Similar Comparative Low and High Doses of Deltamethrin and Acetamiprid Differently Impair the Retrieval of the Proboscis Extension Reflex in the Forager Honey Bee (Apis mellifera)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Exposure Protocols
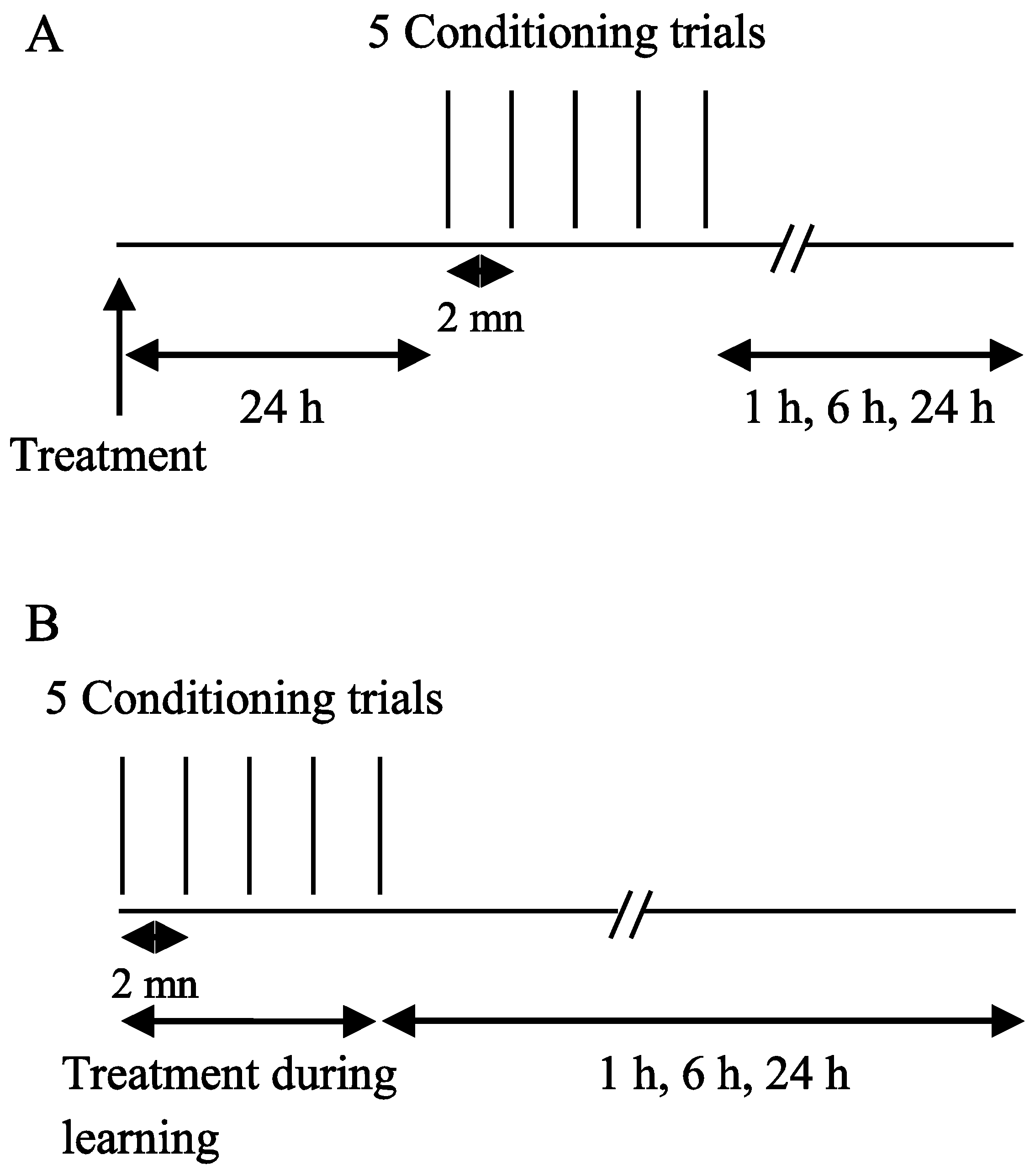
2.3. Drugs
2.4. Behavioral Experiments
2.5. Statistical Analyses
3. Results
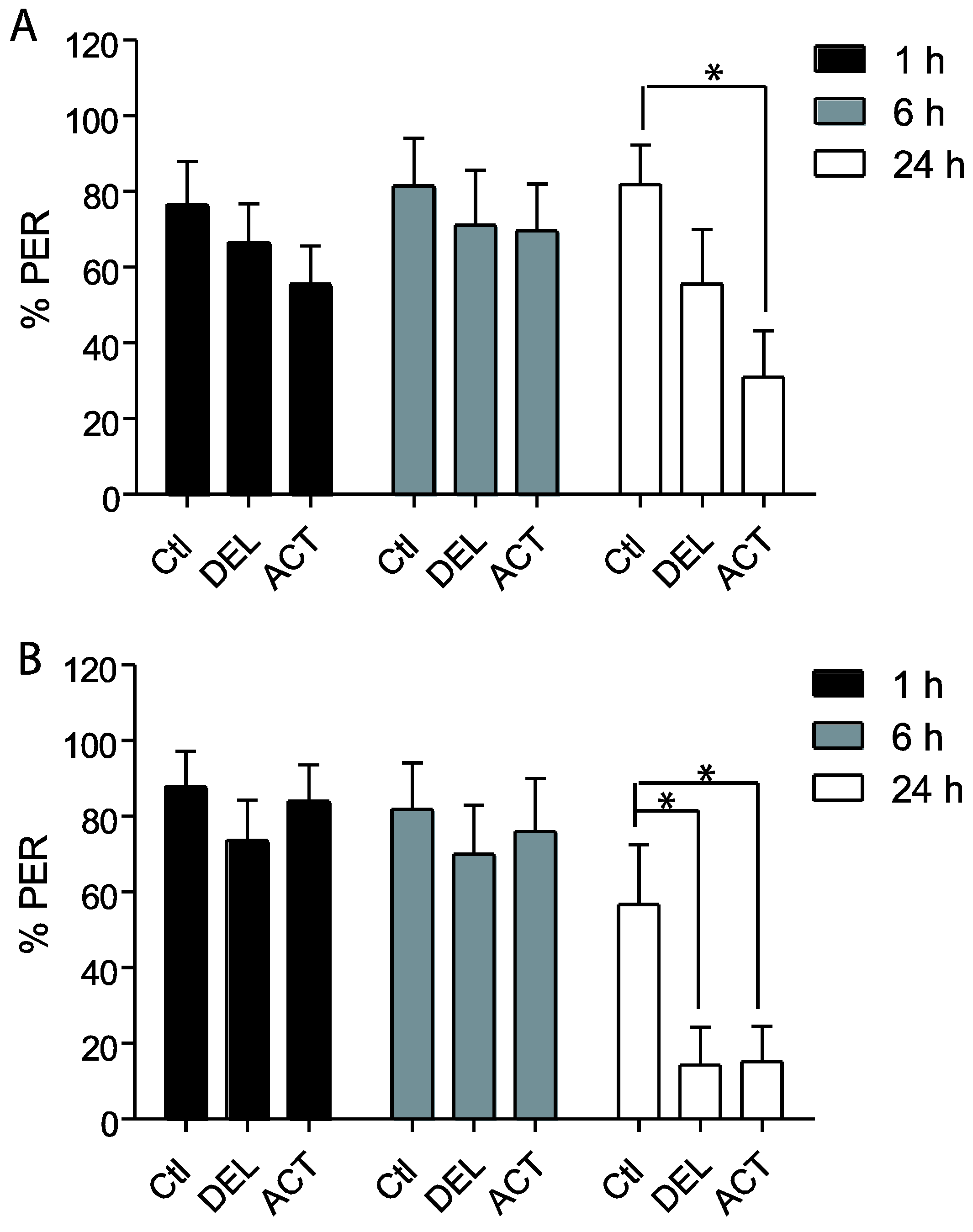
3.1. Effect of ACT and DEL on Acquisition
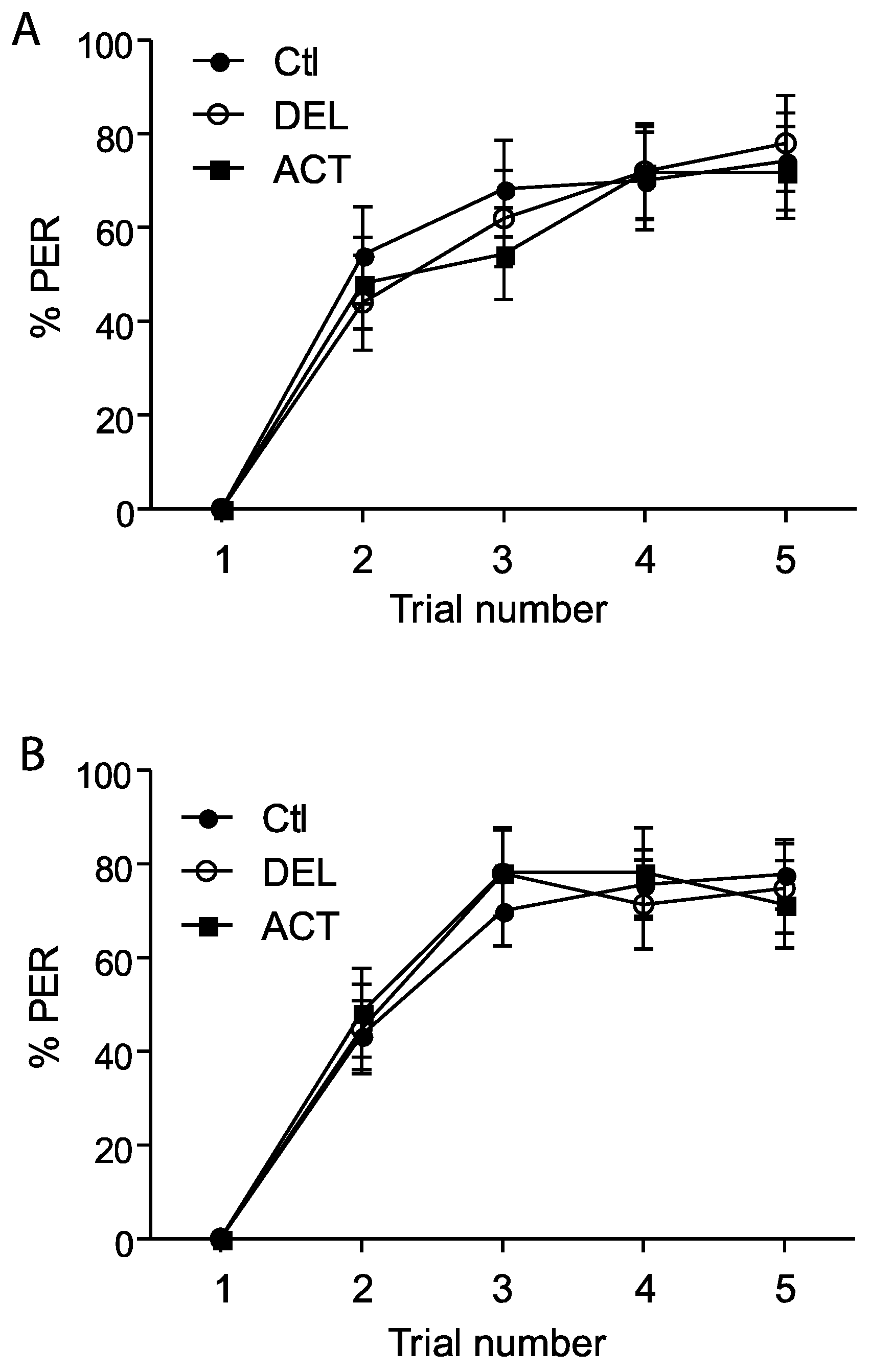
3.2. Effect of ACT and DEL Applied during Learning Trials of the PER
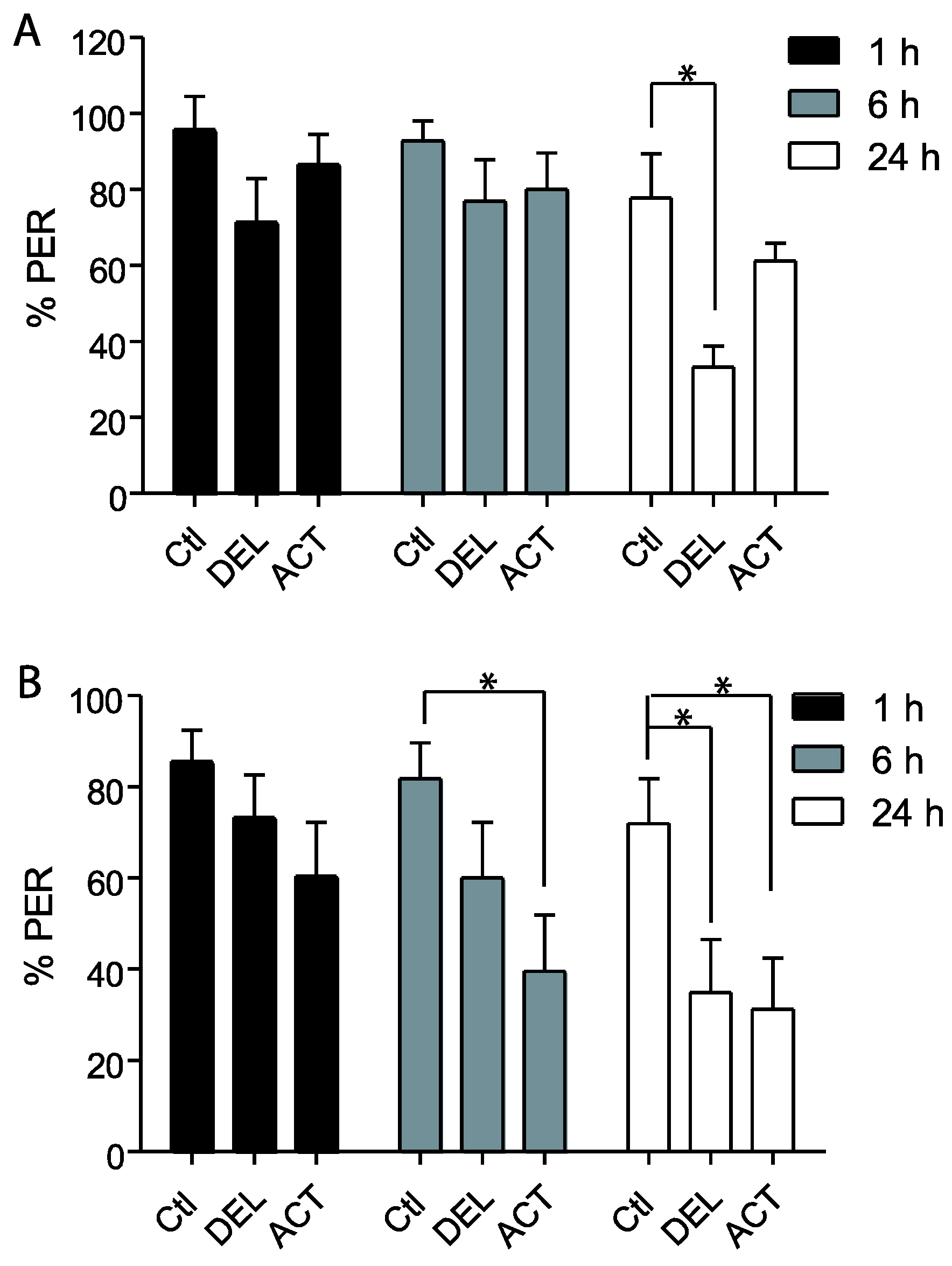
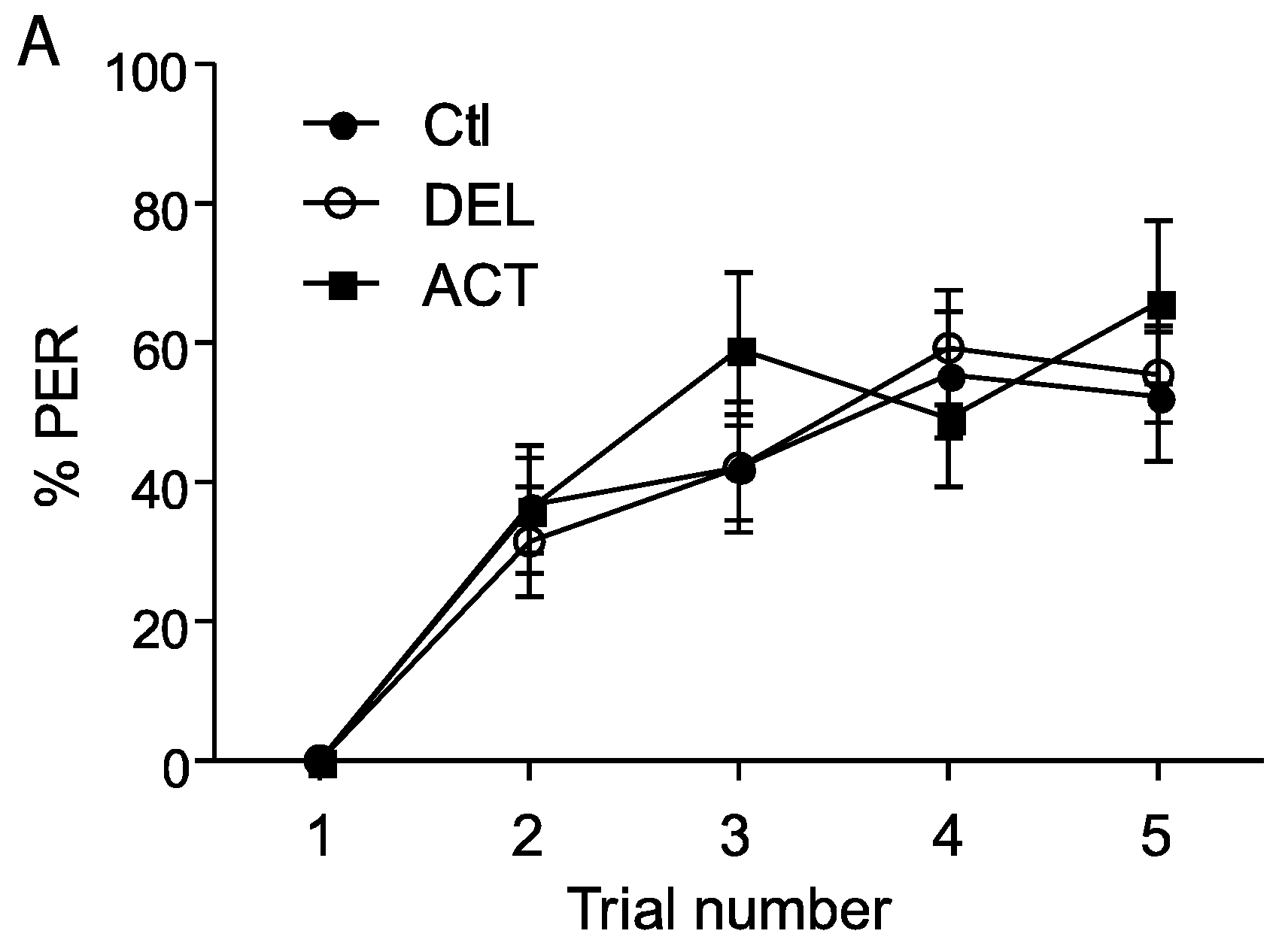

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, A.; Purdy, J.; Anderson, T.; Fell, R. Risks of neonicotinoid insecticides to honeybees. Environ. Toxicol. Chem. 2014, 33, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Rundlof, M.; Andersson, G.K.; Bommarco, R.; Fries, I.; Hederstrom, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayo, F.; Goka, K. Pesticide