The Biology and Control of the Greater Wax Moth, Galleria mellonella
Abstract
:1. Introduction
2. The Biology of the Greater Wax Moth, G. mellonella
2.1. Taxonomy
2.2. Morphology
2.3. Life Cycle
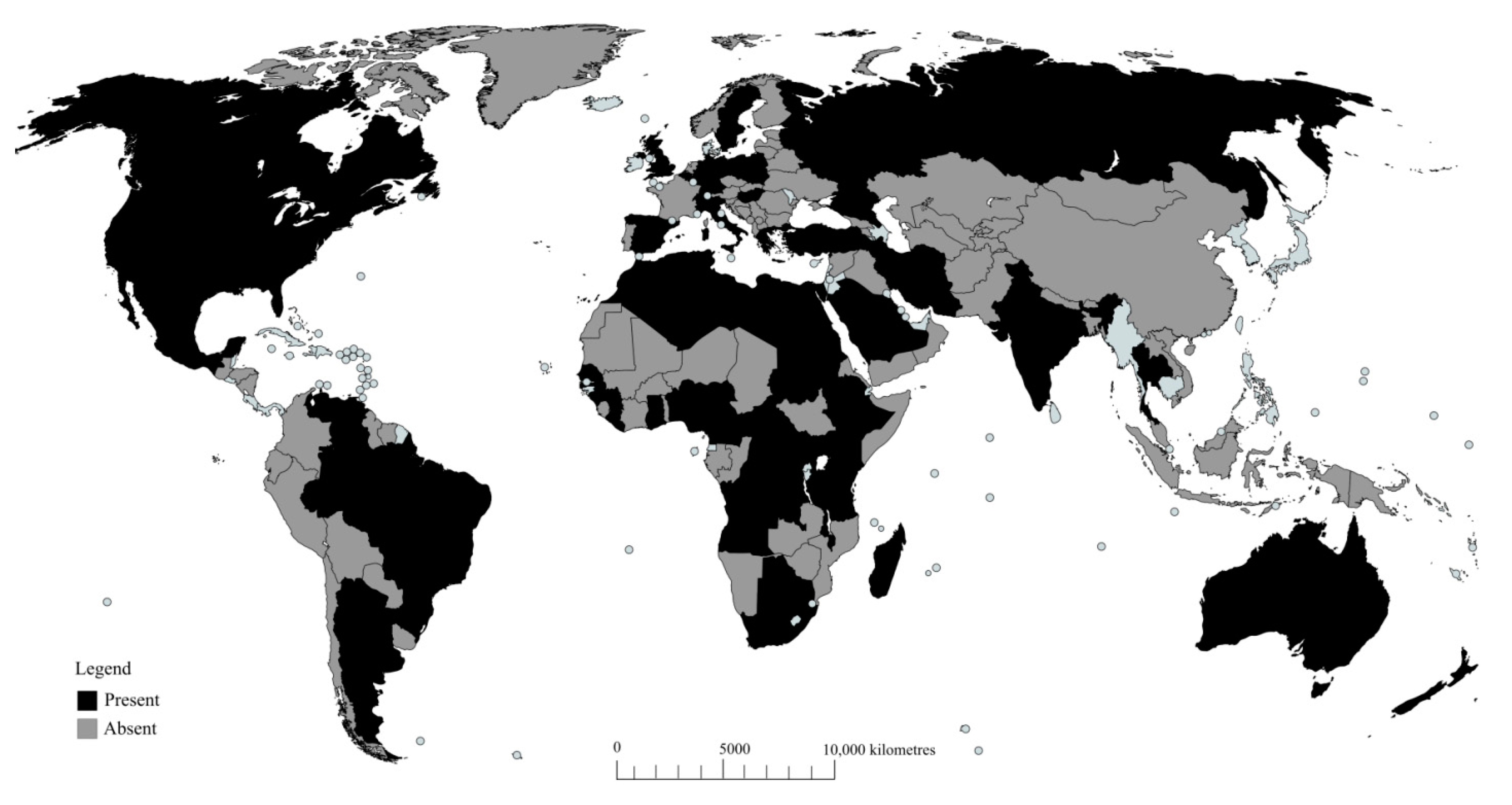
2.4. Distribution
3. Economic Importance of the Greater Wax Moth
4. Management of the Greater Wax Moth
4.1. Cultural Practices
4.2. Temperature Control
4.3. Chemical Control
4.4. Biological Control
4.4.1. Bacillus Thuringiensis H Serotype
4.4.2. Bracon hebetor and Apanteles galleriae
4.4.3. Trichogramma Species
4.4.4. Red Imported Fire Ants (RIFA)
4.4.5. Sterile Insect Technique (SIT)
4.4.6. Semiochemicals
5. Future Prospects
- The potential of utilizing entomopathogens and baculoviruses as biological control agents. For instance, classical application of entomopathogenic nematodes (EPNs) has been applied to control other pests such as the Japanese beetle (Popillia japonica Newman) [118] and black vine weevil (Otiorhynchus sulcatus Fabricius) [119] and offers prospect of controlling the small hive beetle (Aethina tumida) [120,121]. Additionally, entomopathogenic fungi (EPFs) belonging to the genera Hyphomycetes, Zygomycota, and Deuteromycetes, have been significant against management of gypsy moth (Lymantria dispar Linnaeus) in the USA [122]. Recently, Dougherty et al. [123] reported the successful application of propagated baculovirus 1 against a GWM population. However, more studies are needed to optimize formulations and dosages of the virus. In addition, the reported cases of successful application of EPNs represents simple systems of pest-host interactions as opposed to the complex system of the GWM-honeybee colony interactions. Therefore, application of EPNs and EPFs against the GWM should probably focus on suppressing its population “outside” active bee hives, such as in storage facilities. In addition, future exploration of EPs should take into consideration the complex nature of honeybee colonies and the fact that some of these agents might present potential risks to life stages of colony members.
- Semiochemicals including pheromone, kairomone, repellents, and the development of semiochemical-based trapping systems. Such systems should take into consideration compositional variations in the chemical cues brought about by geographic disparities.
- Food and ovipositional baits. Volatiles released from food and oviposition sites of other pests dispensed either individually or in combination, have previously been shown to improve mass trapping [124,125,126]. Identification of such chemical signatures attractive to the greater wax moth would provide an opportunity to develop optimized trapping systems.
- Population modelling. Although the regional reports highlighted in this article are useful in understanding the current spread of GWM, little is known regarding the phenology of this pest in the future. Such knowledge gaps can be filled by using species distribution models (SDMs) [127] which take into account the interplay between the environmental and geographical variables. Recently, an ecological niche (EN) model was used to predict the future distribution of honeybee pests (including GWM) in Kenya [67]. We therefore recommend the application of such models to provide insight into the prospective geographical range of the pest, and in so doing, inform decision making in formulating precautionary measures to prevent and or minimize infestation of new colonies.
