Hydrocarbon Patterns and Mating Behaviour in Populations of Drosophila yakuba
Abstract
:1. Introduction
2. Experimental Section
2.1. Drosophila Strains
2.2. Cuticular Hydrocarbon Extraction and Analyses
2.3. Mating Experiments
2.4. Statistics
2.4.1. CHC
2.4.2. Mating Experiments
3. Results
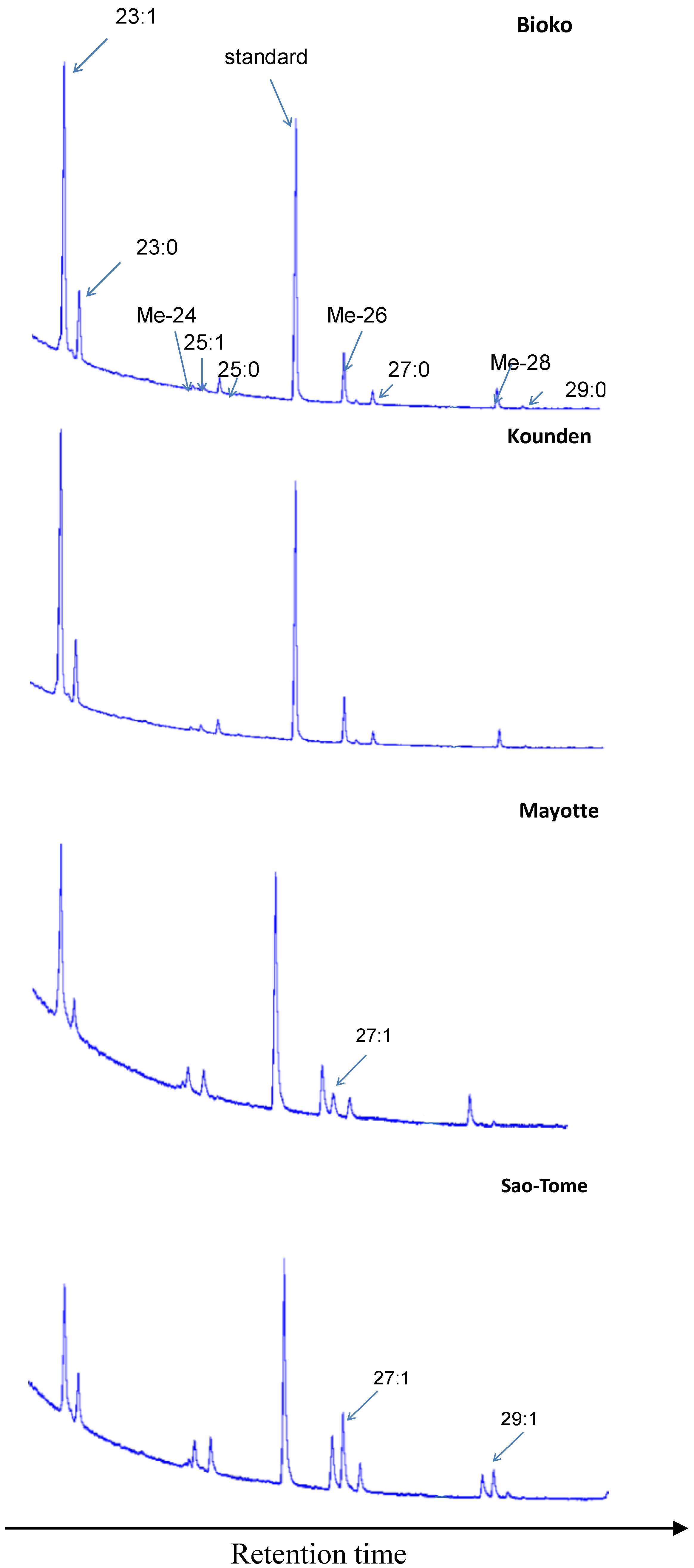
3.1. Cuticular Hydrocarbons
| CHC | Bioko | Kounden | Mayotte | Sao-Tome |
|---|---|---|---|---|
| 23–29 tot | 898.9 ± 52.1 | 877.8 ± 71.8 | 766.3 ± 125.6 | 714.7 ± 58.4 |
| 9-T | 2.17 ± 0.17 | 5.27 ± 1.04 | 4.36 ± 1.04 | 3.69 ± 0.89 |
| 7-T | 57.51 ± 2.84 | 48.56 ± 1.97 | 45.95 ± 1.74 | 27.90 ± 1.82 |
| 5-T | 1.20 ± 0.09 | 1.61 ± 0.14 | 0.94 ± 0.13 | 1.04 ± 0.12 |
| 23:0 | 14.48 ± 0.73 | 14.41 ± 0.63 | 8.21 ± 0.58 | 9.13 ± 0.54 |
| Me-24 | 0.98 ± 0.12 | 1.19 ± 0.11 | 0.53 ± 0.17 | 0.92 ± 0.10 |
| 9-P | 0.36 ± 0.06 | 0.67 ± 0.08 | 1.43 ± 0.13 | 1.52 ± 0.11 |
| 7-P | 1.19 ± 0.14 | 2.82 ± 0.25 | 4.84 ± 0.40 | 5.91 ± 0.27 |
| 25:0 | 3.43 ± 0.80 | 3.79 ± 0.43 | 4.24 ± 0.48 | 5.88 ± 0.47 |
| Me-26 | 10.61 ± 0.85 | 14.37 ± 0.53 | 11.54 ± 1.04 | 14.04 ± 0.48 |
| 7-H | 0.49 ± 0.06 | 0.19 ± 0.05 | 3.66 ± 0.49 | 15.90 ± 1.41 |
| 27:0 | 2.44 ± 0.66 | 1.97 ± 0.39 | 3.67 ± 0.35 | 4.64 ± 0.34 |
| Me-28 | 4.34 ± 0.61 | 3.90 ± 0.93 | 8.73 ± 1.09 | 4.23 ± 0.47 |
| 7-N | 0.0 ± 0.0 | 0.04 ± 0.04 | 0.28 ± 0.07 | 3.84 ± 0.61 |
| 29:0 | 0.42 ± 0.12 | 0.49 ± 0.12 | 0.81 ± 0.12 | 0.66 ± 0.11 |
| CHC | Bioko | Kounden | Mayotte | Sao-Tome |
|---|---|---|---|---|
| 23–29 tot | 1168.8 ± 61.4 | 883.2 ± 64.7 | 803.9 ± 53.6 | 921.1 ± 56.0 |
| 9-T | 3.08 ± 0.26 | 9.69 ± 1.34 | 3.89 ± 0.99 | 2.91 ± 0.62 |
| 7-T | 58.94 ± 1.61 | 46.46 ± 2.62 | 46.11 ± 1.78 | 28.13 ± 2.18 |
| 5-T | 1.44 ± 0.14 | 2.41 ± 0.44 | 0.74 ± 0.10 | 0.85 ± 0.14 |
