Attraction of Culex pipiens to House Sparrows Is Influenced by Host Age but Not Uropygial Gland Secretions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Analysis of Uropygial Gland Secretions
2.1.1. Bird Handling
2.1.2. Statistics
2.2. Bioassays
3. Results
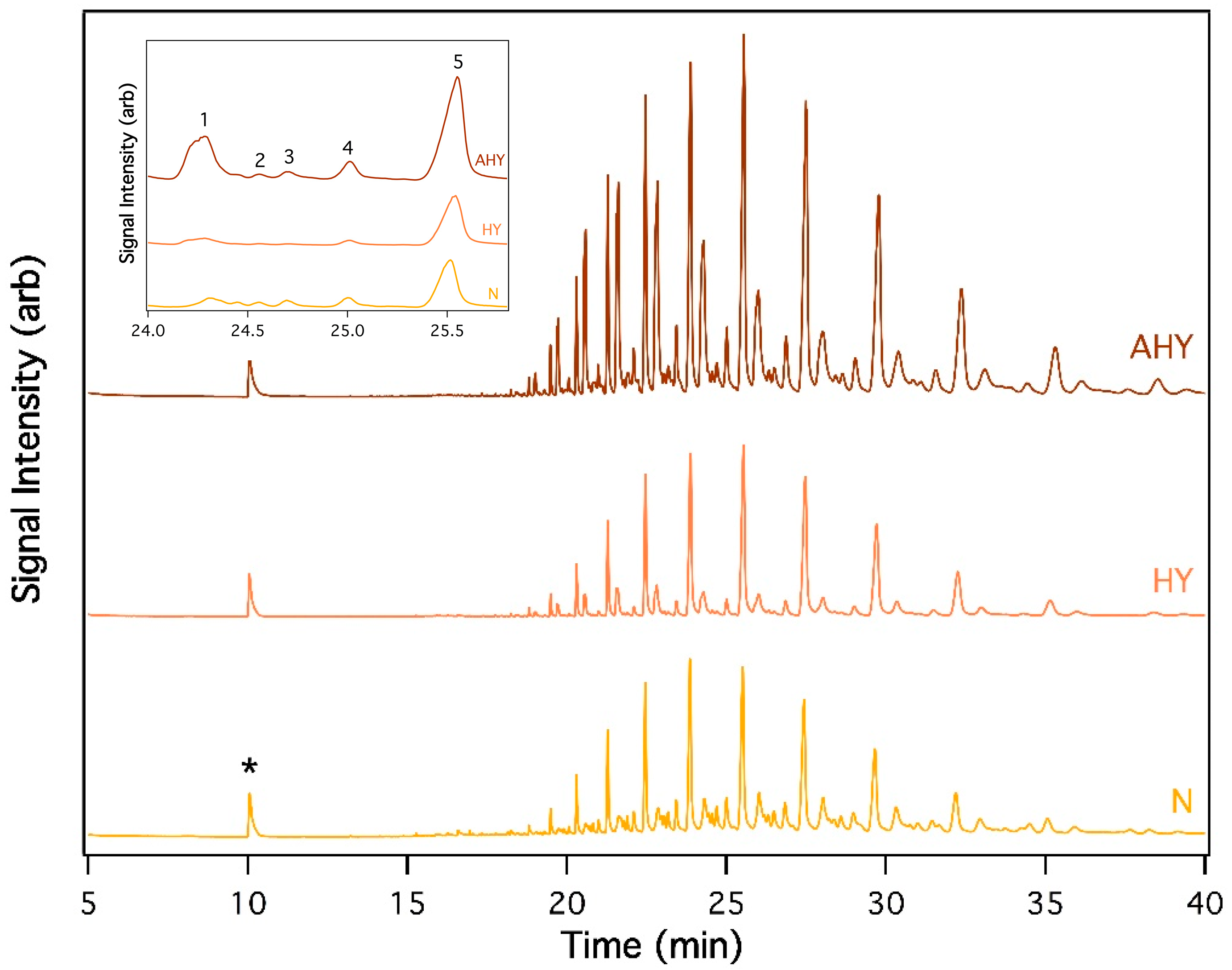
3.1. GC-MS Analysis Comparing Uropygial Secretion Composition of Nestling, Fledgling, and Adult House Sparrows
3.2. Bioassays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Apperson, C.S.; Hassan, H.K.; Harrison, B.A.; Savage, H.M.; Aspen, S.E.; Farajollahi, A.; Crans, W.; Daniels, T.J.; Falco, R.C.; Benedict, M.; et al. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoon. Dis. 2004, 4, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; O’Guinn, M.L.; Dohm, D.J.; Jones, J.W. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J. Med. Entomol. 2001, 38, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Apperson, C.S.; Harrison, B.A.; Unnasch, T.R.; Hassan, H.K.; Irby, W.S.; Savage, H.M.; Aspen, S.E.; Watson, D.W.; Rueda, L.M.; Engber, B.R.; et al. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J. Med. Entomol. 2002, 39, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, J.S.; Dow, R.P. Differential feeding of Culex tarsalis on nesting and adult birds 1958. Mosq. News 1958, 18, 15–18. [Google Scholar]
- Griffing, S.M.; Kilpatrick, A.M.; Clark, L.; Marra, P.P. Mosquito landing rates on nesting American robins (Turdus migratorius). Vector-Borne Zoonotic Dis. 2007, 7, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Kale, H.W.; Edman, J.D.; Webber, L.A. Effect of behavior and age of individual ciconiiform birds on mosquito feeding success. Mosq. News 1972, 32, 343–350. [Google Scholar]
- McLean, R.G.; Crans, W.J.; Caccamise, D.F.; McNelly, J.; Kirk, L.J.; Mitchell, C.J.; Calisher, C.H. Experimental infection of wading birds with eastern equine encephalitis virus. J. Wildl. Dis. 1995, 31, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Burkett-Cadena, N.D.; Ligon, R.A.; Liu, M.; Hassan, H.K.; Hill, G.E.; Eubanks, M.D. Vector-host interactions in avian nests: Do mosquitoes prefer nestlings over adults? Am. J. Trop. Med. Hyg. 2010, 83, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Loss, S.R.; Hamer, G.L.; Goldberg, T.L.; Ruiz, M.O.; Kitron, U.D.; Walker, E.D.; Brawn, J.D. Nestling passerines are not important hosts for amplification of West Nile virus in Chicago, Illinois. Vector-Borne Zoon. Dis 2009, 9, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.W.; Lorenz, L.H.; Edman, J.D. Effects of house sparrow age and arbovirus infection on attraction of mosquitos. J. Med. Entomol. 1990, 27, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Costantini, C.; Sagnon, N.; Della Tori, A.; Diallo, M.; Brady, J.; Gibson, G.; Coluzzi, M. Odor-mediated host preferences of West African mosquitoes, with particular reference to malaria vectors. Am. J. Trop. Med. Hyg. 1998, 58, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Mboera, L.; Takken, W. Odour-mediated host preference of Culex quinquefasciatus in Tanzania. Entomol. Exp. Appl. 1999, 92, 83–88. [Google Scholar] [CrossRef]
