, A.L.S.; Souza, B. Behavioral responses of adult lacewings, Chrysoperla externa, to a rose–aphid–coriander complex. Biol. Control 2015, 80, 103–112. [Google Scholar] [CrossRef]
- Fair, C.G.; Braman, S.K. Assessment of Habitat Modification and Varied Planting Dates to Enhance Potential Natural Enemies of Anasa tristis (Hemiptera: Coreidae) in Squash. Environ. Entomol. 2017, 46, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Bennison, J.A.; Corless, S.P. Biological control of aphids on cucumbers: Further development of open rearing units or “banker plants” to aid establishment of aphid natural enemies. WPRS Bull. 1993, 16, 5. [Google Scholar]
- Trisnawati, I.; Azis, A. The effectiveness of habitat modification schemes for enhancing beneficial insects: Assessing the importance of trap cropping management approach. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2017; Volume 1854, p. 020038. [Google Scholar]
- Jacobson, R.J.; Croft, P. Strategies for the control of Aphis gossypii Glover (Hom.: Aphididae) with Aphidius colemani Viereck (Hym.: Braconidae) in protected cucumbers. Biocontrol. Sci. Technol. 1998, 8, 377–387. [Google Scholar] [CrossRef]
- Brodeur, J. Host specificity in biological control: Insights from opportunistic pathogens. Evol. Appl. 2012, 5, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Gurr, G.M.; Wratten, S.D.; Barbosa, P. Success in conservation biological control of arthropods. Biol. Control Meas. Success 2000, 30, 105–132. [Google Scholar]
| Trap Crop | Crop | Insect Pest | Country | Implementation | References |
|---|---|---|---|---|---|
| African marigold, Tagetes erecta L. (Asteraceae) | Tomato, Solanum lycopersicum L. (Solanaceae) | Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) | India | Field | Srinivasan et al. [56] |
| Alfalfa, Medicago sativa L. (Fabaceae) | Lettuce, Lactuca sativa L. (Asteraceae) | Lygus rugulipennis Hahn (Hemiptera: Miridae) | Italy | Field | Accinelli et al. [57] |
| Arugula, Eruca sativa Mill. (Brassicaceae); | Tomato, S. lycopersicum | Lygus spp. (Hemiptera: Miridae) | United States | Field | Swezey et al. [58] |
| Buckwheat, Fagopyrum esculentum Moench (Polygonaceae) | Onion, Allium cepa L. (Amaryllidaceae) | Thrips tabaci Lindeman (Thysanoptera: Thripidae) | United States | Field | Buckland et al. [45] |
| Buttercup squash, Cucurbita maxima Duchesne (Cucurbitaceae) | Athena muskmelon, Cucumis melo L. (Cucurbitaceae) | Acalymma vittatum (Coleoptera: Chrysomelidae); Diabrotica undecimpunctata L. (Coleoptera: Chrysomelidae) | United States | Field | Cavanagh et al. [59] |
| Cucumber, Cucumis sativus L. (Cucurbitaceae) | Acalymma vittatum (Coleoptera: Chrysomelidae) | United States | Field | Adler and Hazzard [1] | |
| Carrot, Daucus carota Hoffm. (Apiaceae) | Onion, A. cepa | Thrips tabaci Lindeman (Thysanoptera: Thripidae) | United States | Field | Buckland et al. [45] |
| Chinese cabbage, Brassica rapa L. (Brassicaceae) | White cabbage, Brassica oleracea L. (Brassicaceae) | Phyllotreta spp. (Coleoptera: Chrysomelidae) | Slovenia | Field | Trdan et al. [51] |
| Collard cabbage, Brassica oleracea viridis (Brassicaceae) | Cabbage, B. oleracea | Plutella xylostella L. (Lepidoptera: Plutellidae) | United States | Field | Mitchell et al. [60] Shelton and Nault [61] |
| Eggplant, Solanum melongena L. (Solanaceae); | Common bean, Phaseolus vulgaris L. (Fabaceae) | Bemisia argentifolii Gennadius (Hemiptera: Aleyrodidae) | United States | Field | Smith and Mcsorley [62] |
| Ethiopian mustard, Brassica carinata A.Braun (Brassicaceae) | Indian mustard, Brassica juncea (L.) Czern. (Brassicaceae) | Pieris brassicae L. (Lepidoptera: Pieridae) | India | Laboratory and field | Kumar [50] |
| Indian mustard, B. juncea | Cabbage, B. oleracea | Plutella xylostella L. (Lepidoptera: Plutellidae); Crocidolomia binotalis Fabricius (Lepidoptera: Crambidae) | India | Laboratory and field | Srinivasan and Moorthy [48] |
| Crucifer crops, Brassicaceae spp. (Brassicaceae) | Plutella xylostella L. (Lepidoptera: Plutellidae) | South Africa | Field | Charleston and Kfir [49] | |
| Marigold, Calendula officinalis L. (Asteraceae) | Tomato, S. lycopersicum | Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) | India | Laboratory and field | Kumar et al. [63] |
| Mung bean, Vigna radiata L. (Fabaceae) | Bacillus thuringiensis (Bt) cotton, (Bt) Gossypium hirsutum L. (Malvaceae) | Apolygus lucorum Meyer-Dür (Hemiptera: Heteroptera ) | China | Field | Lu et al. [38] |