| 23:0 | 17.65 ± 0.42 | 14.44 ± 0.92 | 6.69 ± 0.77 | 10.28 ± 0.84 |
| Me-24 | 0.41 ± 0.05 | 0.97 ± 0.18 | 0.41 ± 0.05 | 0.82 ± 0.17 |
| 9-P | 0.43 ± 0.04 | 1.28 ± 0.19 | 2.30 ± 0.30 | 2.01 ± 0.09 |
| 7-P | 1.09 ± 0.06 | 2.70 ± 0.35 | 5.29 ± 0.60 | 7.50 ± 0.42 |
| 25:0 | 2.92 ± 0.56 | 3.56 ± 0.43 | 3.01 ± 0.29 | 5.11 ± 0.41 |
| Me-26 | 4.82 ± 0.57 | 8.14 ± 0.83 | 8.14 ± 0.61 | 9.13 ± 0.47 |
| 7-H | 0.77 ± 0.06 | 0.60 ± 0.11 | 6.48 ± 0.97 | 11.98 ± 0.64 |
| 27:0 | 3.10 ± 0.39 | 3.00 ± 0.35 | 6.57 ± 0.81 | 6.11 ± 0.63 |
| Me-28 | 4.26 ± 0.51 | 5.29 ± 0.53 | 8.01 ± 0.50 | 7.22 ± 0.87 |
| 7-N | 0.04 ± 0.02 | 0.04 ± 0.03 | 0.62 ± 0.13 | 5.56 ± 0.53 |
| 29:0 | 0.77 ± 0.10 | 0.56 ± 0.09 | 1.19 ± 0.15 | 1.56 ± 0.21 |
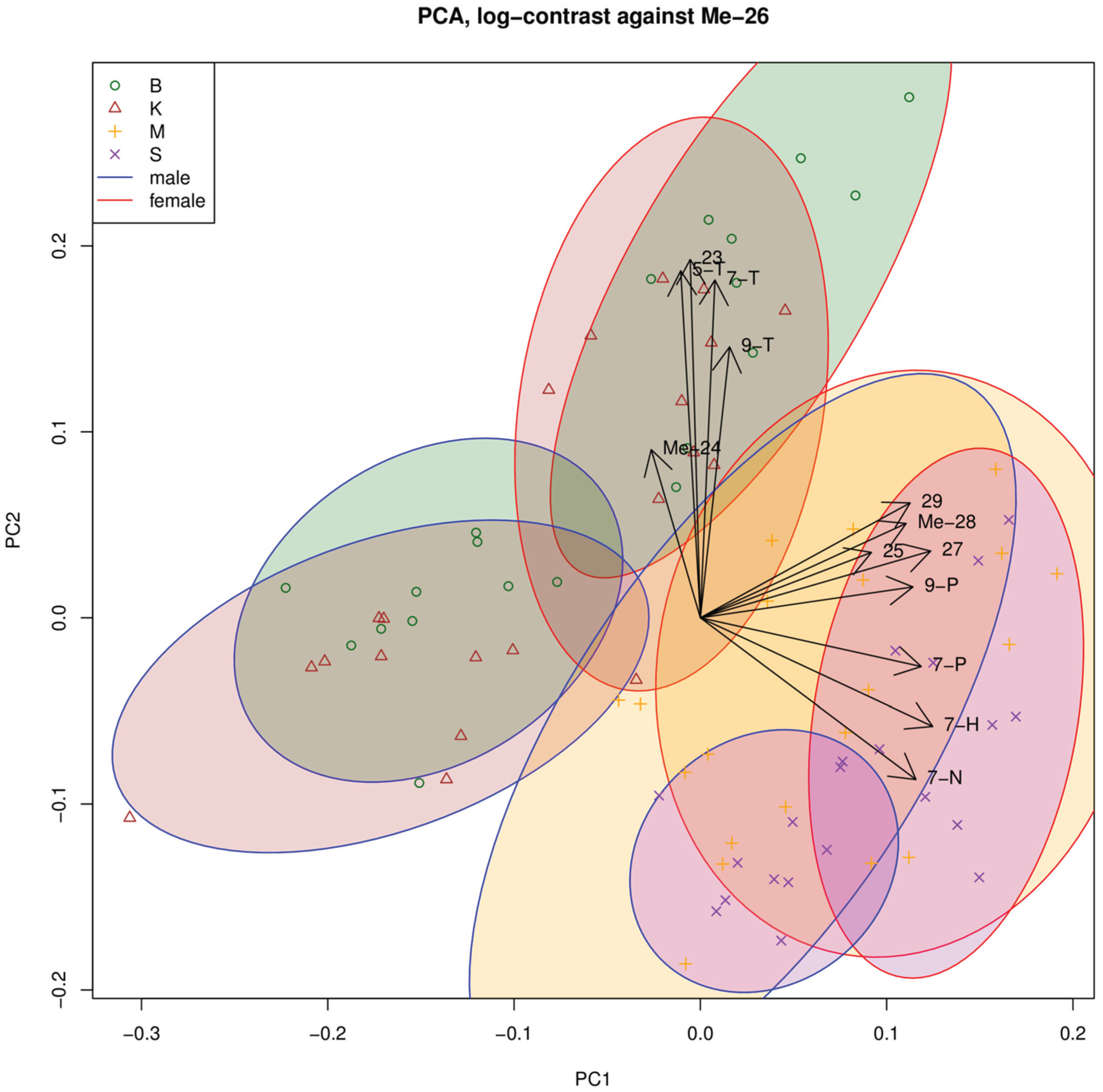

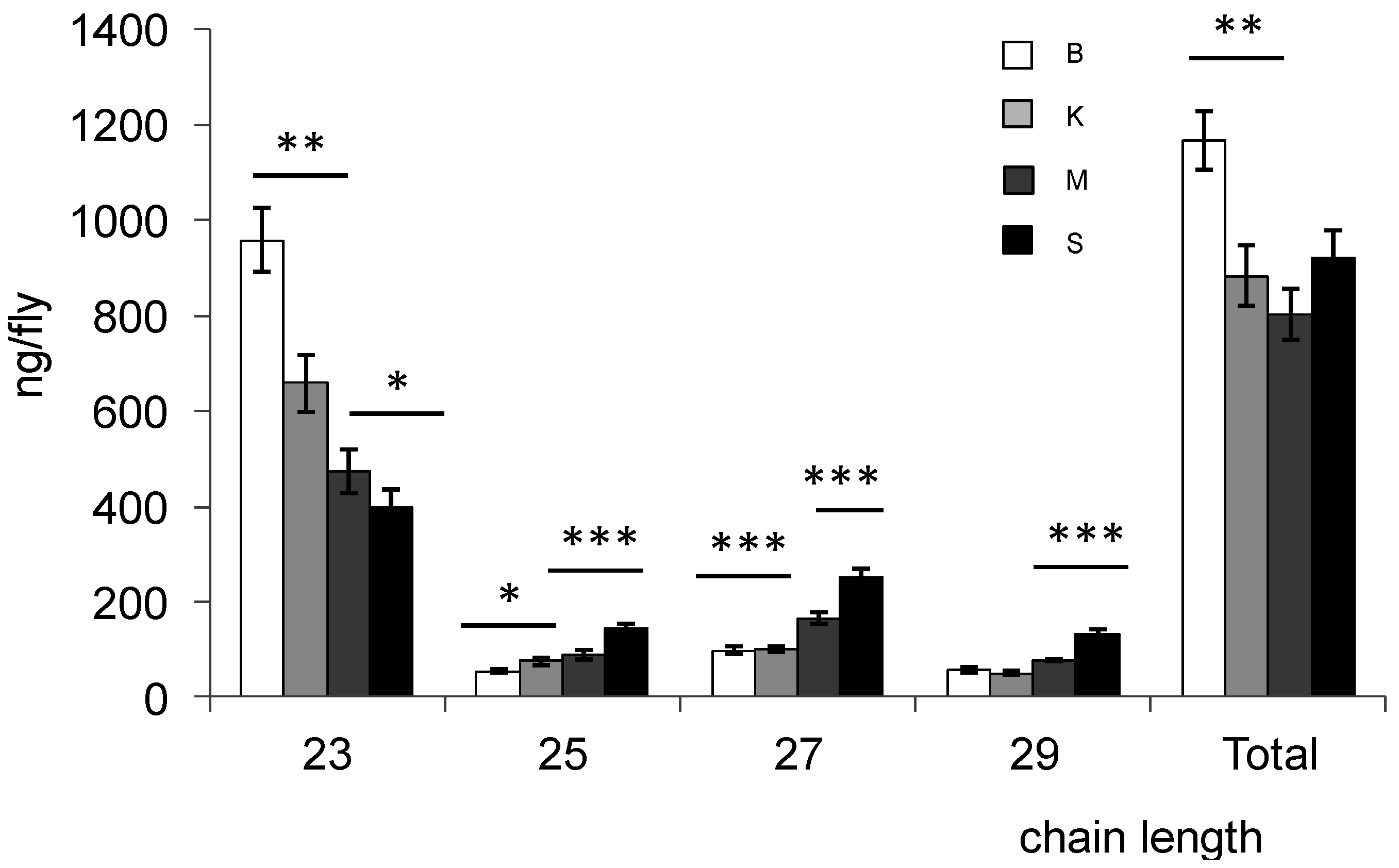
3.2. Mating Behavior and Mate Discrimination between Strains
| Population | Total Number | Copulation Percentage | n | Copulation Latency | Duration of Copulation |
|---|---|---|---|---|---|
| Bioko | 50 | 70 | 35 | 21.56 ± 1.14 ab | 41.50 ± 1.35 a |
| Kounden | 51 | 60.78 | 31 | 18.03 ± 1.07 b | 38.81 ± 1.72 a |
| Mayotte | 54 | 51.85 | 28 | 24.14 ± 1.61 a | 36.19 ± 2.15 a |
| Sao-Tome | 62 | 50 | 31 | 24.53 ± 0.86 a | 40.95 ± 1.67 a |
| Female | Male | Hom | Het | No Mating | p (H0A) | p* (H0A) |
|---|---|---|---|---|---|---|
| B | K | 21 | 17 | 8 | 0.627 | 1.000 |
| B | M | 20 | 14 | 3 | 0.392 | 1.000 |
| B | S | 17 | 20 | 5 | 0.743 | 1.000 |
| K | B | 25 | 27 | 2 | 0.890 | 1.000 |
| K | M | 18 | 20 | 9 | 0.871 | 1.000 |
| K | S | 21 | 11 | 18 | 0.110 | 0.992 |
| M | B | 22 | 13 | 8 | 0.175 | 1.000 |
| M | K | 23 | 11 | 18 | 0.058 | 0.576 |
| M | S | 17 | 13 | 14 | 0.585 | 1.000 |
| S | B | 23 | 7 | 18 | 0.005 | 0.057 |
| S | K | 28 | 7 | 11 | 0.001 | 0.006 |
