Ectomycota Associated with Arthropods from Bat Hibernacula in Eastern Canada, with Particular Reference to Pseudogymnoascus destructans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Collections
2.2. Laboratory Methods
2.3. Statistical Analysis
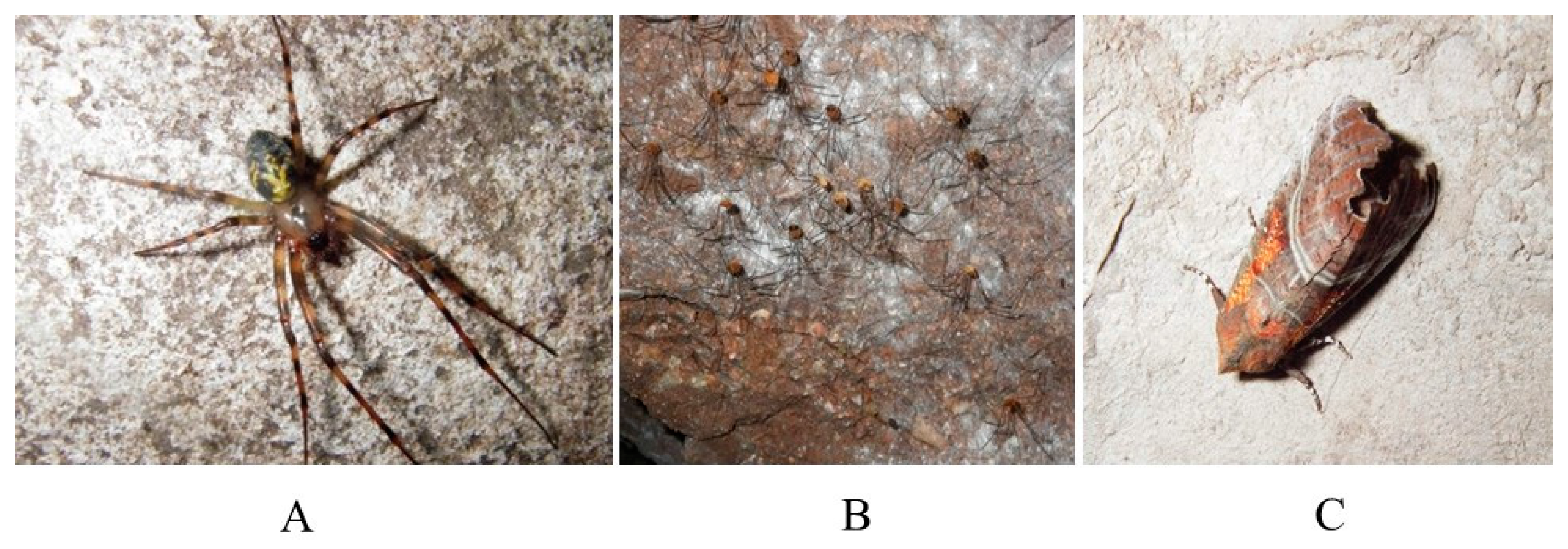
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Pd | Pseudogymnoascus destructans |
| WNS | white-nose syndrome |
| DPYA | dextrose-peptone-yeast extract agar |
| SAB | Sabouraud-dextrose agar |
References
- Lorch, J.M.; Meteyer, C.U.; Behr, M.J.; Boyles, J.G.; Cryan, P.M.; Hicks, A.C.; Ballmann, A.E.; Coleman, J.T.; Redell, D.N.; Reeder, D.M.; et al. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature 2011, 480, 376–378. [Google Scholar] [CrossRef] [PubMed]
- North American bat death toll exceeds 5.5 million from white-nose syndrome. Available online: http://www.batcon.org/pdfs/USFWS_WNS_Mortality_2012_NR_FINAL.pdf (accessed on 1 February 2012).