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kluser, S.; Neumann, P.; Chauzat, M.-P.; Pettis, J.S.; Peduzzi, P.; Witt, R.; Fernandez, N.; Theuri, M. Global Honey Bee Colony Disorders and Other Threats to Insect Pollinators; United Nations Environment Programme: Nairobi, Kenya, 2010. [Google Scholar]
- Meixner, M.D. A historical review of managed honey bee populations in europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Biesmeijer, J.C.; Roberts, S.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.; Potts, S.; Kleukers, R.; Thomas, C. Parallel declines in pollinators and insect-pollinated plants in britain and the netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Status of Pollinators in North America; National Academies Press: Washington, DC, USA, 2007; p. 322. [Google Scholar]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Royal Soc. London B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, M.H.; De Lange, W.J.; Veldtman, R. Valuing insect pollination services with cost of replacement. PLoS ONE 2008, 3, e3128. [Google Scholar] [CrossRef] [PubMed]
- Pirk, C.W.; Strauss, U.; Yusuf, A.A.; Démares, F.; Human, H. Honeybee health in Africa—A review. Apidologie 2015, 47, 276–300. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M. Honey bee toxicology. Annu. Rev. Entomol. 2015, 60, 415. [Google Scholar] [CrossRef] [PubMed]
- Shimanuki, H. Diseases and pests of honey bees. In Bee Keeping in the United States; Science and Education Administration, United States Department of Agriculture: Washington, DC, USA, 1980; Volume 335, pp. 118–128. [Google Scholar]
- Plettner, E.; Eliash, N.; Singh, N.K.; Pinnelli, G.R.; Soroker, V. The chemical ecology of host-parasite interaction as a target of Varroa destructor control agents. Apidologie 2017, 48, 78–92. [Google Scholar] [CrossRef]
- Dietemann, V.; Ellis, J.D.; Neumann, P. The Coloss Beebook Volume I: Standard Methods for Apis mellifera Research; IBRA, International Bee Research Association: Bristol, UK, 2013; Volume 52. [Google Scholar]
- Ritter, W.; Akratanakul, P. Honey Bee Diseases and Pests: A Practical Guide; FAO: Rome, Italy, 2006; Volume 4. [Google Scholar]
- Williams, J.L. Insects:Lepidoptera (moths). In Honey Bee Pests, Predators, and Diseases; Morse, R., Flottum, K., Eds.; AI Root Company: Medina, OH, USA, 1997; pp. 121–141. [Google Scholar]
- Harding, C.R.; Schroeder, G.N.; Collins, J.W.; Frankel, G. Use of Galleria mellonella as a model organism to study Legionella pneumophila infection. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ramarao, N.; Nielsen-Leroux, C.; Lereclus, D. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J. Vis. Exp. 2012, 70, e4392. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.D.; Graham, J.R.; Mortensen, A. Standard methods for wax moth research. J. Apic. Res. 2013, 52, 1–17. [Google Scholar] [CrossRef]
- Chantawannakul, P.; de Guzman, L.I.; Li, J.; Williams, G.R. Parasites, pathogens, and pests of honeybees in Asia. Apidologie 2016, 47, 1–24. [Google Scholar] [CrossRef]
- Paddock, F.B. The Beemoth or Waxworm; Texas Agricultural Experiment Stations: College Station, TX, USA, 1918. [Google Scholar]