| S | M | 18 | 17 | 11 | 1.000 | 1.000 |
| Male | Female | Hom | Het | No Mating | p (H0A) | p* (H0A) |
|---|---|---|---|---|---|---|
| B | K | 16 | 14 | 3 | 0.856 | 1.000 |
| B | M | 21 | 10 | 6 | 0.071 | 0.637 |
| B | S | 23 | 8 | 19 | 0.011 | 0.117 |
| K | B | 10 | 9 | 6 | 1.000 | 1.000 |
| K | M | 25 | 20 | 17 | 0.551 | 1.000 |
| K | S | 28 | 8 | 19 | 0.001 | 0.014 |
| M | B | 16 | 15 | 3 | 1.000 | 1.000 |
| M | K | 9 | 11 | 6 | 0.824 | 1.000 |
| M | S | 13 | 14 | 10 | 1.000 | 1.000 |
| S | B | 17 | 16 | 7 | 1.000 | 1.000 |
| S | K | 17 | 9 | 4 | 0.169 | 1.000 |
| S | M | 24 | 11 | 9 | 0.041 | 0.410 |
| Pop 1 | Pop 2 | Hom 1 | Hom 2 | Het | No | p (H0A) | p* (H0B) | p (H0B) | p* (H0B) | p (H0C) | p* (H0C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | K | 8 | 5 | 18 | 5 | 0.482 | 0.964 | 0.569 | 1.000 | 0.498 | 0.498 |
| B | M | 14 | 9 | 12 | 5 | 0.097 | 0.388 | 0.417 | 1.000 | 0.102 | 0.408 |
| K | M | 13 | 15 | 10 | 2 | 0.003 | 0.018 | 0.843 | 1.000 | 0.016 | 0.096 |
| B | S | 4 | 14 | 17 | 2 | 1.000 | 1.000 | 0.028 | 0.168 | 0.066 | 0.330 |
| K | S | 10 | 12 | 12 | 6 | 0.114 | 0.388 | 0.831 | 1.000 | 0.222 | 0.444 |
| M | S | 10 | 12 | 11 | 6 | 0.073 | 0.365 | 0.836 | 1.000 | 0.146 | 0.438 |
4. Discussion
4.1. Cuticular Hydrocarbons
4.2. Mating Behavior and Reproductive Isolation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dillwith, J.; Blomquist, G.; Nelson, D. Biosynthesis of the hydrocarbon components of the sex pheromone of the housefly, Musca domestica L. Insect Biochem. 1981, 11, 247–253. [Google Scholar] [CrossRef]
- Jallon, J.M. A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet. 1984, 14, 441–478. [Google Scholar] [CrossRef] [PubMed]
