Does a Change from Whole to Powdered Food (Artemia franciscana eggs) Increase Oviposition in the Ladybird Coleomegilla maculata?
Abstract
:1. Introduction
2. Experimental Section
2.1. Insect Colonies and Food Sources
2.2. Powdered vs. Whole Brine Shrimp Eggs with Palmitic Acid
2.3. Statistical Analysis
3. Results
| Parameter | Powder BSE + 5% 16:0 | Whole BSE + 5% 16:0 | Powder BSE | Whole BSE |
|---|---|---|---|---|
| Larval Survival (%) | 66.67 ± 6.15 a | 65.00 ± 5.00 a | 76.67 ± 3.33 a | 63.33 ± 2.11 a |
| Time as Larva (days) | 13.08 ± 0.29 a | 13.54 ± 0.33 a | 13.50 ± 0.27 a | 14.25 ± 0.24 a |
| Time as Pupa (days) | 3.98 ± 0.13 a | 3.99 ± 0.22 a | 4.07 ± 0.08 a | 3.75 ± 0.12 a |
| Pupal Survival (%) | 100 | 100 | 100 | 100 |
| Sex Ratio (% females) | 54.52 ± 3.83 a | 53.85 ± 5.25 a | 56.00 ± 7.22 a | 62.72 ± 5.85 a |
| Male Body Weight (mg) | 11.71 ± 0.18 a | 11.51 ± 0.26 a | 11.82 ± 0.28 a | 11.01 ± 0.52 a |
| Female Body Weight (mg) | 13.58 ± 0.36 a | 13.67 ± 0.20 a | 13.75 ± 0.21 a | 13.42 ± 0.28 a |
| Parameter | Powder BSE + 5% 16:0 | Whole BSE + 5% 16:0 | Powder BSE | Whole BSE |
|---|---|---|---|---|
| Pre-oviposition Time (days) | 14.0 a (10.0; 25.0) | 14.5 a (10.0; 26.0) | 12.0 a (10.0; 18.0) | 15.0 a (12.0; 31.0) |
| Oviposition (eggs per female *) | 69.0 a (30.0; 122.0) | 23.0 bc (15.2; 47.5) | 33.0 ab (28.0; 63.0) | 15.0 c (10.5; 25.5) |
| Oviposition Rate/Day (eggs per female/day) | 15.0 a (12.0; 18.0) | 14.75 a (9.9; 19.5) | 13.3 a (12.0; 16.8) | 13.0 a (8.4; 19.0) |
| Hatch Rate (%) | 67.2 a (60.0; 77.8) | 62.8 a (58.3; 72.4) | 68.0 a (62.4; 74.6) | 52.9 a (43.3; 65.6) |
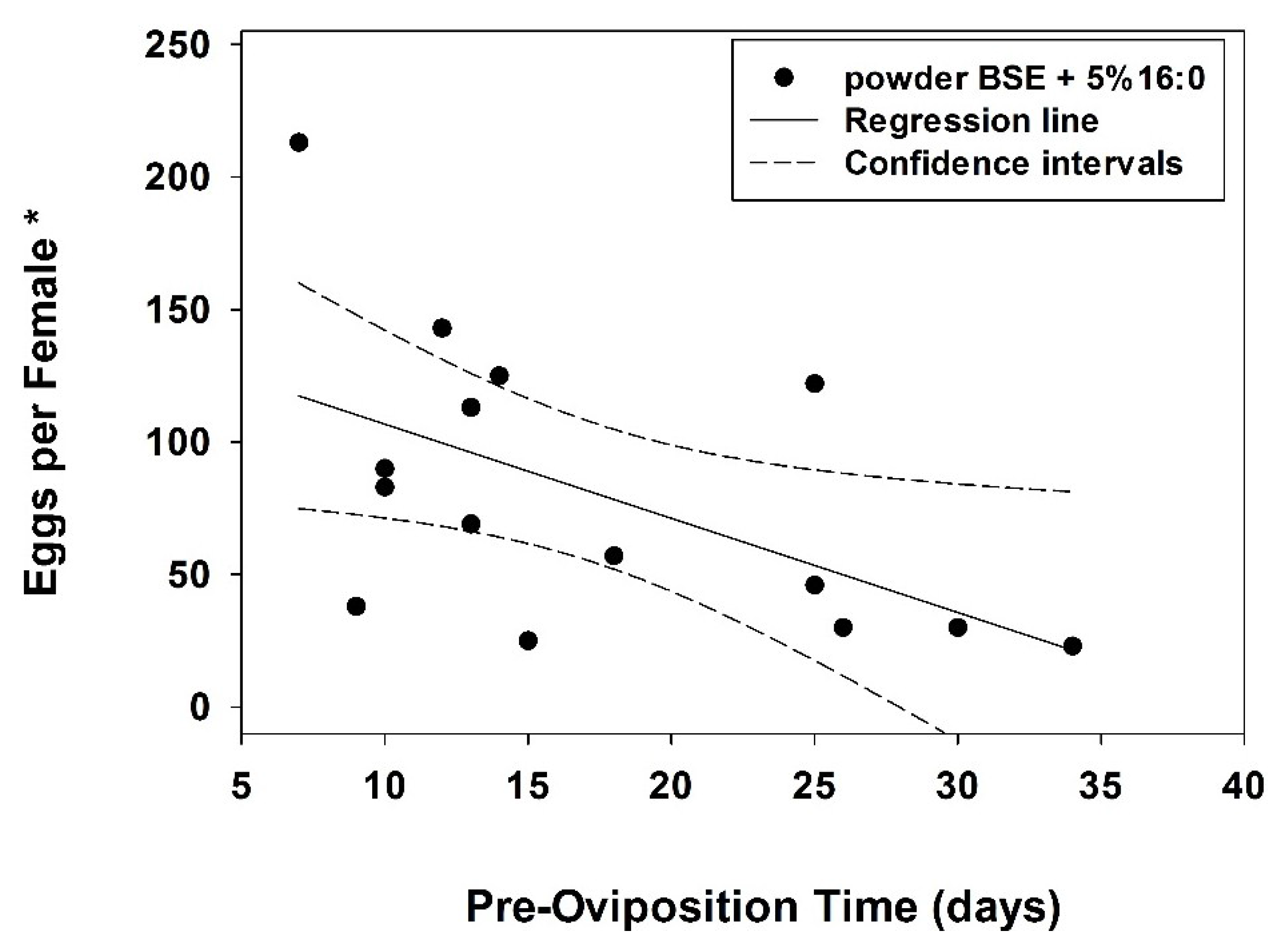
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gordon, R.D. The Coccinellidae (Coleoptera) of America north of Mexico. J. N. Y. Entomol. Soc. 1985, 93, 1–912. [Google Scholar]
- Coll, M.; Mendoza, L.C.; Roderick, G.K. Population structure of a predatory beetle: The importance of gene flow for intertrophic level interactions. Heredity 1994, 72, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Munyaneza, J.; Obrycki, J.J. Development of three populations of Coleomegilla maculata (Coleoptera: Coccinellidae) feeding on eggs of Colorado potato beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 1998, 27, 117–122. [Google Scholar] [CrossRef]
- Krafsur, E.S.; Obrycki, J.J. Coleomegilla maculata (Coleoptera: Coccinellidae) is a species complex. Ann. Entomol. Soc. Am. 2000, 93, 1156–1163. [Google Scholar] [CrossRef]
- Conrad, M.S. The spotted lady beetle, Coleomegilla maculata (De Geer) as a predator of European corn borer eggs. J. Econ. Entomol. 1959, 52, 843–847. [Google Scholar] [CrossRef]
- Michaud, J.P.; Jyoti, J.L. Dietary complementation across life stages in the polyphagous lady beetle Coleomegilla maculata. Entomol. Exp. Appl. 2008, 126, 40–45. [Google Scholar]