- Turner, G.G.; Reeder, D.M.; Coleman, J.T. A five-year assessment of mortality and geographic spread of white-nose syndrome in North American bats and a look to the future. Bat Res. News 2011, 52, 13–27. [Google Scholar]
- Lucan, R.K.; Bandouchova, H.; Bartonicka, T.; Pikula, J.; Zahradnikova Jr, A.; Zukal, J.; Martinkova, N. Ectoparasites may serve as vectors for the white-nose syndrome fungus. Parasit. Vectors 2016. [Google Scholar] [CrossRef]
- Raudabaugh, D.B.; Miller, A.N. Nutritional capability of and substrate suitability for Pseudogymnoascus destructans, the causal agent of bat white-nose syndrome. PLoS ONE 2013, 8, e78300. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Labruna, M.B.; Mans, B.J.; Maruyama, S.R.; Francischetti, I.M.; Barizon, G.C.; de Miranda Santos, I.K. The sialotranscriptome of Antricola delacruzi female ticks is compatible with non-hematophagous behavior and an alternative source of food. Insect Biochem. Mol. Biol. 2012, 42, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Vanderwolf, K.J.; Malloch, D.; McAlpine, D.F.; Forbes, G.J. A world review of fungi, yeasts and slime molds in caves. Int. J. Spel. 2013, 42, 77–96. [Google Scholar] [CrossRef]
- Benoit, J.B.; Yoder, J.A.; Zettler, L.W.; Hobbs, H.H. Mycoflora of a trogloxenic Cave Cricket, Hadenoecus cumberlandicus (Orthoptera: Rhaphidophoridae), from two small caves in northeastern Kentucky. Ann. Entomol. Soc. Am. 2004, 97, 989–993. [Google Scholar] [CrossRef]
- Yoder, J.A.; Benoit, J.B.; Christensen, B.S.; Croxall, T.J.; Hobbs III, H.H. Entomopathogenic fungi carried by the cave orb weaver spider, Meta ovalis (Araneae, Tetragnathidae), with implications for mycoflora transfer to cave crickets. J. Cave Karst Stud. 2009, 71, 116–120. [Google Scholar]
- Pugsley, C. Ecology of the New Zealand glowworm, Arachnocampa luminosa (Diptera: Keroplatidae), in the Glowworm Cave, Waitomo. J. R. Soc. NZ 1984, 14, 387–407. [Google Scholar] [CrossRef]
- Yoder, J.A.; Benoit, J.B.; Hobbs, H.H., III; Nelson, B.W.; Main, L.R.; Gibas, C.F. The entomopathogenic fungus Beauveria caledonica, a newly identified pathogen of cave crickets, Hadenoecus spp. (Orthoptera: Rhaphidophoridae). Speleobiology Notes 2015, 7, 1–9. [Google Scholar]
- Smrž, J.; Kováč, L.; Mikeš, J.; Šustr, V.; Lukešová, A.; Tajovský, K.; Nováková, A.; Režňáková, P. Food sources of selected terrestrial cave arthropods. Subterr. Biol. 2015, 16, 37–46. [Google Scholar] [CrossRef]
- Estrada-Bárcenas, D.A.; Palacios-Vargas, J.G.; Estrada-Venegas, E.; Klimov, P.B.; Martínez-Mena, A.; Taylor, M.L. Biological activity of the mite Sancassania sp. (Acari: Acaridae) from bat guano associated with the pathogenic fungus Histoplasma capsulatum. Mem. Inst. Oswaldo. Cruz. 2010, 105, 127–131. [Google Scholar]
- Stephenson, S.; Slay, M.; Slay, C.; Tuggle, A. Cave crickets (Orthoptera: Rhaphidophoridae) as vectors of Dictyostelids (Protista: Dictyosteliida). Entomol. News 2007, 118, 292–295. [Google Scholar] [CrossRef]
- Boyer-Lefevre, N.H. Les Laboulbéniales des Trechinae cavernicoles pyrénéens. Ann. Spéléo. 1966, 21, 775–794. [Google Scholar]
- Enghoff, H.; Santamaria, S. Infectious intimacy and contaminated caves––Three new species of ectoparasitic fungi (Ascomycota: Laboulbeniales) from blaniulid millipedes (Diplopoda: Julida) and inferences about their transmittal mechanisms. Org. Divers. Evol. 2015, 15, 249–263. [Google Scholar] [CrossRef]
- Langwig, K.E.; Frick, W.F.; Reynolds, R.; Parise, K.L.; Drees, K.P.; Hoyt, J.R.; Cheng, T.L.; Kunz, T.H.; Foster, J.T.; Kilpatrick, A.M. Host and pathogen ecology drive the seasonal dynamics of a fungal disease, white-nose syndrome. Proc. R. Soc. B. 2014. [Google Scholar] [CrossRef] [PubMed]