- Bontonou, G.; Wicker-Thomas, C. Sexual communication in the Drosophila genus. Insects 2014, 5, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Jallon, J.M.; David, J.R. Variations in cuticular hydrocarbons among the eight species of the Drosophila melanogaster subgroup. Evolution 1987, 4, 294–302. [Google Scholar] [CrossRef]
- Coyne, J.A. Genetics of differences in pheromonal hydrocarbons between Drosophila melanogaster and D. simulans. Genetics 1996, 143, 353–364. [Google Scholar] [PubMed]
- Coyne, J.A.; Charlesworth, B. Genetics of a pheromonal difference affecting sexual isolation between Drosophila mauritiana and D. sechellia. Genetics 1997, 145, 1015–1030. [Google Scholar] [PubMed]
- Coyne, J.A. Genetics of a difference in male cuticular hydrocarbons between two sibling species, Drosophila simulans and D. sechellia. Genetics 1996, 143, 1689–1698. [Google Scholar] [PubMed]
- Dallerac, R.; Labeur, C.; Jallon, J.M.; Knipple, D.C.; Roelofs, W.L.; Wicker-Thomas, C. A delta 9 desaturase gene with a different substrate specificity is responsible for the cuticular diene hydrocarbon polymorphism in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 9449–9454. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.I.; Hollocher, H.; Begun, D.J.; Aquadro, C.F.; Xu, Y.; Wu, M.L. Sexual isolation in Drosophila melanogaster: A possible case of incipient speciation. Proc. Natl. Acad. Sci. USA 1995, 92, 2519–2523. [Google Scholar] [CrossRef] [PubMed]
- Mas, F.; Jallon, J.M. Sexual isolation and cuticular hydrocarbon differences between Drosophila santomea and Drosophila yakuba. J. Chem. Ecol. 2005, 31, 2747–2752. [Google Scholar] [CrossRef] [PubMed]