- Zwiebel, L.J.; Takken, W. Olfactory regulation of mosquito-host interactions. Insect Biochem. Mol. Biol. 2004, 34, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Haribal, M.; Dhondt, A.; Rosane, D.; Rodriguez, E. Chemistry of preen gland secretions of passerines: Different pathways to same goal? Why? Chemoecology 2005, 15, 251–260. [Google Scholar] [CrossRef]
- Giraudeau, M.; Duval, C.; Guillon, N.; Bretagnolle, V.; Gutierrez, C.; Heeb, P. Effects of access to preen gland secretions on mallard plumage. Naturwissenschaften 2010, 97, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Rajchard, J. Intraspecific and interspecific chemosignals in birds: A review. Vet. Med. Czech. 2007, 52, 385–391. [Google Scholar] [CrossRef]
- Hagelin, J.C.; Jones, I.L. Bird odors and other chemical substances: A defense mechanism or overlooked mode of intraspecific communication? Auk 2007, 124, 741–761. [Google Scholar] [CrossRef]
- Russell, C.B.; Hunter, F.F. Attraction of Culex pipiens/restuans (Diptera: Culicidae) mosquitoes to bird uropygial gland odors at two elevations in the Niagara region of Ontario. J. Med. Entomol. 2005, 42, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Bernier, U.R.; Allan, S.A.; Quinn, B.P.; Kline, D.L.; Barnard, D.R.; Clark, G.G. Volatile compounds from the integument of White Leghorn Chickens (Gallus gallus domesticus L.): Candidate attractants of ornithophilic mosquito species. J. Sep. Sci. 2008, 31, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.A.; Bernier, U.R.; Kline, D.L. Laboratory evaluation of avian odors for mosquito (Diptera: Culicidae) attraction. J. Med. Entomol. 2006, 43, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.; Double, M.C.; Orr, K.; Dawson, R.A. DNA test to sex most birds. Mol. Ecol. 1998, 7, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Garvin, M.C.; Austin, A.L.; Stracker, N.H.; Slowinski, S.P.; Rutter, J.E.; Butler, M.; Michel, M.; Whelan, R.J. Attraction of Cx. pipiens to uropygial gland secretions of American Robins. J. Vector Ecol. 2018, 43, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Pyle, P. Identification Guide to the North American Birds; Slate Creek Press: Bolinas, CA, USA, 1997. [Google Scholar]
- Posey, K.H.; Barnard, D.R.; Schreck, C.E. Triple cage olfactometer for evaluating mosquito (Diptera: Culicidae) attraction responses. J. Med. Entomol. 1998, 35, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.J.; Lovette, I.J.; Harvey, E.L. Evolutionary variation in feather waxes of passerine birds. Auk 2004, 121, 435–445. [Google Scholar] [CrossRef]
