Do Aphids Alter Leaf Surface Temperature Patterns During Early Infestation?
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Experimental Design
2.3. Statistical Analysis
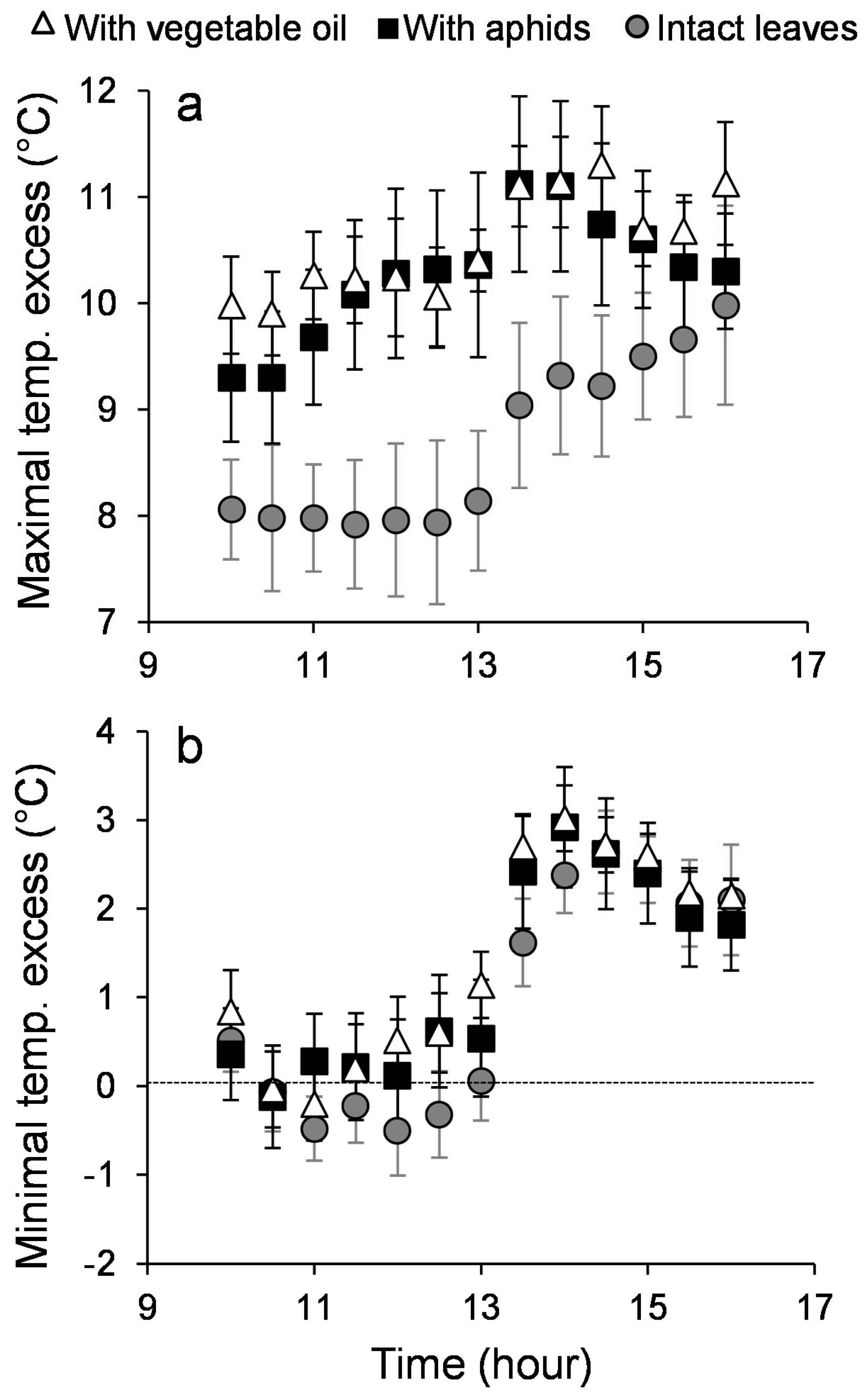
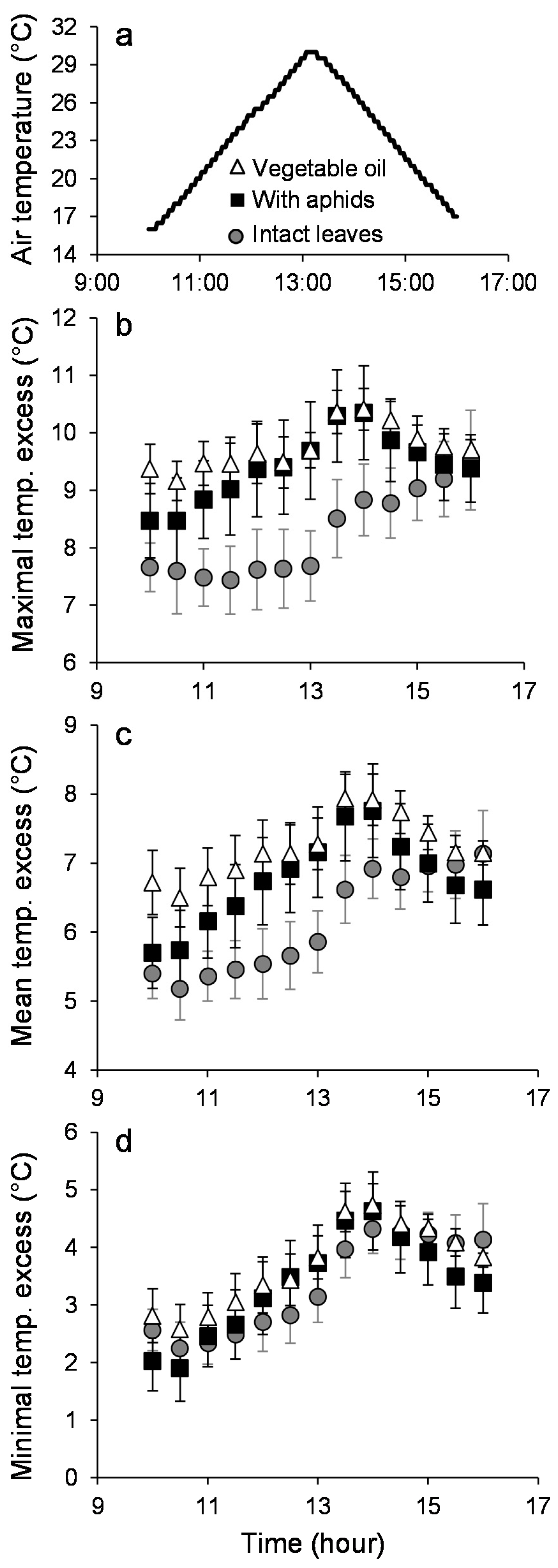
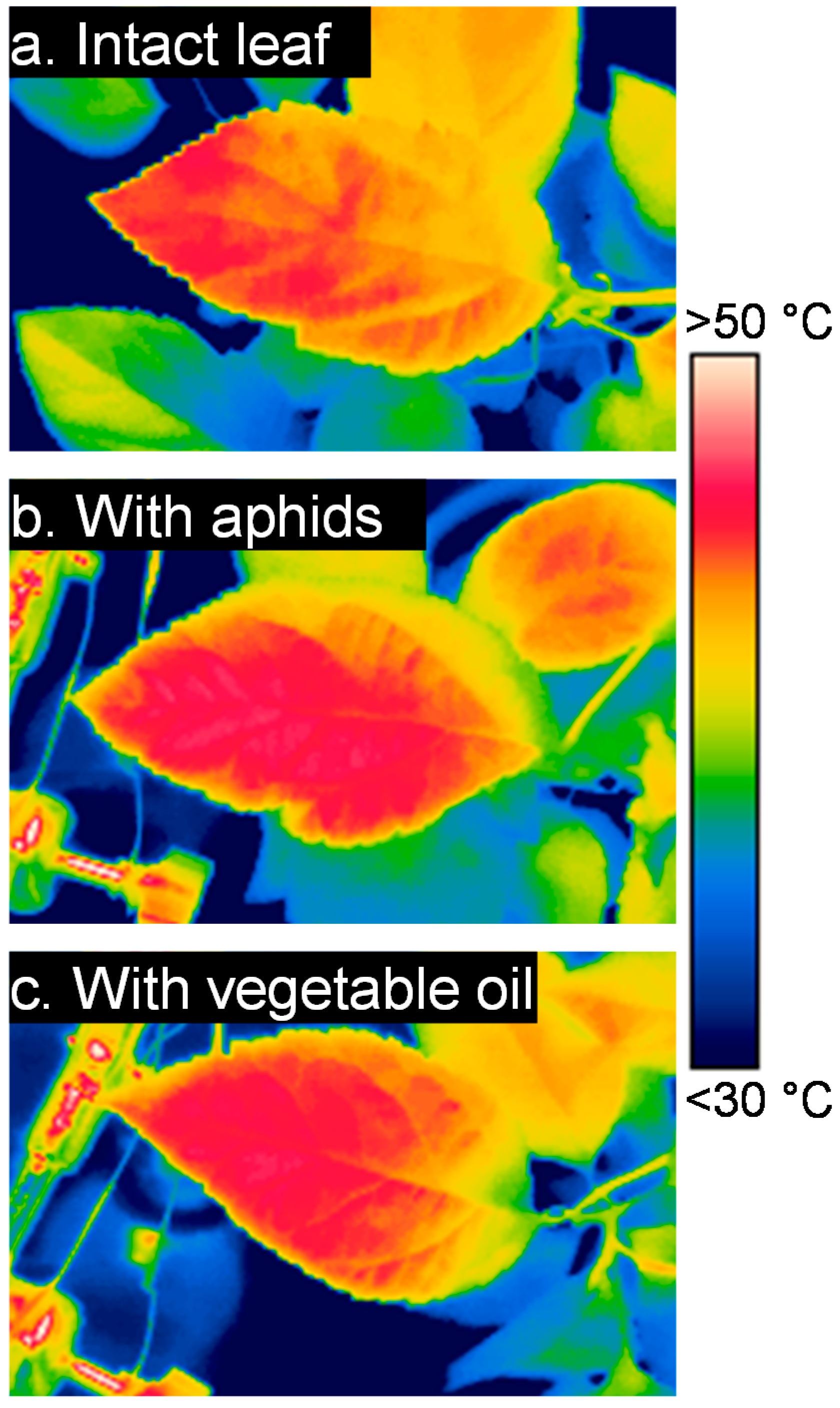
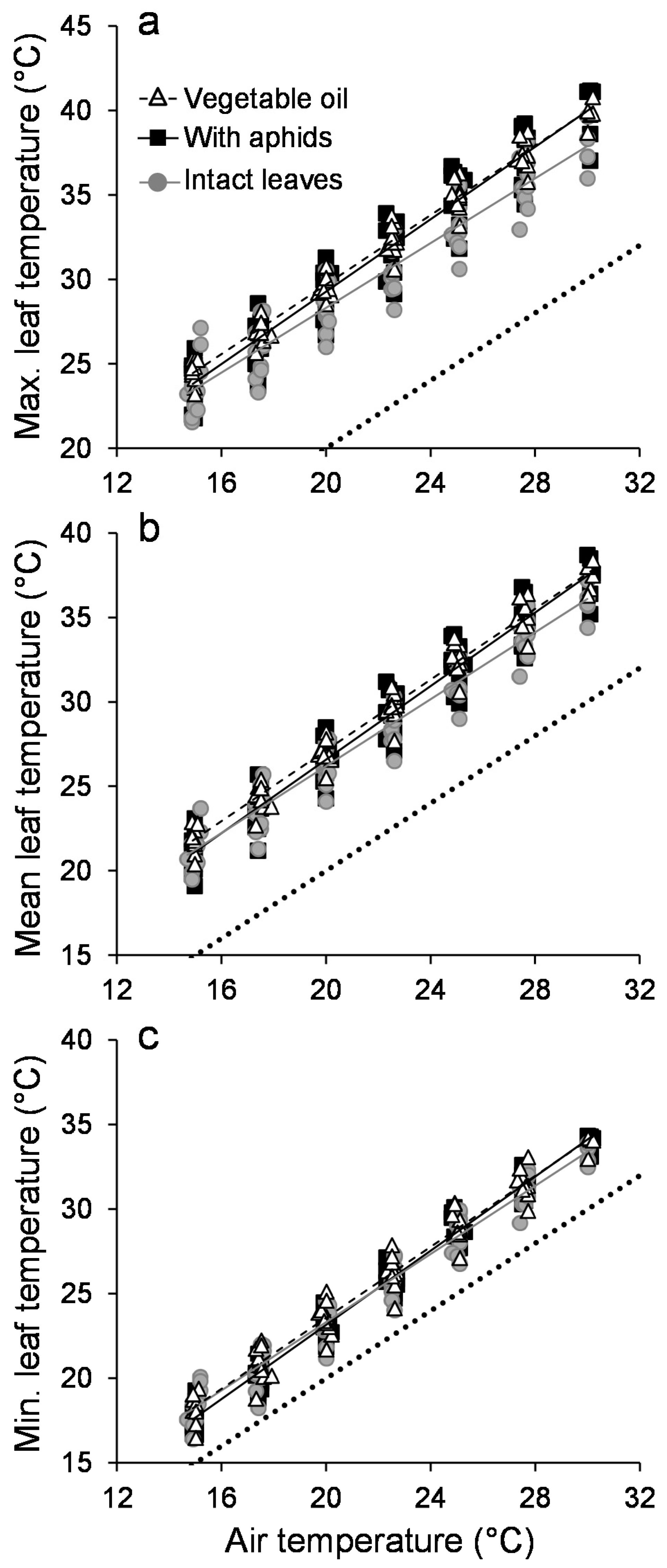
3. Results
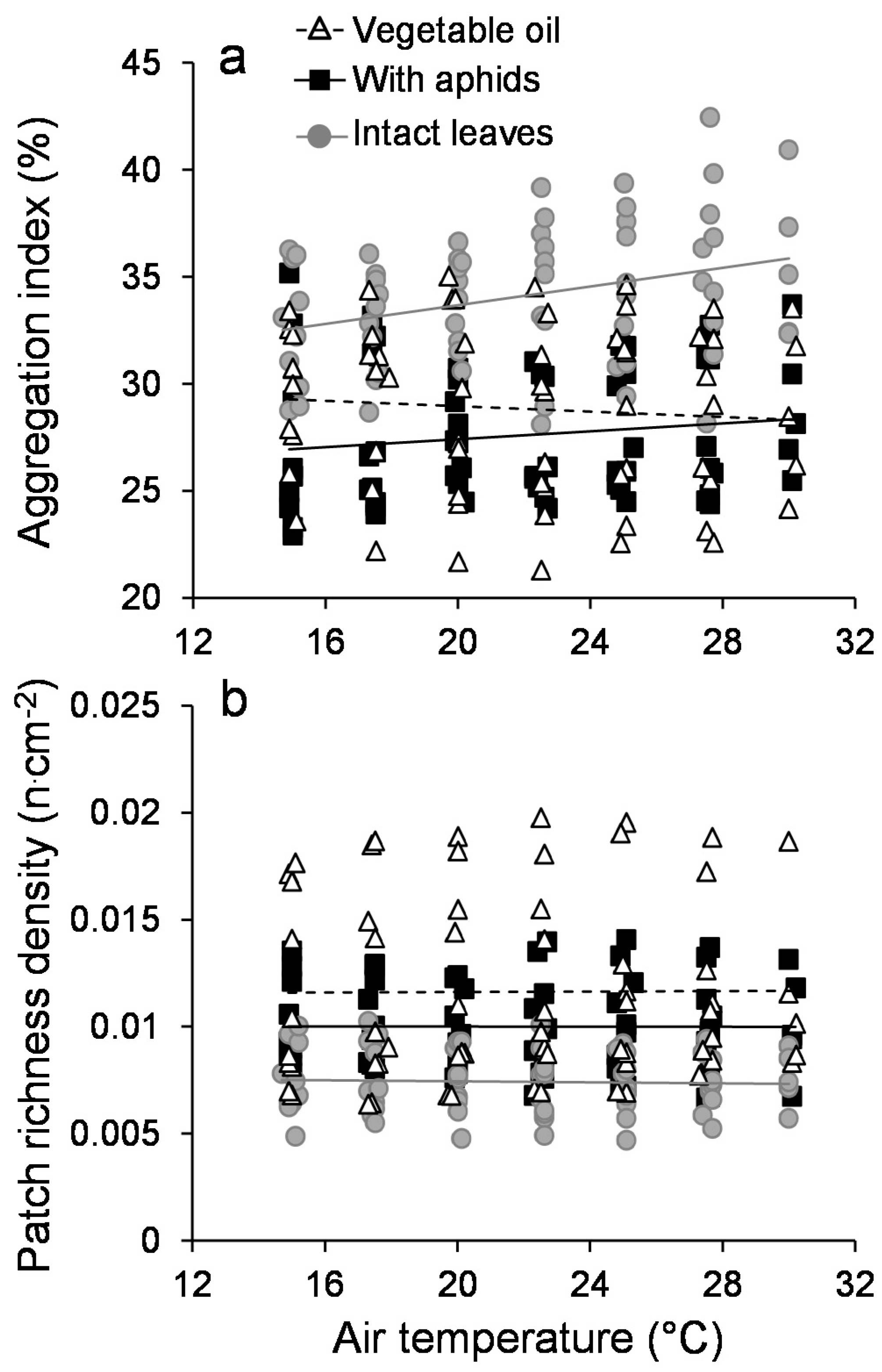
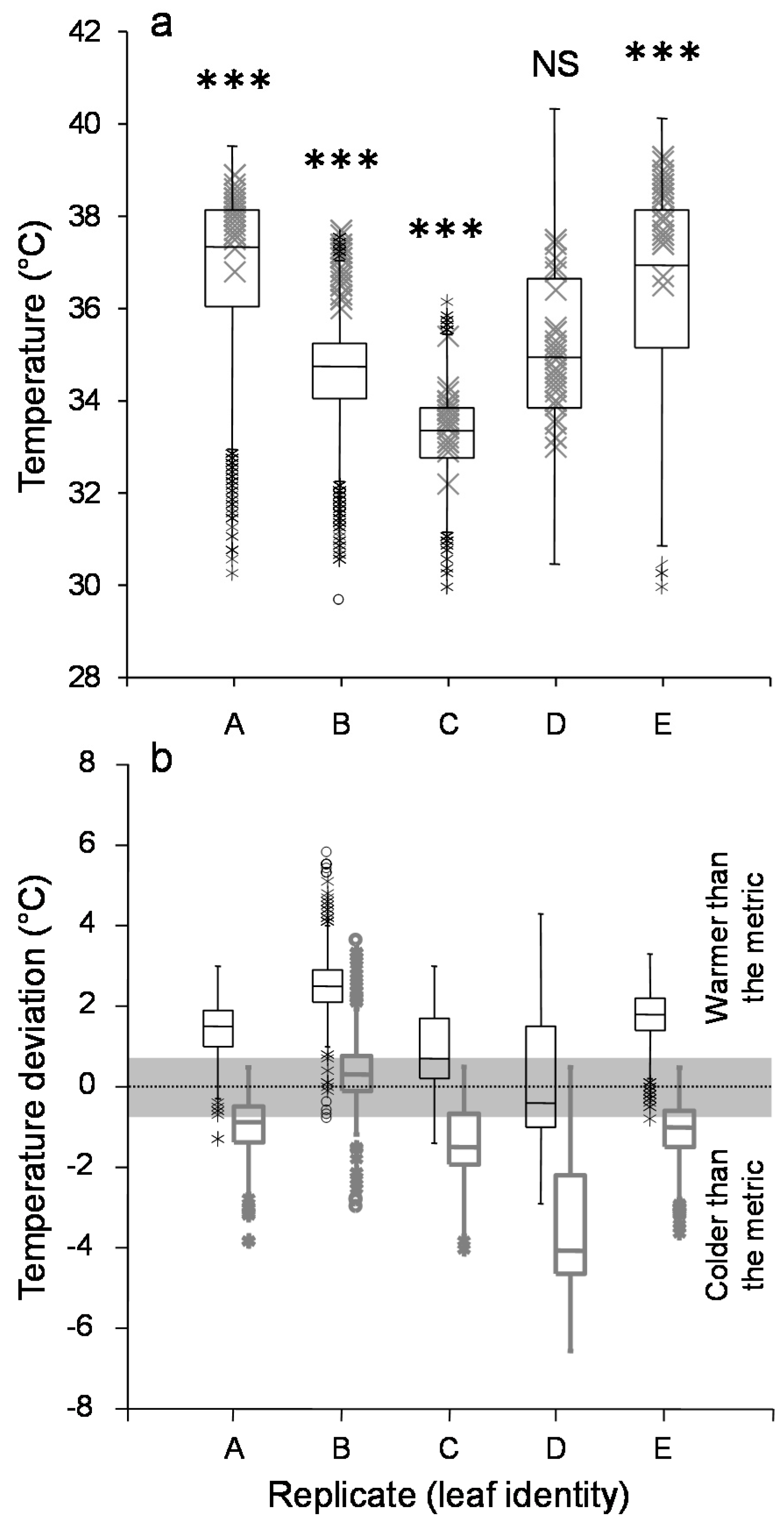
3.1. Leaf Temperature Heterogeneity: Composition
3.2. Leaf Temperature Heterogeneity: Configuration
3.3. Aphid Position within the Leaf Thermal Pattern
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A




References
- Somero, G.N. Proteins and temperature. Ann. Rev. Physiol. 1995, 57, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanism and Process in Physiological Evolution; Oxford University Press: Oxford, UK, 2002; ISBN 0-195-11703-4. [Google Scholar]
- Scherrer, D.; Körner, C. Infra-red thermometry of alpine landscapes challenges climatic warming projections. Glob. Chang. Biol. 2010, 16, 2602–2613. [Google Scholar] [CrossRef]
- Sears, M.W.; Raskin, E.; Angilletta, M.J. The world is not flat: Defining relevant thermal landscapes in the context of climate change. Integr. Comp. Biol. 2011, 51, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Koussoroplis, A.-M.; Pincebourde, S.; Wacker, A. Understanding and predicting physiological performance of organisms in fluctuating and multifactorial environments. Ecol. Monogr. 2017, 87, 178–197. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Edwards, D.P.; Diesmos, A.; Williams, S.E.; Evans, T.A. Microhabitats reduce animal’s exposure to climate extremes. Glob. Chang. Biol. 2013, 20, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Benedetti-Cecchi, L.; Bertocci, I.; Vaselli, S.; Maggi, E. Temporal variance reverses the impact of high mean intensity of stress in climate change experiments. Ecology 2006, 87, 2489–2499. [Google Scholar] [CrossRef]