- Grillet, M.; Everaerts, C.; Houot, B.; Ritchie, M.G.; Cobb, M.; Ferveur, J.F. Incipient speciation in Drosophila melanogaster involves chemical signals. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Bontonou, G.; Denis, B.; Wicker-Thomas, C. Male pheromone polymorphism and reproductive isolation in populations of Drosophila simulans. Ecol. Evol. 2012, 2, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Lachaise, D.; Cariou, M.L.; David, J.R.; Lemeunier, F.; Tsacas, L.; Ashburner, M. Historical biogeography of the Drosophila melanogaster species subgroup. Evol. Biol. 1988, 22, 159–225. [Google Scholar]
- David, J.R.; Lemeunier, F.; Tsacas, L.; Yassin, A. The historical discovery of the nine species in the Drosophila melanogaster species subgroup. Genetics 2007, 177, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Kwan, L.; Rundle, H.D. Adaptation to desiccation fails to generate pre- and post-mating isolation in replicate Drosophila melanogaster laboratory populations. Evolution 2010, 64, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.L.; Cridland, J.M.; Shao, L.; Hu, T.T.; Andolfatto, P.; Thornton, K.R. Landscape of standing variation for tandem duplications in Drosophila yakuba and Drosophila simulans. Mol. Biol. Evol. 2014, 31, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Conant, G.C.; Wolfe, K.H. Turning a hobby into a job: How duplicated genes find new functions. Nat. Rev. Genet. 2008, 9, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Chertemps, T.; Duportets, L.; Labeur, C.; Ueda, R.; Takahashi, K.; Saigo, K.; Wicker-Thomas, C. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2007, 104, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Lachaise, D.; Harry, M.; Solignac, M.; Lemeunier, F.; Benassi, V.; Cariou, M.L. Evolutionary novelties in islands: Drosophila santomea, a new melanogaster sister species from Sao Tome. Proc. Biol. Sci. 