- Dickson, G.W. A preliminary study of heterotrophic microorganisms as factors in substrate of troglobitic invertebrates. NSS. Bull. 1975, 37, 89–93. [Google Scholar]
- Malloch, D.; Blackwell, M. Dispersal biology of the ophiostomatoid fungi. In Certatocystis and Ophiostoma. Taxonomy, Ecology and Pathogenicity; Wingfield, M.J., Seifert, K.A., Webber, J.F., Eds.; APS: Saint Paul, MN, USA, 1993; pp. 195–206. [Google Scholar]
- Agrios, G.M. Plant pathology, 3rd ed.; Academic Press Inc.: San Diego, CA, USA, 1988; pp. 416–422. [Google Scholar]
- Vanderwolf, K.J.; McAlpine, D.F.; Malloch, D.; Forbes, G.J. Ectomycota associated with hibernating cave bats in eastern Canada prior to the emergence of white-nose syndrome. Northeast. Nat. 2013, 20, 115–130. [Google Scholar] [CrossRef]
- Vanderwolf, K.J.; Malloch, D.; McAlpine, D.F. Fungi associated with over-wintering Tricolored bats, Perimyotis subflavus, in a white-nose syndrome region of Eastern Canada. J. Cave Karst Stud. 2015, 77, 145–151. [Google Scholar]
- McAlpine, D.F.; Vanderwolf, K.J.; Forbes, G.J.; Malloch, D. Consumption of bats (Myotis spp.) by raccoons (Procyon lotor) during an outbreak of white-nose syndrome in New Brunswick: Implications for bat mortality estimates. Can. Field Nat. 2011, 125, 257–260. [Google Scholar]
- Peck, S.B. A review of the cave fauna of Canada, and the composition and ecology of the invertebrate fauna of caves and mines in Ontario. Can. J. Zool. 1988, 66, 1197–1213. [Google Scholar] [CrossRef]
- Moseley, M. Acadian biospeleology: composition and ecology of cave fauna of Nova Scotia and southern New Brunswick, Canada. Int. J. Spel. 2007, 36, 1–21. [Google Scholar] [CrossRef]
- Vanderwolf, K.J.; McAlpine, D.F.; Forbes, G.J.; Malloch, D. Winter bat populations and cave microclimate prior to and at the onset of white-nose syndrome in New Brunswick. Can. Field Nat. 2012, 126, 125–134. [Google Scholar]
- United States Fish and Wildlife Service. Revised decontamination protocol (June 25, 2012). Available online: http://www.whitenosesyndrome.org/resource/revised-decontamination-protocol-june-25-2012 (accessed on 15 December 2015).
- Holmberg, R.G.; Angerilli, N.P.; LaCasse, L.J. Overwintering aggregations of Leiobunum paessleri in caves and mines (Arachnida, Opiliones). J. Arachnol. 1984, 12, 195–204. [Google Scholar]
- Vockeroth, J.R. Mycetophilidae. In Manual of Nearctic Diptera Volume 1; McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J., Vockeroth, J.R., Wood, D.M., Eds.; Biosystematics Research Institute Agriculture Canada: Ottawa, Canada, 1981; pp. 223–246. [Google Scholar]
- Papavizas, G.C.; Davey, C.B. Evaluation of various media and antimicrobial agents for isolation of soil fungi. Soil. Sci. 1958, 88, 112–117. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag: Regensburg, Germany, 2007; p. 672. [Google Scholar]
- Seifert, K.; Morgan-Jones, G.; Gams, W.; Kendrick, B. The Genera of Hyphomycetes; CBS-KNAW Fungal Biodiversity Centre: Utrecht, the Netherlands, 2011; p. 997. [Google Scholar]
- Khankhet, J.; Vanderwolf, K.J.; McAlpine, D.F.; McBurney, S.; Overy, D.P.; Slavic, D.; Xu, J. Clonal expansion of the Pseudogymnoascus destructans genotype in North America is accompanied by significant variation in phenotypic expression. PLoS ONE 2014, 9, e104684. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.; Drees, K.; University of New Hampshire. Personal Communitcation, 2014.