- Jacob, J.; Poltz, J. Composition of uropygial gland secretions of birds of prey. Lipids 1975, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Sawant, S.B. Physico-chemical properties of wax esters synthesised from corresponding alcohols using hydrobromic acid and hydrogen peroxide action. Eur. J. Lipid Sci. Technol. 2002, 104, 387–393. [Google Scholar] [CrossRef]
- Epand, R.M.; Rychnovsky, S.D.; Belani, J.D.; Epand, R.F. Role of chirality in peptide-induced formation of cholesterol-rich domains. Biochem. J. 2005, 390, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Schultz, G.W.; Axelrod, H.; Kramer, W.L.; Mulla, M.S. Ovisposition repellency of fatty acids and their derivatives against Culex and Aedes mosquitoes. Environ. Entomol. 2005, 11, 223–226. [Google Scholar] [CrossRef]
- Sharma, K.R.; Seenivasagan, T.; Rao, A.N.; Ganesan, K.; Agrawal, O.P.; Prakash, S. Mediation of oviposition responses in the malaria mosquito Anopheles stephensi Liston by certain fatty acid esters. Parasitol. Res. 2009, 104, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rueda, G. Uropygial gland size correlates with feather holes, body condition and wingbar size in the house sparrow Passer domesticus. J. Avian Biol. 2010, 41, 229–236. [Google Scholar] [CrossRef]
| Trial Type | Assay Type | Number of Trials | Number of Mosquitoes Choosing | Mean % (s.d.) Choosing | F-Value (d.f.) | p-Value | |
|---|---|---|---|---|---|---|---|
| Adult HOSP | Juvenile HOSP | Adult HOSP | |||||
| Adult vs. nestling | live birds | 4 | 76 | 15 | 84 (0.091) | 31.609 (1, 89) | <0.001 |
| secretions | 5 | 11 | 22 | 36 (0.165) | 2.809 (1, 13) | 0.188 | |
| Adult vs. fledgling | live birds | 5 | 71 | 66 | 51 (0.193) | 0.260 (1, 135) | 0.611 |
| secretions | 12 | 63 | 60 | 51 (0.225) | 0.020 (1, 126) | 0.887 | |
| Principal Component | ||||
|---|---|---|---|---|
| Compound | 1 | 2 | 3 | 4 |
| C24 | 0.187 | 0.543 | 0.053 | 0.748 |
| C24 | 0.157 | 0.173 | 0.25 | 0.881 |
| C24 | 0.047 | 0.549 | 0.279 | 0.57 |
| C25 | 0.228 | 0.54 | 0.144 | 0.773 |
| C26-1 | 0.182 | 0.114 | 0.454 | 0.84 |
| C26-2 | 0.237 | 0.628 | 0.379 | 0.295 |
| C26-3 | −0.2 | 0.631 | −0.118 | 0.405 |
| C26-4 | 0.011 | 0.814 | 0.278 | 0.367 |
| C26-5 | 0.314 | 0.498 | 0.238 | 0.756 |
| C27-1 | 0.236 | 0.063 | 0.622 | 0.72 |
| C27-2 | 0.181 | 0.669 | 0.282 | 0.261 |
| C27-3 | −0.103 | 0.852 | 0.126 | 0.297 |
| C27-4 | 0.087 | 0.657 | 0.355 | 0.551 |
| C27-5 | 0.401 | 0.489 | 0.334 | 0.676 |
| C28-1 | 0.248 | 0.068 | 0.751 | 0.584 |
| C28-2 | 0.117 | 0.793 | 0.107 | 0.35 |
| C28-3 | −0.135 | 0.836 | 0.193 | 0.245 |
| C28-4 | 0.206 | 0.654 | 0.394 | 0.477 |
| C28-5 | 0.478 | 0.443 | 0.38 | 0.62 |