- Denny, M.; Hunt, L.; Miller, L.; Harley, C. On the prediction of extreme ecological events. Ecol. Monogr. 2009, 79, 397–421. [Google Scholar] [CrossRef]
- Pincebourde, S.; Sanford, E.; Casas, J.; Helmuth, B. Temporal coincidence of environmental stress events modulates predation rates. Ecol. Lett. 2012, 15, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Shine, R.; Porter, W. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc. Natl. Acad. Sci. USA 2009, 106, 3835–3840. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009; ISBN 978-0-19-857087-5. [Google Scholar]
- Pike, D.A.; Webb, J.K.; Shine, R. Hot mothers, cool eggs: Nest-site selection by egg-guarding spiders accommodates conflicting thermal optima. Funct. Ecol. 2012, 26, 469–475. [Google Scholar] [CrossRef]
- Pincebourde, S.; Woods, H.A. Climate uncertainty on leaf surfaces: The biophysics of leaf microclimates and their consequences for leaf-dwelling organisms. Funct. Ecol. 2012, 26, 844–853. [Google Scholar] [CrossRef]
- Jones, H.G. Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant Cell Environ. 1999, 22, 1043–1055. [Google Scholar] [CrossRef]
- Leigh, A.; Sevanto, S.; Close, J.D.; Nicotra, A.B. The influence of leaf size and shape on leaf thermal dynamics: Does theory hold up under natural conditions? Plant Cell Environ. 2017, 40, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Saudreau, M.; Ezanic, A.; Adam, B.; Caillon, R.; Walser, P.; Pincebourde, S. Temperature heterogeneity over leaf surfaces: the contribution of the lamina microtopography. Plant Cell Environ. 2017, 40, 2174–2188. [Google Scholar] [CrossRef] [PubMed]