- Gulati, R.; Kaushik, H. Enemies of honeybees and their management—A review. Agric. Rev. 2004, 25, 189–200. [Google Scholar]
- Smith, T. External morphology of the larva, pupa, and adult of the wax moth, Galleria mellonella L. J. Kans. Entomol. Soc. 1965, 38, 287–310. [Google Scholar]
- Shimanuki, H. Controlling the Greater Wax Moth: A Pest of Honeycombs; Science and Education Administration, US: United States Department of Agriculture, Washington, DC, USA, 1981; Volume 2217, pp. 1–13. [Google Scholar]
- Angelini, D.R.; Kaufman, T.C. Insect appendages and comparative ontogenetics. Dev. Biol. 2005, 286, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Leyrer, R.; Monroe, R. Isolation and identification of the scent of the moth, Galleria mellonella, and a revaluation of its sex pheromone. J. Insect Physiol. 1973, 19, 2267–2271. [Google Scholar] [CrossRef]
- Nielsen, R.A.; Brister, C. Greater wax moth: Behavior of larvae. Ann. Entomol. Soc. Am. 1979, 72, 811–815. [Google Scholar] [CrossRef]
- Charriere, J.-D.; Imdorf, A. Protection of honey combs from wax moth damage. Am. Bee J. 1999, 139, 627–630. [Google Scholar]
- Krams, I.; Kecko, S.; Kangassalo, K.; Moore, F.R.; Jankevics, E.; Inashkina, I.; Krama, T.; Lietuvietis, V.; Meija, L.; Rantala, M.J. Effects of food quality on trade-offs among growth, immunity and survival in the greater wax moth Galleria mellonella. Insect Sci. 2015, 22, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Banville, N.; Browne, N.; Kavanagh, K. Effect of nutrient deprivation on the susceptibility of Galleria mellonella larvae to infection. Virulence 2012, 3, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Huddleston, P. Life history of the greater wax moth, Galleria mellonella L., in arkansas. J. Kans. Entomol. Soc. 1962, 35, 212–216. [Google Scholar]
- Kwadha, C.A.; Fombong, A.T. Identification of larval aggregation pheromone components in the greater wax moth, Galleria mellonella. 2017; under preparation. [Google Scholar]
- Nielsen, R.A.; Brister, D. The greater wax moth: Adult behavior. Ann. Entomol. Soc. Am. 1977, 70, 101–103. [Google Scholar] [CrossRef]
- Spangler, H.G. Sound and the moths that infest beehives. Fla. Entomol. 1988, 71, 467–477. [Google Scholar] [CrossRef]
- Akratanakul, P. Honeybee Diseases and Enemies in Asia: A Practical Guide; Food & Agriculture Organization: Rome, Italy, 1987. [Google Scholar]
- Hussein, M.H. Beekeeping in Africa: I. North, east, north-east and west African countries. Apiacta 2000, 1, 32–48. [Google Scholar]
- Al-Ghamdi, A. Survey of Honeybee Diseases, Pests and Predators in Saudi Arabia. Master’s Thesis, University of Wales, Cardiff, UK, 1990. [Google Scholar]
- Carroll, T. A Beginners Guide to Beekeeping in Kenya; Baraka Agricultural Training College: Nakuru, Kenya, 2006. [Google Scholar]
- El-Niweiri, M.A.A. Survey of the Pests and Diseases of Honeybees in Sudan; UOFK: Khartoum, Sudan, 2015. [Google Scholar]
- Kajobe, R.; Agea, J.G.; Kugonza, D.R.; Alioni, V.; Otim, A.; Rureba, T.; Marris, G. National Beekeeping Calendar, Honeybee Pest and Disease Control Methods for Improved Production of Honey and Other Hive Products in Uganda; National Agricultural Research Organization: Entebbe, Uganda, 2009. [Google Scholar]
- Kebede, E.; Redda, Y.T.; Hagos, Y.; Ababelgu, N.A. Prevalence of wax moth in modern hive with colonies in kafta humera. Anim. Vet. Sci. 2015, 3, 132–135. [Google Scholar] [CrossRef]