- Moseley, M.; Hebda, A. Overwintering Leiobunum elegans (Opiliones: Phalangiidae) in Caves and Mines in Nova Scotia. Proc. N. S. Inst. Sci. 2001, 41, 216–218. [Google Scholar]
- Dickson, G.W.; Kirk, P.W. Distribution of heterotrophic microorganisms in relation to detritivores in Virginia caves (with supplemental bibliography on cave mycology and microbiology). In the Distributional History of the Biota of the Southern Appalachians. IV. Algae and Fungi; Parker, B.C., Roane, M.K., Eds.; University of Virginia Press: Charlottesville, VA, USA, 1976; pp. 205–226. [Google Scholar]
- Goodnight, C.J.; Goodnight, M.L. Speciation among cave opilionids of the United States. Am. Midland. Nat. 1960, 64, 34–38. [Google Scholar] [CrossRef]
- Balazy, S.; Wisniewski, J.; Kaczmarek, S. Some noteworthy fungi occurring on mites. B. Pol. Acad. Sci. Biol. 1987, 35, 199–224. [Google Scholar]
- Kubatova, A.; Cerny, M.; Novakova, A. New records of micromycetes from the Czech Republic. IV. Acrodontium salmoneum, Chaunopycnis alba, and Cylindrocarpostylus gregarious, and notes on Dactylaria lanosa and Trichoderma saturnisporum. Czech. Mycol. 2001, 53, 237–255. [Google Scholar]
- Hwang, S.C.; Chen, C.L. A new leaf-speckle disease of banana caused by Acrodontium simplex in Taiwan. Plant. Protect. Bull. 1986, 28, 413–416. [Google Scholar]
- Cabello, M.N. Deuteromycotina from Antarctica––New species of hyphomycetes from Danco coast, Antarctic peninsula. Mycotaxon 1989, 36, 91–94. [Google Scholar]
- Meyer-Rochow, V.B.; Liddle, A.R. Structure and function of the eyes of two species of opilionid from New Zealand glow-worm caves (Megalopsalis tumida: Palpatores, and Hendea myersi cavernicola: Laniatores). Proc. R. Soc. Lond. B. 1988, 233, 293–319. [Google Scholar] [CrossRef]
- Machado, G.; Raimundo, R.L.; Oliveira, P.S. Daily activity schedule, gregariousness, and defensive behaviour in the Neotropical harvestman Goniosoma longipes (Opiliones: Gonyleptidae). J. Nat. Hist. 2000, 34, 587–596. [Google Scholar] [CrossRef]
- Mains, E.B. Entomogenous species of Akanthomyces, Hymenostilbe and Insecticola in north America. Mycologia 1950, 42, 566–589. [Google Scholar] [CrossRef]
- Leatherdale, D. The arthropod hosts of entomogenous fungi in Britain. Entomophaga 1970, 15, 419–435. [Google Scholar] [CrossRef]
- Greenstone, M.H.; Ignoffo, C.M.; Samson, R.A. Susceptibility of spider species to fungus Nomuraea atypicola. J. Arachnol. 1987, 15, 266–268. [Google Scholar]
- Mitov, P.G. Harvestmen (Opiliones, Arachnida)––Carriers of plant and fungus spores. Acta. Zool. Bulg. 1992, 43, 75–77. [Google Scholar]
- Cokendolpher, J.C. Pathogens and parasites of Opiliones (Arthropoda: Arachnida). J. Arachnol. 1993, 21, 120–146. [Google Scholar]
- Cokendolpher, J.C.; Mitov, P.G. Natural enemies. In Harvestmen: The Biology of Opiliones; Pinto-da-Rocha, R., Machado, G., Giribet, G., Eds.; Harvard University Press: Cambridge, MA, USA, 2007; pp. 339–373. [Google Scholar]
- Balazy, S. A new species of entomophthoraceae (Mycophyta: Entomophthorales) from Poland. J. Invert. Path. 1978, 31, 275–279. [Google Scholar] [CrossRef]
- Keller, S. Arthropod-pathogenic Entomophthorales of Switzerland. I. Conidiobolus, Entomophaga, and Entomophthora. Sydowia 1987, 40, 122–167. [Google Scholar]
- McKillop, W.B. Scoliopteryx libatrix (Noctuidae) and Triphosa haesitata (Geometridae) in caves in Manitoba, Canada. J. Lepid. Soc. 1993, 47, 106–113. [Google Scholar]