- Willmer, P. Microcimatic effects on insects at the plant surface. In Insects and the Plant Surface; Juniper, B., Southwood, R., Eds.; Edward Arnold: London, UK, 1986; pp. 65–80. ISBN 0-7131-2909-3. [Google Scholar]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics; Springer: New York, NK, USA, 1998; ISBN 978-1-4612-1626-1. [Google Scholar]
- Mott, K.A.; Buckley, T.N. Patchy stomatal conductance: Emergent collective behaviour of stomata. Trends Plant Sci. 2000, 5, 258–262. [Google Scholar] [CrossRef]
- Burrage, S.W. The microclimate at the leaf surface. In Ecology of the Leaf Surface Micro-Organisms; Preece, T.F., Dickinson, C.H., Eds.; Academic Press: London, UK, 1971; pp. 91–101. ISBN 0-12-563950-3. [Google Scholar]
- West, J.D.; Peak, D.; Peterson, J.Q.; Mott, K.A. Dynamics of stomatal patches for a single surface of Xanthium strumarium L. leaves observed with fluorescence and thermal images. Plant Cell Environ. 2005, 28, 633–641. [Google Scholar] [CrossRef]
- Potter, K.; Davidowitz, G.; Woods, H.A. Insect eggs protected from high temperatures by limited homeothermy of plant leaves. J. Exp. Biol. 2009, 212, 3448–3454. [Google Scholar] [CrossRef] [PubMed]
- Woods, H.A. Ontogenetic changes in the body temperature of an insect herbivore. Funct. Ecol. 2013, 27, 1322–1331. [Google Scholar] [CrossRef]
- Caillon, R.; Suppo, C.; Casas, J.; Woods, H.A.; Pincebourde, S. Warming decreases thermal heterogeneity of leaf surfaces: Implications for behavioural thermoregulation by arthropods. Funct. Ecol. 2014, 28, 1449–1458. [Google Scholar] [CrossRef]