- Keshlaf, M. Beekeeping in libya. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2014, 8, 32–35. [Google Scholar]
- Lawal, O.; Banjo, A. A checklist of pests and visitors of Apis mellifera adansonii (honeybee) in the six states of south Western Nigeria. Apiacta 2007, 42, 39–63. [Google Scholar]
- Swart, J.; Johannsmeier, M.; Tribe, G.; Kryger, P. Diseases and pests of honeybees. In Beekeeping in South Africa, 3rd ed.; Science and Education Administration: United States Department of Agriculture, Washington, DC, USA, 2001; pp. 198–222. [Google Scholar]
- Philips, J. News around the World. Available online: http://www.beesfordevelopment.org/media/3245/bfdj-98-news-recent-research.pdf (accessed on 30 November 2016).
- Al-Chzawi, A.A.; Zaitoun, S.T.; Shannag, H.K. Incidence and geographical distribution of honeybee (Apis mellifera L.) pests in Jordan. In Annales de la Société Entomologique de France; Taylor & Francis: Abingdon, UK, 2009; pp. 305–308. [Google Scholar]
- Al-Ghamdi, A.; Nuru, A. Beekeeping in the kingdom of Saudi Arabia opportunities and challenges. Bee World 2013, 90, 54–57. [Google Scholar] [CrossRef]
- Shrestha, J.; Shrestha, K. Beekeeping in Nepal: Problems and potentials. In Asian bees and beekeeping, Progress of Research and Development, Proceeding of the Fourth Asian Apiculture Association International Conference, Nepal-Kathmandu, Kathmandu, Nepal, 23–28 March 1998; Science: New York, NY, USA, 2000; pp. 262–265. [Google Scholar]
- Suwannapong, G.; Benbow, M.; Nieh, J. Biology of thai honeybees: Natural history and threats. In Bees: Biology, Threats and Colonies; Nova Science: Hauppauge, NY, USA, 2012. [Google Scholar]
- Traiyasut, P.; Mookhploy, W.; Kimura, K.; Yoshiyama, M.; Khongphinitbunjong, K.; Chantawannakul, P.; Buawangpong, N.; Saraithong, P.; Burgett, M.; Chukeatirote, E. First detection of honey bee viruses in wax moth. Chiang Mai J. Nat. Sci. 2016, 43, 695–698. [Google Scholar]
- Lebedeva, K.; Vendilo, N.; Ponomarev, V.; Pletnev, V.; Mitroshin, D. Identification of pheromone of the greater wax moth Galleria mellonella from the different regions of Russia. IOBC Wprs Bull. 2002, 25, 229–232. [Google Scholar]
- Szabo, T.I.; Nelson, D.L. Beekeeping in Western Canada; Agriculture Canada: Ottawa, Canada, 1986. [Google Scholar]
- Mondragón, L.; Spivak, M.; Vandame, R. A multifactorial study of the resistance of honeybees Apis mellifera to the mite Varroa destructor over one year in Mexico. Apidologie 2005, 36, 345–358. [Google Scholar] [CrossRef]
- Eischen, F.A.; Rinderer, T.E.; Dietz, A. Nocturnal defensive responses of Africanized and european honey bees to the greater wax moth (Galleria mellonella L.). Anim. Behav. 1986, 34, 1070–1077. [Google Scholar] [CrossRef]
- Katzenelson, M. Argentine beekeeping. Apiacta 1968, 1, 1–2. [Google Scholar]
- Corrêa-Marques, M.-H.; De Jong, D. Uncapping of worker bee brood, a component of the hygienic behavior of Africanized honey bees against the mite Varroa jacobsoni Oudemans. Apidologie 1998, 29, 283–290. [Google Scholar] [CrossRef]
- White, B. Small hive beetle. Apiacta 2004, 38, 295–301. [Google Scholar]
- Base, B. Wax moth. Available online: http://www.nationalbeeunit.com/index.cfm?pageid=207 (accessed on 6 September 2016).