- Kowalski, W. Ethological and ecological observations on Lepidoptera in their subterranean hibernating places in the vicinity of Cracow. Zeszyty Naukowe Uniwersytetu Jagiellonskiego. Prace Zoologiczne, Zeszyt 1965, 103, 97–157. [Google Scholar]
- Roederk, K.D.; Fenton, M.B. Acoustic responsiveness of Scoliopteryx libatrix L. (Lepidoptera: Noctuidae), a moth that shares hibernacula with some insectivorous bats. Can. J. Zool. 2011, 51, 681–685. [Google Scholar] [CrossRef]
- Kubatova, A.; Dvorak, L. Entomopathogenic fungi associated with insects hibernating in underground shelters. Czech. Mycol. 2005, 57, 221–237. [Google Scholar]
- Rector, M.A. Foraging in the Cave Environment: The Ecology of the Cave Spider Meta ovalis (Araneae: tetragnathidae). Master’s Thesis, Ohio State University, Columbus, OH, USA, 2009. [Google Scholar]
- Smithers, P. The early life history and dispersal of the cave spider Meta menardi (Latreille, 1804) (Araneae: Tetragnathidae). Bull. Br. Arachnol. Soc. 2005, 13, 213–216. [Google Scholar]
- Del Fiol, F.; Solveig, T.; Riccardo, G. Fungal spores and pollen as potential nutritional additives for the cross spider Araneus diadematus Clerck (Araneae, Araneidae). Boletin Micologico 2007, 22, 47–50. [Google Scholar]
- Reed, C.F.; Witt, P.N. Growth rate and longevity in two species of orb-weaving spiders (Araneae: Argiopidae). Bull. Brit. Arach. Soc. 1972, 2, 111–112. [Google Scholar]
- Gnaspini, P. Development. In Harvestmen: The Biology of Opiliones; Pinto-da-Rocha, R., Machado, G., Giribet, G., Eds.; Harvard University Press: Cambridge, MA, USA, 2007; pp. 455–472. [Google Scholar]
- Keates, S.E.; Sturrock, R.N.; Sutherland, J.R. Populations of adult fungus gnats and shore flies in British Columbia container nurseries as related to nursery environment, and incidence of fungi on the insects. New Forest. 1989, 3, 1–9. [Google Scholar] [CrossRef]
- Teernstra-Eeken, M.H.; Engel, A. Notes on entomophthorous fungi on Heleomyzidae and Culicidae (Diptera). J. Invert. Path. 1967, 9, 431–432. [Google Scholar] [CrossRef]
- Weiser, J.; Batko, A. A new parasite of Culex pipiens Entomophthora destruens new species Phycomycetes Entomophthoraceae. Folia. Parasitol. Praha. 1966, 13, 144–149. [Google Scholar]
- Roberts, D.W.; Campbell, A.S. Stability of entomopathogenic fungi. Misc. Publ. Entomol. Soc. Am. 1977, 10, 19–76. [Google Scholar]
- Fernandes, E.K.; Rangel, D.E.; Moraes, A.M.; Bittencourt, V.R.; Roberts, D.W. Cold activity of Beauveria and Metarhizium, and thermotolerance of Beauveria. J. Invert. Path. 2008, 98, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, J.; Thomsen, L.; Jensen, A.B. A third way for entomophthoralean fungi to survive the winter: Slow disease transmission between individuals of the hibernating host. Insects 2013, 4, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, J.E. Mycophagy in Lepidoptera. In Fungus-Insect Relationships: Perspectives in Ecology and Evolution; Wheeler, Q., Blackwell, M., Eds.; Columbia University Press: New York, NY, USA, 1984; pp. 382–423. [Google Scholar]
- Steinkraus, D.C. Factors affecting transmission of fungal pathogens of aphids. J. Invert. Path. 2006, 92, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Mulec, J.; Vaupotic, J.; Walochnik, J. Prokaryotic and eukaryotic airborne micoorganisms as tracers of microclimatic changes in the underground (Postojna Cave, Slovenia). Environ. Microbiol. 2012, 64, 654–667. [Google Scholar]