2000, 267, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Llopart, A.; Lachaise, D.; Coyne, J.A. Multilocus analysis of introgression between two sympatric sister species of Drosophila: Drosophila yakuba and D. santomea. Genetics 2005, 171, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Cariou, M.L.; Silvain, J.F.; Daubin, V.; da Lage, J.L.; Lachaise, D. Divergence between Drosophila santomea and allopatric or sympatric populations of D. yakuba using paralogous amylase genes and migration scenarios along the Cameroon volcanic line. Mol. Ecol. 2001, 10, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Llopart, A.; Elwyn, S.; Lachaise, D.; Coyne, J.A. Genetics of a difference in pigmentation between Drosophila yakuba and Drosophila santomea. Evolution 2002, 56, 2262–2277. [Google Scholar] [CrossRef] [PubMed]
- Moehring, A.J.; Llopart, A.; Elwyn, S.; Coyne, J.A.; Mackay, T.F. The genetic basis of prezygotic reproductive isolation between Drosophila santomea and D. yakuba due to mating preference. Genetics 2006, 173, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Coyne, J.A.; Kim, S.Y.; Chang, A.S.; Lachaise, D.; Elwyn, S. Sexual isolation between two sibling species with overlapping ranges: Drosophila santomea and Drosophila yakuba. Evolution 2002, 56, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.A.; Ortiz-Barrientos, D. Simulating natural conditions in the laboratory: A re-examination of sexual isolation between sympatric and allopatric populations of Drosophila pseudoobscura and D. persimilis. Behav. Genet. 2006, 36, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Coyne, J.A.; Elwyn, S.; Rolan-Alvarez, E. Impact of experimental design on Drosophila sexual isolation studies: Direct effects and comparison to field hybridization data. Evolution 2005, 59, 2588–2601. [Google Scholar] [CrossRef] [PubMed]