| C29-1 | 0.19 | 0.104 | 0.869 | 0.412 |
| C29-2 | 0.152 | 0.769 | 0.143 | 0.307 |
| C29-3 | −0.072 | 0.713 | 0.3 | 0.413 |
| C29-4 | 0.35 | 0.589 | 0.379 | 0.496 |
| C29-5 | 0.592 | 0.362 | 0.405 | 0.545 |
| C30-1 | 0.167 | 0.13 | 0.908 | 0.306 |
| C30-2 | 0.365 | 0.625 | 0.057 | 0.338 |
| C30-3 | 0.088 | 0.696 | 0.413 | 0.288 |
| C30-4 | 0.511 | 0.511 | 0.402 | 0.412 |
| C30-5 | 0.667 | 0.286 | 0.423 | 0.471 |
| C31-1 | 0.186 | 0.182 | 0.913 | 0.197 |
| C31-2 | 0.465 | 0.467 | 0.118 | 0.258 |
| C31-3 | 0.224 | 0.632 | 0.317 | 0.211 |
| C31-4 | 0.63 | 0.371 | 0.386 | 0.355 |
| C31-5 | 0.727 | 0.202 | 0.439 | 0.398 |
| C32-1 | 0.299 | 0.236 | 0.842 | 0.202 |
| C32-2 | 0.481 | 0.316 | 0.205 | 0.156 |
| C32-3 | 0.144 | 0.554 | 0.363 | 0.133 |
| C32-4 | 0.681 | 0.266 | 0.392 | 0.33 |
| C32-5 | 0.757 | 0.135 | 0.475 | 0.333 |
| C33-1 | 0.42 | 0.314 | 0.771 | 0.151 |
| C33-2 | 0.371 | 0.111 | 0.118 | −0.036 |
| C33-3 | 0.177 | 0.441 | 0.398 | 0.006 |
| C33-4 | 0.754 | 0.189 | 0.392 | 0.262 |
| C33-5 | 0.789 | 0.067 | 0.489 | 0.285 |
| C34-1 | 0.532 | 0.367 | 0.624 | 0.205 |
| C34-2 | 0.216 | −0.134 | 0.058 | 0.028 |
| C34-3 | −0.169 | 0.273 | 0.052 | 0.095 |
| C34-4 | 0.747 | 0.16 | 0.44 | 0.237 |
| C34-5 | 0.82 | 0.013 | 0.466 | 0.253 |
| C35-1 | 0.634 | 0.334 | 0.539 | 0.201 |
| C35-2 | −0.093 | 0.216 | 0.002 | −0.105 |
| C35-3 | 0.85 | 0.096 | 0.262 | 0.2 |
| C35-4 | 0.849 | −0.049 | 0.406 | 0.239 |
| C35-5 | 0.779 | 0.208 | 0.372 | 0.191 |
| C36 | 0.826 | 0.01 | 0.266 | 0.175 |
| C36 | 0.856 | −0.09 | 0.336 | 0.243 |
| C36 | 0.778 | 0.156 | 0.206 | 0.234 |
| C37 | 0.693 | −0.159 | −0.019 | 0.281 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garvin, M.C.; Austin, A.; Boyer, K.; Gefke, M.; Wright, C.; Pryor, Y.; Soble, A.; Whelan, R.J. Attraction of Culex pipiens to House Sparrows Is Influenced by Host Age but Not Uropygial Gland Secretions. Insects 2018, 9, 127. https://doi.org/10.3390/insects9040127
Garvin MC, Austin A, Boyer K, Gefke M, Wright C, Pryor Y, Soble A, Whelan RJ. Attraction of Culex pipiens to House Sparrows Is Influenced by Host Age but Not Uropygial Gland Secretions. Insects. 2018; 9(4):127. https://doi.org/10.3390/insects9040127
Chicago/Turabian StyleGarvin, Mary C., Amy Austin, Kevin Boyer, Madeleine Gefke, Celestina Wright, Yemko Pryor, Anah Soble, and Rebecca J. Whelan. 2018. "Attraction of Culex pipiens to House Sparrows Is Influenced by Host Age but Not Uropygial Gland Secretions" Insects 9, no. 4: 127. https://doi.org/10.3390/insects9040127
APA StyleGarvin, M. C., Austin, A., Boyer, K., Gefke, M., Wright, C., Pryor, Y., Soble, A., & Whelan, R. J. (2018). Attraction of Culex pipiens to House Sparrows Is Influenced by Host Age but Not Uropygial Gland Secretions. Insects, 9(4), 127. https://doi.org/10.3390/insects9040127