- Vaughan, M.J.; Nelson, W.; Soderlund, C.; Maier, R.M.; Pryor, B.M. Assessing fungal community structure from mineral surfaces in Kartchner Caverns using multiplexed 454 pyrosequencing. Environ. Microbiol. 2015, 70, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.T.; Ingersoll, T.; Barton, H.A. Modeling the environmental growth of Pseudogymnoascus destructans and its impact on the white-nose syndrome epidemic. J. Wildl. Dis. 2015, 51, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Verant, M.L.; Boyles, J.G.; Waldrep, W., Jr.; Wibbelt, G.; Blehert, D.S. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS ONE 2012, 7, e46280. [Google Scholar] [CrossRef] [PubMed]
| Arthropod & Bat Species | Glebe Mine | Dorchester Mine | Markhamville Mine | Dallings Cave | All sites | Range |
|---|---|---|---|---|---|---|
| Meta ovalis | 7.0 ± 1.9 (8) BC | 2.8 ± 2.2 (9) | ND | ND | 4.76 ± 2.95 (17) * | 0–10 |
| Scoliopteryx libatrix | 9.7 ± 4.3 (6) ABC | 8.4 ± 2.5 (5) | 6.0 ± 1.4 (2) | 6.5 ± 4.2 (4) | 8.12 ± 3.59 (17) & | 2–15 |
| Nelima elegans | 13.6 ± 3.0 (5) A | 8.3 ± 3.1 (9) | 7 (1) | ND | 10.00 ± 3.89 (15) & | 4–18 |
| Exechiopsis/Anatella sp. | 5.8 ± 1.8 (11) C | 3.3 ± 1.2 (11) | ND | ND | 4.55 ± 1.97 (23) * | 1–9 |
| Perimyotis subflavus | 10.2 ± 2.2 (9) AB | ND | ND | ND | ND | 7–14 |
| Myotis lucifugus | 7.7 ± 3.7 (6) BC | ND | ND | ND | ND | 2–12 |
| Myotis septentrionalis | 9.3 ± 4.0 (4) ABC | ND | ND | ND | ND | 6–15 |
| All arthropod species | 8.2 ± 3.8 (30) | 5.2 ± 3.4 (34) | 6.3 ± 1.2 (3) | 6.5 ± 4.2 (4) | 6.61 ± 3.78 (72) | 0–18 |
| Fungal Taxon | Meta ovalis | Scoliopteryx libatrix | Nelima elegans | Exechiopsis/Anatella sp. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gl | Do | Gl | Do | Ma | Da | Gl | Do | Ma | Gl | Do | all | |
| Ascomycota | ||||||||||||
| Acremonium sp. | 2 | 0 | 3 | 4 | 0 | 1 | 3 | 6 | 0 | 4 | 1 | 24 |
| Acremonium sp. (hyaline) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Acrodontium spp. | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 9 | 0 | 0 | 2 | 16 |
| Alternaria sp. | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Aureobasidium sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| A. pullulans (De Bary) G. Arnaud ex Cif., Ribaldi & Corte | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Beauveria bassiana (Bals.-Criv.) Vuill. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Beauveria sp. (penicillate) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Botrytis sp. | 0 | 0 | 3 | 3 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 9 |
| Cephalotrichum stemonitis (Pers.) Link | 8 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 8 | 0 | 21 |
| Chaetomidium sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Cladosporium sp. | 0 | 0 | 6 | 5 | 2 | 4 | 5 | 4 | 1 | 4 | 7 | 38 |
| C. cladosporioides complex (Fresen.) G.A. de Vries | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| cf. Conioscypha sp. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| cf. Cylindrocarpon sp. | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Dactylella sp. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Dendryphiella sp. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Exophiala sp. | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 3 |
| Fusarium sp. | 0 | 0 | 2 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 5 |
| Fusicladium cf. carpophilum (Thum.) Oudem | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hormonema sp. | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| Humicula cf. UAMH 11595 | 2 | 1 | 1 | 0 | 2 | 0 | 2 | 0 | 1 | 3 | 1 | 13 |