- Welter, S.C. Arthropod impact on plant gas exchange. In Insect-Plant Interactions; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 135–150. ISBN 0849341213. [Google Scholar]
- Raimondo, F.; Ghirardelli, L.A.; Nardini, A.; Salleo, S. Impact of the leaf miner Cameraria ohridella on photosynthesis, water relations and hydraulics of Aesculus hippocastanum. Trees 2003, 17, 376–382. [Google Scholar] [CrossRef]
- Pincebourde, S.; Frak, E.; Sinoquet, H.; Regnard, J.L.; Casas, J. Herbivory mitigation through increased water use efficiency in a leaf mining moth-apple tree relationship. Plant Cell Environ. 2006, 29, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, B.; Pena, J.E.; Coils, A.M.; Hunsberger, A. Citrus leafminer (Lepidoptera: Gracillariidae) in lime: Assessment of leaf damage and effects on photosynthesis. Crop Protect. 1997, 16, 337–343. [Google Scholar] [CrossRef]
- Zangerl, A.R.; Hamilton, J.G.; Miller, T.J.; Crofts, A.R.; Oxborough, K.; Berenbaum, M.R.; de Lucia, E.H. Impact of folivory on photosynthesis is greater than the sum of its holes. Proc. Natl. Acad. Sci. USA 2002, 99, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Reddall, A.; Sadras, V.O.; Wilson, L.J.; Gregg, P.C. Physiological responses of cotton to two-spotted spider mite damage. Crop Sci. 2004, 44, 835–846. [Google Scholar] [CrossRef]
- Peterson, R.K.D.; Shannon, C.L.; Lenssen, A.W. Photosynthetic responses of legume species to leaf-mass consumption injury. Environ. Entomol. 2004, 33, 450–456. [Google Scholar] [CrossRef]
- Aldea, M.; Hamilton, J.G.; Resti, J.P.; Zangerl, A.R.; Berenbaum, M.R.; Delucia, E.H. Indirect effects of insect herbivory on leaf gas exchange in soybean. Plant Cell Environ. 2005, 28, 402–411. [Google Scholar] [CrossRef]