residues and bees—A risk assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Beguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Aptel1, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Muller, U. Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron 2000, 27, 159–168. [Google Scholar] [CrossRef]
- Giurfa, M.; Menzel, R. Human spatial representation derived from a honeybee compass. Trends Cogn. Sci. 2003, 7, 59–60. [Google Scholar] [CrossRef]
- Giurfa, M. Cognitive neuroethology: Dissecting non-elemental learning in a honeybee brain. Curr. Opin. Neurobiol. 2003, 13, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Benard, J.; Giurfa, M. A test of transitive inferences in free-flying honeybees: Unsuccessful performance due to memory constraints. Learn. Mem. 2004, 11, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Guez, D.; Belzunces, L.P.; Maleszka, R. Effects of imidacloprid metabolites on habituation in honeybees suggest the existence of two subtypes of nicotinic receptors differentially expressed during adult development. Pharmacol. Biochem. Behav. 2003, 75, 217–222. [Google Scholar] [CrossRef]
- Decourtye, A.; Devillers, J.; Cluzeau, S.; Charreton, M.; Pham-Delegue, M.H. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol. Environ. Saf. 2004, 57, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Decourtye, A.; Devillers, J.; Genecque, E.; le Menach, K.; Budzinski, H.; Cluzeau, S.; Pham-Delègue, M.H. Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Arch. Environ. Contam. Toxicol. 2005, 48, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Badiou, A.; Meled, M.; Belzunces, L.P. Honeybee Apis mellifera acetylcholinesterase—A biomarker to detect deltamethrin exposure. Ecotoxicol. Environ. Saf. 2008, 69, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Chauzat, M.P.; Faucon, J.P. Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera L.) in France. Pest. Manag. Sci. 2007, 63, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Decourtye, A.; Armengaud, C.; Renou, M.; Devillers, J.; Cluzeau, S.; Gauthierb, M.; Pham-Delègue, M.-H. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pest. Biochem. Physiol. 2004, 78, 83–92. [Google Scholar] [CrossRef]
- Aliouane, Y.; el Hassani, A.K.; Gary, V.; Armengaud, C.; Lambin, M.; Gauthier, M. Subchronic exposure of honeybees to sublethal doses of pesticides: Effects on behavior. Environ. Toxicol. Chem. 2009, 28, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.; Dacher, M.; Thany, S.H.; Niggebrugge, C.; Deglise, P.; Kljucevic, P.; Armengaud, C.; Grünewald, B. Involvement of alpha-bungarotoxin-sensitive nicotinic receptors in long-term memory formation in the honeybee (Apis mellifera). Neurobiol. Learn. Mem. 2006, 86, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Bertrand, C.; le Feon, V.; Requier, F.; Odoux, J.F.; Aupinel, P.; Bretagnolle, V.; Decourtye, A. Pesticide risk assessment in free-ranging bees is weather and landscape dependent. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thany, S.H.; Bourdin, C.M.; Graton, J.; Laurent, A.D.; Mathé-Allainmat, M.; Lebreton, J.; Le Questel, J.-Y. Similar Comparative Low and High Doses of Deltamethrin and Acetamiprid Differently Impair the Retrieval of the Proboscis Extension Reflex in the Forager Honey Bee (Apis mellifera). Insects 2015, 6, 805-814. https://doi.org/10.3390/insects6040805
Thany SH, Bourdin CM, Graton J, Laurent AD, Mathé-Allainmat M, Lebreton J, Le Questel J-Y. Similar Comparative Low and High Doses of Deltamethrin and Acetamiprid Differently Impair the Retrieval of the Proboscis Extension Reflex in the Forager Honey Bee (Apis mellifera). Insects. 2015; 6(4):805-814. https://doi.org/10.3390/insects6040805
Chicago/Turabian StyleThany, Steeve H., Céline M. Bourdin, Jérôme Graton, Adèle D. Laurent, Monique Mathé-Allainmat, Jacques Lebreton, and Jean-Yves Le Questel. 2015. "Similar Comparative Low and High Doses of Deltamethrin and Acetamiprid Differently Impair the Retrieval of the Proboscis Extension Reflex in the Forager Honey Bee (Apis mellifera)" Insects 6, no. 4: 805-814. https://doi.org/10.3390/insects6040805
APA StyleThany, S. H., Bourdin, C. M., Graton, J., Laurent, A. D., Mathé-Allainmat, M., Lebreton, J., & Le Questel, J.-Y. (2015). Similar Comparative Low and High Doses of Deltamethrin and Acetamiprid Differently Impair the Retrieval of the Proboscis Extension Reflex in the Forager Honey Bee (Apis mellifera). Insects, 6(4), 805-814. https://doi.org/10.3390/insects6040805