| Hyalodendriella sp. | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Isaria farinosa (Holmsk.) Fr. | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Lecanicillium sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| L. muscarium (Petch) Zare & W. Gams | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Leuconeurospora capsici (J.F.H. Beyma) Malloch, Sigler & Hambleton | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 1 | 5 |
| L. cf. pulcherrima (G. Winter) Malloch & Cain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| L. polypaeciloides Malloch, Sigler & Hambleton | 4 | 1 | 1 | 0 | 1 | 0 | 4 | 1 | 1 | 7 | 1 | 21 |
| Malbranchea sp. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mammaria sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Microascus sp. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| M. caviariformis Malloch & Hubart | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| M. cf. giganteus Malloch | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| Monodictys sp. | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Myceliophthora sp. | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| Oidiodendron sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| O. truncatum G.L. Barron | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 5 |
| Paecilomyces sp. | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| P. inflatus (Burnside) J.W. Carmich. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 |
| cf. Penicillifer sp. | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Penicillium sp. | 4 | 2 | 3 | 4 | 0 | 2 | 2 | 5 | 0 | 5 | 2 | 29 |
| P. cf. brevicompactum Dierckx | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| P. cf. decumbens Thom | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| P. cf. soppii K.M. Zalessky | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| P. cf. thomii Maire | 0 | 0 | 2 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 7 |
| Phaeotrichum hystricinum Cain & M.E. Barr | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 4 |
| Phialemonium sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Phoma sp. | 0 | 0 | 4 | 3 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 13 |
| Preussia sp. | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 4 |
| Pseudogymnoascus destructans (Blehert & Gargas) Minnis & D.L. Lindner | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 0 | 11 |
| P. pannorum (Link) Minnis & D.L. Lindner senso lato | 5 | 1 | 1 | 2 | 0 | 2 | 4 | 0 | 0 | 3 | 2 | 20 |
| Sarocladium strictum (W. Gams) Summerbell | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Scopulariopsis sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 |
| Simplicillium sp. | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 5 |
| Stachybotrys sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| sterile | 1 | 4 | 3 | 2 | 0 | 1 | 2 | 4 | 0 | 1 | 2 | 20 |
| Streptomyces sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Thelebolus sp. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Thelebolus crustaceus (Fuckel) Kimbr. | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| Thysanophora sp. | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| T. penicilliodes (Roum.) W.B. Kendr. | 0 | 2 | 1 | 0 | 0 | 0 | 2 | 4 | 0 | 1 | 1 | 11 |
| Tolypocladium cf. cylindrosporum W. Gams | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| T. inflatum W. Gams | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 3 |
| Trichoderma sp. | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 |
| Trichosporiella sp. | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 3 |