- Tang, J.Y.; Zielinski, R.E.; Zangerl, A.R.; Crofts, A.R.; Berenbaum, M.R.; Delucia, E.H. The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana. J. Exp. Bot. 2006, 57, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, H.; Yuan, L.; Wei, J.; Zhang, W.; Ge, F. Plant stomatal closure improves aphid feeding under elevated CO2. Glob. Change Biol. 2015, 21, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Shannag, H.K.; Thorvilson, H.; El-Shatnawimk, M.K. Changes in photosynthetic and transpiration rates of cotton leaves infested with the cotton aphid, Aphis gossypii: Unrestricted infestation. Ann. Appl. Biol. 1998, 132, 13–18. [Google Scholar] [CrossRef]
- Shannag, H.K. Effect of black bean aphid, Aphis fabae, on transpiration, stomatal conductance and crude protein content of faba bean. Ann. Appl. Biol. 2007, 151, 183–188. [Google Scholar] [CrossRef]
- Kennedy, R.A.; Johnson, D. Changes in photosynthetic characteristics during leaf development in apple. Photosynth. Res. 1981, 2, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Faye, E.; Dangles, O.; Pincebourde, S. Distance makes the difference in thermography for ecological studies. J. Therm. Biol. 2016, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pincebourde, S.; Casas, J. Multitrophic biophysical budgets: Thermal ecology of an intimate herbivore insect-plant interaction. Ecol. Monogr. 2006, 76, 175–194. [Google Scholar] [CrossRef]
- McGarigal, K.; Marks, B.J. Fragstats: Spatial Pattern Analysis Program for Quantifying Landscape Structure; Oregon State University: Corvallis, OR, USA, 1994. [Google Scholar]