- Simon-Delso, N.; San Martin, G.; Bruneau, E.; Minsart, L.-A.; Mouret, C.; Hautier, L. Honeybee colony disorder in crop areas: The role of pesticides and viruses. PLoS ONE 2014, 9, e103073. [Google Scholar] [CrossRef] [PubMed]
- Topolska, G.; Gajda, A.; Hartwig, A. Polish honey bee colony-loss during the winter of 2007/2008. J. Apic. Sci 2008, 52, 95–104. [Google Scholar]
- The British Beekeepers Association. Wax moth in the apiary. Available online: http://www.bbka.org.uk/files/library/wax_moth_l020_(data)_r2_1342860174.pdf (accessed on 6 September 2016).
- Anderson, D.L. Pests and pathogens of the honeybee (Apis mellifera L.) in Fiji. J. Apic. Res. 1990, 29, 53–59. [Google Scholar] [CrossRef]
- Rasolofoarivao, H.; Clémencet, J.; Ravaomanarivo, L.H.R.; Razafindrazaka, D.; Reynaud, B.; Delatte, H. Spread and strain determination of Varroa destructor (Acari: Varroidae) in Madagascar since its first report in 2010. Exp. Appl. Acarol. 2013, 60, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Murray, R. Diseases of honeybees in New Zealand. N. Z. Entomol. 1988, 15. [Google Scholar] [CrossRef]
- Kato, M.; Shibata, A.; Yasui, T.; Nagamasu, H. Impact of introduced honeybees, Apis mellifera, upon native bee communities in the Bonin (Ogasawara) islands. Res. Popul. Ecol. 1999, 41, 217–228. [Google Scholar] [CrossRef]
- Makori, D.M.; Fombong, A.T.; Abdel-Rahman, E.M.; Nkoba, K.; Ongus, J.; Irungu, J.; Mosomtai, G.; Makau, S.; Mutanga, O.; Odindi, J. Predicting spatial distribution of key honeybee pests in Kenya using remotely sensed and bioclimatic variables: Key honeybee pests distribution models. ISPRS Int. J. Geo-Inf. 2017, 6, 66. [Google Scholar] [CrossRef]
- Türker, L.; Togan, I.; Ergezen, S.; Özer, M. Novel attractants of Galleria mellonella L. (Lepidoptera Pyralidae Galleriinae). Apidologie 1993, 24, 425–430. [Google Scholar] [CrossRef]
- Hood, W.M.; Horton, P.M.; McCreadie, J.W. Field evaluation of the red imported fire ant (Hymenoptera: Formicidae) for the control of wax moths (Lepidoptera: Pyralidae) in stored honey bee comb. J. Agric. Urban Entomol. 2003, 20, 93–103. [Google Scholar]
- Jafari, R.; Goldasteh, S.; Afrogheh, S. Control of the wax moth Galleria mellonella L. (Lepidoptera: Pyralidae) by the male sterile technique (mst). Arch. Biol. Sci. 2010, 62, 309–313. [Google Scholar] [CrossRef]
- Carson, R. Silent Spring; Houghton Mifflin Harcourt: Boston, MA, USA, 2002. [Google Scholar]
- Burges, H. Control of wax moth Galleria mellonella on beecomb by h-serotype Bacillus turingiensis and the effect of chemical additives. Apidologie 1977, 8, 155–168. [Google Scholar] [CrossRef]
- Basedow, T.; Shafie, H.; Abo-El-Saad, M.; Al Ajlan, A. Evaluation of Bacillus thuringiensis aizawi and neem for controlling the larvae of the greater wax moth, Galleria mellonela (Lepidopthera: Pyralidae). Int. J. Agric. Biol. 2012, 14, 60–63. [Google Scholar]
- Burges, H.D. Control of wax moths: Physical, chemical and biological methods. Bee World 1978, 59, 129–138. [Google Scholar] [CrossRef]
- Hanley, A.V.; Huang, Z.Y.; Pett, W.L. Effects of dietary transgenic bt corn pollen on larvae of Apis mellifera and Galleria mellonella. J. Apic. Res. 2003, 42, 77–81. [Google Scholar] [CrossRef]
- McGaughey, W.; Johnson, D. Influence of crystal protein composition of Bacillus thuringiensis strains on cross-resistance in indianmeal moths (Lepidoptera: Pyralidae). J. Econ. Entomol. 1994, 87, 535–540. [Google Scholar] [CrossRef]