| Verticillium sp. | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 5 |
| Verticillium cf. alboatrum Reinke & Berthold | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Verticillium sp. (cf. Gabarnaudia) | 4 | 6 | 1 | 1 | 0 | 0 | 2 | 7 | 0 | 4 | 1 | 26 |
| Wardomyces sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| W. cf. columbinus (Demelius) Hennebert | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| W. humicola Hennebert & G.L. Barron | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| W. inflatus (Marchal) Hennebert | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 5 |
| Zopfiella pleuropora Malloch & Cain | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| Zythia sp. | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Basidiomycota | ||||||||||||
| Asterotremella sp. | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 4 |
| Basidiomycete unidentified | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 4 |
| Cystofilobasidium sp. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Trichosporon sp. | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 6 |
| T. dulcitum (Berkhout) Weijman | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Zygomycota | ||||||||||||
| Mortierella sp. | 3 | 1 | 3 | 1 | 0 | 1 | 3 | 8 | 1 | 4 | 3 | 28 |
| Mucor sp. | 4 | 0 | 1 | 3 | 2 | 3 | 2 | 2 | 1 | 4 | 1 | 23 |
| Thamnidium elegans Link | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| Umbelopsis isabellina (Oudem.) W. Gams | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| Bat Hibernacula | 2012 | 2013 | 2014 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pd yield | # of bats | Temperature | Pd yield | # of bats | Temperature | Pd yield | # of bats | Temperature | |
| Glebe Mine | 30% (M, A, S) | 174 | 5.83 ± 1.48 a (D) | 0% (M, N) | 22 | 5.98 ± 1.94 (D) | 7.1% (M, N, A, S) | 0 | 5.33 ± 2.09 (D) 0.06 ± 1.33 b (E) −2.67 ± 7.66 (O) |
| Dorchester Mine | 0% (M, N, A, S) | 1 | 6.63 ± 0.00 c (D) 1.08 ± 2.67 c (E) −0.97 ± 7.12 c (O) | 54.5% (M, N, S) | 0 | 6.59 ± 0.12 (D) −2.19 ± 6.91 c (O) | 0% (M, N, A, S) | 2 | 6.61 ± 0.07 (D) −3.93 ± 7.29 c (O) |
| Markhamville Mine | ND | 5.33 ± 0.52 a (D) | 0% (N, S) | 16 | 5.28 ± 0.37 (D) | ND | 4.89 ± 0.69 (D) −0.64 ± 2.68 (E) −3.71 ± 7.69 (O) | ||
| Dallings Cave | ND | 3.55 ± 1.29 d (D) | 0% (S) | 2 | 4.26 ± 2.07 (D) −0.12 ± 6.03 (O) | ND | 3.59 ± 2.26 (D) 0.84 ± 3.20 (E) −2.96 ± 7.79 (O) | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanderwolf, K.J.; Malloch, D.; McAlpine, D.F. Ectomycota Associated with Arthropods from Bat Hibernacula in Eastern Canada, with Particular Reference to Pseudogymnoascus destructans. Insects 2016, 7, 16. https://doi.org/10.3390/insects7020016
Vanderwolf KJ, Malloch D, McAlpine DF. Ectomycota Associated with Arthropods from Bat Hibernacula in Eastern Canada, with Particular Reference to Pseudogymnoascus destructans. Insects. 2016; 7(2):16. https://doi.org/10.3390/insects7020016
Chicago/Turabian StyleVanderwolf, Karen J., David Malloch, and Donald F. McAlpine. 2016. "Ectomycota Associated with Arthropods from Bat Hibernacula in Eastern Canada, with Particular Reference to Pseudogymnoascus destructans" Insects 7, no. 2: 16. https://doi.org/10.3390/insects7020016
APA StyleVanderwolf, K. J., Malloch, D., & McAlpine, D. F. (2016). Ectomycota Associated with Arthropods from Bat Hibernacula in Eastern Canada, with Particular Reference to Pseudogymnoascus destructans. Insects, 7(2), 16. https://doi.org/10.3390/insects7020016