- He, H.S.; DeZonia, B.E.; Mladenoff, D.J. An aggregation index (AI) to quantify spatial patterns of landscapes. Land. Ecol. 2000, 15, 591–601. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-0. [Google Scholar]
- Hilker, M.; Fatouros, N.E. Plant responses to insect egg deposition. Ann. Rev. Entomol. 2015, 60, 493–515. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, M.; Tooker, J.F.; Luthe, D.S.; Felton, G.W. Plants on early alert: Glandular trichomes as sensors for insect herbivores. New Phytol. 2009, 184, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Woods, H.A. Water loss and gas exchange by eggs of Manduca sexta: Trading off costs and benefits. J. Insect Physiol. 2010, 56, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Phytophages of xylem and phloem: A comparison of animal and plant sap-feeders. Adv. Ecol. Res. 1993, 13, 135–234. [Google Scholar] [CrossRef]
- Pollard, D.G. Plant penetration by feeding aphids (Hemiptera, Aphidoidea): A review. Bull. Entomol. Res. 1973, 62, 631–714. [Google Scholar] [CrossRef]
- Mugford, S.T.; Barclay, E.; Drurey, C.; Findlay, K.C.; Hogenhout, S.A. An immuno-suppressive aphid saliva protein is delivered into the cytosol of plant mesophyll cells during feeding. MPMI 2016, 29, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Cheng, L. Photosystem 2 is more tolerant to high temperature in apple (Malus domestica Borkh.) leaves than in fruit peel. Photosynthetica 2009, 47, 112–120. [Google Scholar] [CrossRef]
- Pincebourde, S.; Casas, J. Hypoxia and hypercarbia in endophagous insects: Larval position in the plant gas exchange network is key. J. Insect Physiol. 2016, 84, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Ann. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Arbab, A.; Kontodimas, D.C.; Sahragard, A. Estimating development of Aphis pomi (DeGeer) (Homoptera: Aphididae) using linear and nonlinear models. Environ. Entomol. 2006, 35, 1208–1215. [Google Scholar] [CrossRef][Green Version]