- Shelton, A.M.; Robertson, J.; Tang, J.; Perez, C.; Eigenbrode, S.; Preisler, H.; Wilsey, W.; Cooley, R. Resistance of diamondback moth (Lepidoptera: Plutellidae) to Bacillus thuringiensis subspecies in the field. J. Econ. Entomol. 1993, 86, 697–705. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Finson, N.; Johnson, M.W.; Heckel, D.G. Cross-resistance to Bacillus thuringiensis toxin cryif in the diamondback moth (Plutella xylostella). Appl. Environ. Microbiol. 1994, 60, 4627–4629. [Google Scholar] [PubMed]
- Dubovskiy, I.M.; Grizanova, E.V.; Whitten, M.M.; Mukherjee, K.; Greig, C.; Alikina, T.; Kabilov, M.; Vilcinskas, A.; Glupov, V.V.; Butt, T.M. Immuno-physiological adaptations confer wax moth Galleria mellonella resistance to Bacillus thuringiensis. Virulence 2016, 7, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Betz, F.S.; Hammond, B.G.; Fuchs, R.L. Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul. Toxicol. Pharmacol. 2000, 32, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Romero, R.; Desneux, N.; Decourtye, A.; Chaffiol, A.; Pham-Delègue, M. Does cry1ab protein affect learning performances of the honey bee Apis mellifera L.(Hymenoptera, Apidae)? Ecotoxicol. Environ. Saf. 2008, 70, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Niu, C.-Y.; Lei, C.-L.; Cui, J.-J.; Desneux, N. Quantification of toxins in a cry1ac+ cpti cotton cultivar and its potential effects on the honey bee Apis mellifera L. Ecotoxicology 2010, 19, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Niu, C.-Y.; Biondi, A.; Desneux, N. Does transgenic cry1ac+ cpti cotton pollen affect hypopharyngeal gland development and midgut proteolytic enzyme activity in the honey bee Apis mellifera L. (hymenoptera, apidae)? Ecotoxicology 2012, 21, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Devos, Y.; De Schrijver, A.; De Clercq, P.; Kiss, J.; Romeis, J. Bt-maize event mon 88017 expressing cry3bb1 does not cause harm to non-target organisms. Transgenic Res. 2012, 21, 1191–1214. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.; Dively, G.P.; Pettis, J. Effects of bt corn pollen on honey bees: Emphasis on protocol development. Apidologie 2007, 38, 368–377. [Google Scholar] [CrossRef]
- Ghimire, M.N.; Phillips, T.W. Suitability of different lepidopteran host species for development of bracon hebetor (hymenoptera: Braconidae). Environ. Entomol. 2010, 39, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Dweck, H.K.; Svensson, G.P.; Gündüz, E.A.; Anderbrant, O. Kairomonal response of the parasitoid, Bracon hebetor Say, to the male-produced sex pheromone of its host, the greater waxmoth, Galleria mellonella (L.). J. Chem. Ecol. 2010, 36, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, D.P.; Allen, C.R.; Brenner, R.J.; Forys, E.A.; Jouvenaz, D.P.; Lutz, R.S. Red imported fire ants: Impact on biodiversity. Am. Entomol. 2001, 47, 16–23. [Google Scholar] [CrossRef]
- Boldt, P.T.; Marston, N. Eggs of the greater wax moth as a host for trichogramma. Environ. Entomol. 1974, 3, 545–548. [Google Scholar] [CrossRef]
- Van Lenteren, J.; Babendreier, D.; Bigler, F.; Burgio, G.; Hokkanen, H.; Kuske, S.; Loomans, A.; Menzler-Hokkanen, I.; Van Rijn, P.; Thomas, M. Environmental risk assessment of exotic natural enemies used in inundative biological control. BioControl 2003, 48, 3–38. [Google Scholar] [CrossRef]
- Knipling, E. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 1955, 48, 459–462. [Google Scholar] [CrossRef]
- Bloem, K.; Bloem, S.; Carpenter, J. Impact of moth suppression/eradication programmes using the sterile insect technique or inherited sterility. In Sterile Insect Technique; Springer: Dordrecht, The Netherlands, 2005; pp. 677–700. [Google Scholar]
- North, D.T. Inherited sterility in lepidoptera. Annu. Rev. Entomol. 1975, 20, 167–182. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A. The Pherobase: Database of Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 6 September 2016).