- Ewing, A.W.; Bennet-Clark, H.C. The courtship songs of Drosophila. Behaviour 1968, 31, 288–301. [Google Scholar] [CrossRef]
- Cowling, D.E.; Burnet, B. Courtship song and genetic control of their acoustic characteristics in sibling species of D. melanogaster subgroup. Anim. Behav. 1981, 29, 924–935. [Google Scholar] [CrossRef]
- Watson, E.T.; Rodewald, E.; Coyne, J.A. The courtship song of Drosophila santomea and a comparison to its sister species D. yakuba (Diptera: Drosophilidae). Eur. J. Entomol. 2007, 104, 145–148. [Google Scholar] [CrossRef]
- Cande, J.; Andolfatto, P.; Prud’homme, B.; Stern, D.L.; Gompel, N. Evolution of multiple additive loci caused divergence between Drosophila yakuba and D. santomea in wing rowing during male courtship. PLoS ONE 2012, 7, e43888. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denis, B.; Rouzic, A.L.; Wicker-Thomas, C. Hydrocarbon Patterns and Mating Behaviour in Populations of Drosophila yakuba. Insects 2015, 6, 897-911. https://doi.org/10.3390/insects6040897
Denis B, Rouzic AL, Wicker-Thomas C. Hydrocarbon Patterns and Mating Behaviour in Populations of Drosophila yakuba. Insects. 2015; 6(4):897-911. https://doi.org/10.3390/insects6040897
Chicago/Turabian StyleDenis, Béatrice, Arnaud Le Rouzic, and Claude Wicker-Thomas. 2015. "Hydrocarbon Patterns and Mating Behaviour in Populations of Drosophila yakuba" Insects 6, no. 4: 897-911. https://doi.org/10.3390/insects6040897
APA StyleDenis, B., Rouzic, A. L., & Wicker-Thomas, C. (2015). Hydrocarbon Patterns and Mating Behaviour in Populations of Drosophila yakuba. Insects, 6(4), 897-911. https://doi.org/10.3390/insects6040897