- Niehaus, A.C.; Angilletta, M.J.; Sears, M.W.; Franklin, C.E.; Wilson, R.S. Predicting the physiological performance of ectotherms in fluctuating thermal environments. J. Exp. Biol. 2012, 215, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Ma, C.S. Effect of acclimation on heat-escape temperatures of two aphid species: Implications for estimating behavioral response of insects to climate warming. J. Insect Physiol. 2011, 58, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Ma, C.S. Climate warming may increase aphids’ dropping probabilities in response to high temperatures. J. Insect Physiol. 2012, 58, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, J.J.A.; Casas, J.; Pincebourde, S. Nutritional ecology of insect-plant interactions: Persistent handicaps and the need for innovative approaches. Oikos 2005, 108, 194–201. [Google Scholar] [CrossRef]
- Pincebourde, S.; van Baaren, J.; Rasmann, S.; Rasmont, P.; Rodet, G.; Martinet, B.; Calatayud, P.-A. Plant-insect interactions in a changing world. Adv. Bot. Res. 2017, 81, 289–332. [Google Scholar] [CrossRef]
- Woods, H.A.; Dillon, M.E.; Pincebourde, S. The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change. J. Therm. Biol. 2015, 54, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Pincebourde, S.; Suppo, C. The vulnerability of tropical ectotherms to warming is modulated by the microclimatic heterogeneity. Integr. Comp. Biol. 2016, 56, 85–97. [Google Scholar] [CrossRef] [PubMed]
| Variable | Effect | Sum sq | df | F-value | p-Value |