- Agelopoulos, N.; Birkett, M.A.; Hick, A.J.; Hooper, A.M.; Pickett, J.A.; Pow, E.M.; Smart, L.E.; Smiley, D.W.; Wadhams, L.J.; Woodcock, C.M. Exploiting semiochemicals in insect control. Pestic. Sci. 1999, 55, 225–235. [Google Scholar] [CrossRef]
- Tumlinson, J.H. The importance of volatile organic compounds in ecosystem functioning. J. Chem. Ecol. 2014, 40, 212. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.A.; Pickett, J.A. Aphid sex pheromones: From discovery to commercial production. Phytochemistry 2003, 62, 651–656. [Google Scholar] [CrossRef]
- Borden, J.H.; Lindgren, B.S. The role of semiochemicals in IPM of the mountain pine beetle. In Integrated Control of Scolytid Bark Beetles; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 1988. [Google Scholar]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Ann. Rev. Entomol. 2006, 52, 375. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, P.; Stelinski, L.; Gut, L.; Thomson, D. Codling moth management and chemical ecology. Annu. Rev. Entomol. 2008, 53, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Nakagawa, T.; Mitsuno, H.; Mori, H.; Endo, Y.; Tanoue, S.; Yasukochi, Y.; Touhara, K.; Nishioka, T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. USA 2004, 101, 16653–16658. [Google Scholar] [CrossRef] [PubMed]
- Fombong, A.T.; Mumoki, F.N.; Muli, E.; Masiga, D.K.; Arbogast, R.T.; Teal, P.E.; Torto, B. Occurrence, diversity and pattern of damage of Oplostomus species (coleoptera: Scarabaeidae), honey bee pests in Kenya. Apidologie 2013, 44, 11–20. [Google Scholar] [CrossRef]
- Torto, B.; Suazo, A.; Alborn, H.; Tumlinson, J.H.; Teal, P.E. Response of the small hive beetle (Aethina tumida) to a blend of chemicals identified from honeybee (Apis mellifera) volatiles. Apidologie 2005, 36, 523–532. [Google Scholar] [CrossRef]
- Suazo, A.; Torto, B.; Teal, P.E.; Tumlinson, J.H. Response of the small hive beetle (Aethina tumida) to honey bee (Apis mellifera) and beehive-produced volatiles. Apidologie 2003, 34, 525–534. [Google Scholar] [CrossRef]
- Fombong, A.T.; Teal, P.E.; Arbogast, R.T.; Ndegwa, P.N.; Irungu, L.W.; Torto, B. Chemical communication in the honey bee scarab pest Oplostomus haroldi: Role of (z)-9-pentacosene. J. Chem. Ecol. 2012, 38, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Pernal, S.; Baird, D.; Higo, H.; Slessor, K.; Winston, M. Semiochemicals influencing the host-finding behaviour of Varroa destructor. Exp. Appl. Acarol. 2005, 37, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Donzé, G.; Schnyder-Candrian, S.; Bogdanov, S.; Diehl, P.A.; Guerin, P.M.; Kilchenman, V.; Monachon, F. Aliphatic alcohols and aldehydes of the honey bee cocoon induce arrestment behavior in Varroa jacobsoni (Acari: Mesostigmata), an ectoparasite of Apis mellifera. Arch. Insect Biochem. Physiol. 1998, 37, 129–145. [Google Scholar] [CrossRef]
- Rickli, M.; Diehl, P.A.; Guerin, P.M. Cuticle alkanes of honeybee larvae mediate arrestment of bee parasite Varroa jacobsoni. J. Chem. Ecol. 1994, 20, 2437–2453. [Google Scholar] [CrossRef] [PubMed]
- Nazzi, F.; Le Conte, Y. Ecology of varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu. Rev. Entomol. 2016, 61, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, B.P.; Allsopp, M.H. Risk assessment for large African hive beetles (Oplostomus spp.)—A review. Apidologie 2017. [Google Scholar] [CrossRef]
- Roller, H.; Biemann, K.; Bjerke, J.; Norgard, D.; McShan, W. Sex pheromones of pyralid moths. I. Isolation and identification of sex-attractant of Galleria mellonella l (greater wax moth). Acta Entomol. Bohemoslov. 1968, 65, 208–211. [Google Scholar]
- Flint, H.; Merkle, J. Mating behavior, sex pheromone responses, and radiation sterilization of the greater wax moth (Lepidoptera: Pyralidae). J. Econ. Entomol. 1983, 76, 467–472. [Google Scholar] [CrossRef]
- Greenfield, M.D. Moth sex pheromones: An evolutionary perspective. Fla. Entomol. 1981, 64, 4–17. [Google Scholar] [CrossRef]