| Napier grass, Pennisetum purpureum Schumach (Poaceae) | Sorghum, Sorghum bicolor L. (Poaceae) | Busseola fusca Fuller (Lepidoptera: Noctuidae) | United States | Field | Khan et al. [64] |
| Non-flowering Barbarea, Barbarea spp. (Brassicaceae) | Cabbage, B. oleracea | Plutella xylostella L. (Lepidoptera: Plutellidae) | Spain | Field | Badenes-Pérez et al. [65] |
| Sorghum, S. bicolor | Maize, Zea mays L. (Poaceae) | Chilo partellus Swinhoe (Lepidoptera: Crambidae) | Kenya | Field | Midega et al. [66] |
| Cotton, G. hirsutum | Nezara viridula L. (Hemiptera: Pentatomidae) | United States | Field | Tillman [67] | |
| Summer squash, Cucurbita pepo L. (Cucurbitaceae) | Bean, P. vulgaris | Bemisia argentifolii Gennadius (Hemiptera: Aleyrodidae) | United States | Field | Smith et al. [68] |
| Sunflower, Helianthus annuus L. (Asteraceae); grain sorghum, S. bicolor | Bell peppers, Capsicum annuum L. (Solanaceae) | Halyomorpha halys Stål (Hemiptera: Pentatomidae) | United States | Field | Blaauw et al. [16,46] |
| Yellow rocket, Barbarea vulgaris W. T. Aiton (Brassicaceae) | Cabbage, B. oleracea | Plutella xylostella L. (Lepidoptera: Plutellidae) | United States | Field | Badenes-Perez et al. [69] |
| Indian mustard, B. juncea; white mustard, Sinapis alba L. (Brassicaceae) | Chinese Cabbage, B. rapa; Oilseed rape, Brassica napus L. (Brassicaceae) | Ceutorhynchus obstrictus Marsham (Coleoptera: Curculionidae) | Estonia | Field | Kovács et al. [70] |
| Black mustard, Brassica nigra L. (Brassicaceae); radish, Raphanus sativus Pers. (Brassicaceae); arugula, E. sativa | Oilseed rape, B. napus | Meligethes aeneus Fabricius (Coleoptera: Nitidulidae) | Estonia | Field | Kaasik et al. [71] |
| Oilseed rape, B. napus; Chinese cabbage, B. rapa; black mustard, B. nigra; Indian mustard, B. juncea | White mustard, S. alba; Radish, R. sativus | M. aeneus | Estonia | Field | Veromann et al. [72,73] |
| Trap Crop | Crop | Natural Enemy | Country | Implementation | Reference |
|---|---|---|---|---|---|
| Alfalfa, M. sativa L. | Maize, Z. mays | Chrysopidae | United States | Field | Zhu et al. [97] |
| Borage, Borago officinalis L. (Boraginaceae) | Tomatoes, S. lycopersicum | A. colemani; Syrphidae; Chrysopidae | Japan, United States | Greenhouse, field | Fujinuma et al. [78] Hogg et al. [108] |
| Coriander, Coriandrum sativum L. (Apiaceae); | Banana, Musa balbisiana L. (Musaceae) | Chrysopidae; Coleomegilla maculata De Geer (Coleoptera: Coccinellidae) | Brazil | Greenhouse | Salamanca et al. [109] |
| Cornflower, Centaurea cyanus L. (Asteraceae) | Squash, C. pepo | Spiders; Carabidae | United States | Field | Fair and Braman [110] |
| Maize, Z. mays | Cucumber, C. sativus | A. colemani | United States | Field | Bennison and Corless [111] |
| Sunflower, H. annuus | Banana, M. balbisiana | Chrysopidae | United States | Field | Zhu et al. [97] |
| Sunflower, H. annuus | Cotton, G. hirsutum | Chrysopidae; Coccinellidae | United States | Field | Williams et al. [80] |
| Sunn hemp, Crotalaria juncea L. (Fabaceae) | Tobacco, Nicotiana tabacum L. (Solanaceae) | Crocothemis servilia Drury (Odonata: Libellulidae); Orthetrum Sabina Drury (Odonata: Libellulidae) | Indonesia | Field | Trisnawati and Azis [112] |
| Sweet alyssum, Lobularia maritime L. (Brassicaceae) | Cruciferous vegetables, Brassica spp. (Brassicaceae) | Syrphidae | United States | Field | Hogg et al. [79] |
| Wheat, Triticum aestivum L. (Poaceae) | Cucumber, C. sativus | A. colemani | United Kingdom | Greenhouse | Jacobson and Croft [113] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, S.C.; Wang, E.; Wu, S.; Lei, Z. Application of Trap Cropping as Companion Plants for the Management of Agricultural Pests: A Review. Insects 2018, 9, 128. https://doi.org/10.3390/insects9040128
Sarkar SC, Wang E, Wu S, Lei Z. Application of Trap Cropping as Companion Plants for the Management of Agricultural Pests: A Review. Insects. 2018; 9(4):128. https://doi.org/10.3390/insects9040128
Chicago/Turabian StyleSarkar, Shovon Chandra, Endong Wang, Shengyong Wu, and Zhongren Lei. 2018. "Application of Trap Cropping as Companion Plants for the Management of Agricultural Pests: A Review" Insects 9, no. 4: 128. https://doi.org/10.3390/insects9040128
APA StyleSarkar, S. C., Wang, E., Wu, S., & Lei, Z. (2018). Application of Trap Cropping as Companion Plants for the Management of Agricultural Pests: A Review. Insects, 9(4), 128. https://doi.org/10.3390/insects9040128