|---|---|---|---|---|---|
| Tmax | Treatment | 80.9 | 2 | 22.83 | <0.01 |
| Tair | 4548.6 | 1 | 2565.42 | <0.01 | |
| Treatment*Tair | 9.9 | 2 | 2.79 | 0.064 | |
| Residuals | 333.3 | 188 | |||
| Tmean | Treatment | 35.4 | 2 | 13.91 | <0.01 |
| Tair | 4755 | 1 | 3732.65 | <0.01 | |
| Treatment*Tair | 7.5 | 2 | 2.93 | 0.056 | |
| Residuals | 239.5 | 188 | |||
| Tmin | Treatment | 4.8 | 2 | 2.66 | 0.073 |
| Tair | 4840.9 | 1 | 5371.81 | <0.01 | |
| Treatment*Tair | 5.7 | 2 | 3.14 | 0.046 | |
| Residuals | 169.4 | 188 | |||
| AI | Treatment | 1542.4 | 2 | 67.32 | <0.01 |
| Tair | 30.7 | 1 | 2.68 | 0.103 | |
| Treatment*Tair | 57.4 | 2 | 2.50 | 0.084 | |
| Residuals | 2153.7 | 188 | |||
| PRD | Treatment | 5.8 × 10−4 | 2 | 34.74 | <0.01 |
| Tair | 3.0 × 10−7 | 1 | 3.1 × 10−3 | 0.955 | |
| Treatment*Tair | 2.5 × 10−6 | 2 | 0.01 | 0.985 | |
| Residuals | 1.6 × 10−3 | 188 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cahon, T.; Caillon, R.; Pincebourde, S. Do Aphids Alter Leaf Surface Temperature Patterns During Early Infestation? Insects 2018, 9, 34. https://doi.org/10.3390/insects9010034
Cahon T, Caillon R, Pincebourde S. Do Aphids Alter Leaf Surface Temperature Patterns During Early Infestation? Insects. 2018; 9(1):34. https://doi.org/10.3390/insects9010034
Chicago/Turabian StyleCahon, Thomas, Robin Caillon, and Sylvain Pincebourde. 2018. "Do Aphids Alter Leaf Surface Temperature Patterns During Early Infestation?" Insects 9, no. 1: 34. https://doi.org/10.3390/insects9010034
APA StyleCahon, T., Caillon, R., & Pincebourde, S. (2018). Do Aphids Alter Leaf Surface Temperature Patterns During Early Infestation? Insects, 9(1), 34. https://doi.org/10.3390/insects9010034