- Spangler, H.G.; Greenfield, M.D.; Takessian, A. Ultrasonic mate calling in the lesser wax moth. Physiol. Entomol. 1984, 9, 87–95. [Google Scholar] [CrossRef]
- Svensson, G.P.; Gündüz, E.A.; Sjöberg, N.; Hedenström, E.; Lassance, J.-M.; Wang, H.-L.; Löfstedt, C.; Anderbrant, O. Identification, synthesis, and behavioral activity of 5, 11-dimethylpentacosane, a novel sex pheromone component of the greater wax moth, Galleria mellonella (L.). J. Chem. Ecol. 2014, 40, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Light, D.M.; Knight, A.L.; Henrick, C.A.; Rajapaska, D.; Lingren, B.; Dickens, J.C.; Reynolds, K.M.; Buttery, R.G.; Merrill, G.; Roitman, J. A pear-derived kairomone with pheromonal potency that attracts male and female codling moth, Cydia pomonella (L.). Naturwissenschaften 2001, 88, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Jumean, Z.; Gries, R.; Unruh, T.; Rowland, E.; Gries, G. Identification of the larval aggregation pheromone of codling moth, Cydia pomonella. J. Chem. Ecol. 2005, 31, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, R.; Campbell, J.F.; Selvan, S.; Lewis, E.E. Large-scale inoculative releases of the entomopathogenic nematode steinernema glaseri: Assessment 50 years later. Biol. Control 1992, 2, 181–187. [Google Scholar] [CrossRef]
- Haukeland, S.; Lola-Luz, T. Efficacy of the entomopathogenic nematodes Steinernema kraussei and Heterorhabditis megidis against the black vine weevil Otiorhynchus sulcatus in open field-grown strawberry plants. Agric. For. Entomol. 2010, 12, 363–369. [Google Scholar] [CrossRef]
- Ellis, J.; Spiewok, S.; Delaplane, K.; Buchholz, S.; Neumann, P.; Tedders, W. Susceptibility of Aethina tumida (Coleoptera: Nitidulidae) larvae and pupae to entomopathogenic nematodes. J. Econ. Entomol. 2010, 103, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, A.G.; Mathers, J.J.; Blackburn, L.F.; Powell, M.E.; Marris, G.; Pietravalle, S.; Brown, M.A.; Budge, G.E. Screening commercially available entomopathogenic biocontrol agents for the control of Aethina tumida (Coleoptera: Nitidulidae) in the UK. Insects 2012, 3, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Pell, J. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, E.M.; Cantwell, G.E.; Kuchinski, M. Biological control of the greater wax moth (Lepidoptera: Pyralidae), utilizing in vivo-and in vitro-propagated baculovirus. J. Econ. Entomol. 1982, 75, 675–679. [Google Scholar] [CrossRef]
- Landolt, P.; Adams, T.; Rogg, H. Trapping spotted wing drosophila, Drosophila suzukii (Matsumura)(Diptera: Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. J. Appl. Entomol. 2012, 136, 148–154. [Google Scholar] [CrossRef]
- Heath, R.R.; Epsky, N.D.; Guzman, A.; Dueben, B.D.; Manukian, A.; Meyer, W.L. Development of a dry plastic insect trap with food-based synthetic attractant for the mediterranean and mexican fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 1995, 88, 1307–1315. [Google Scholar] [CrossRef]
- El-Sayed, A.; Suckling, D.; Wearing, C.; Byers, J. Potential of mass trapping for long-term pest management and eradication of invasive species. J. Econ. Entomol. 2006, 99, 1550–1564. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwadha, C.A.; Ong’amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects 2017, 8, 61. https://doi.org/10.3390/insects8020061
Kwadha CA, Ong’amo GO, Ndegwa PN, Raina SK, Fombong AT. The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects. 2017; 8(2):61. https://doi.org/10.3390/insects8020061
Chicago/Turabian StyleKwadha, Charles A., George O. Ong’amo, Paul N. Ndegwa, Suresh K. Raina, and Ayuka T. Fombong. 2017. "The Biology and Control of the Greater Wax Moth, Galleria mellonella" Insects 8, no. 2: 61. https://doi.org/10.3390/insects8020061
APA StyleKwadha, C. A., Ong’amo, G. O., Ndegwa, P. N., Raina, S. K., & Fombong, A. T. (2017). The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects, 8(2), 61. https://doi.org/10.3390/insects8020061