- Hodek, I.; Evans, E.W. Food relationships, Chapter 8. In Ecology and Behaviour of Ladybird Beetles (Coccinellidae); Hodek, I., van Emden, H.F., Honěk, A., Eds.; Blackwell Publishing Ltd.: Chichester, West Sussex, UK, 2012; pp. 141–274. [Google Scholar]
- Smith, B.C. A technique for rearing coccinellid beetles on dry foods, and influence of various pollens on the development of Coleomegilla maculata lengi Timb. Can. J. Zool. 1960, 38, 1047–1049. [Google Scholar] [CrossRef]
- Smith, B.C. Growth and development of coccinellid larvae on dry foods (Coleoptera: Coccinellidae). Can. Entomol. 1965, 97, 760–768. [Google Scholar] [CrossRef]
- Andow, D.A.; Risch, S.J. Predation in diversified agroecosystems: Relations between a coccinellid predator Coleomegilla maculata and its food. J. Appl. Ecol. 1985, 22, 357–372. [Google Scholar] [CrossRef]
- Phoofolo, M.W.; Obrycki, J.J.; Lewis, L.C. Quantitative assessment of biotic mortality factors of the European corn borer (Lepidoptera: Crambidae) in field corn. J. Econ. Entomol. 2001, 94, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Musser, F.R.; Shelton, A.M. Predation of Ostrinia nubilalis (Lepidoptera: Crambidae) eggs in sweet corn by generalist predators and the impact of alternative foods. Environ. Entomol. 2003, 32, 1131–1138. [Google Scholar] [CrossRef]
- Li, Y.; Ostrem, J.; Romeis, J.; Chen, M.; Liu, X.; Hellmich, R.L.; Shelton, A.M.; Peng, Y. Development of a Tier-1 assay for assessing the toxicity of insecticidal substances against Coleomegilla maculata. Environ. Entomol. 2011, 40, 496–502. [Google Scholar] [CrossRef]
- Riddick, E.W.; Wu, Z.; Rojas, M.G. Is Tetranychus urticae suitable prey for development and reproduction of naïve Coleomegilla maculata? Insect Sci. 2014, 21, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Riddick, E.W.; Wu, Z.; Rojas, M.G. Potential utilization of Artemia franciscana eggs as food for Coleomegilla maculata. BioControl 2014, 59, 575–583. [Google Scholar] [CrossRef]
- Riddick, E.W.; Wu, Z.; Rojas, M.G.; Morales-Ramos, J.A. Potential utilization of Spirulina microalga as a dietary supplement for the ladybird beetle Coleomegilla maculata. Trends Entomol. 2014, 10, 39–48. [Google Scholar]
- Hagen, K. Nutritional ecology of terrestrial insect predators, Chapter 17. In Nutritional Ecology of Insects, Mites, Spiders, and Related Invertebrates; Slansky, F., Jr., Rodriguez, J.G., Eds.; Wiley & Sons: New York, NY, USA, 1987; pp. 533–577. [Google Scholar]
- Riddick, E.W.; Chen, H. Production of Coleopteran Predators, Chapter 2. In Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens; Morales-Ramos, J., Rojas, M.G., Shapiro-Ilan, D.I., Eds.; Academic Press: London, UK, 2014; pp. 17–55. [Google Scholar]
- Smirnoff, W.A. An artificial diet for rearing coccinellid beetles. Can. Entomol. 1958, 90, 563–565. [Google Scholar] [CrossRef]
- Atallah, Y.H.; Newsom, L.D. Ecological and nutritional studies on Coleomegilla maculata. J. Econ. Entomol. 1966, 59, 1173–1179. [Google Scholar]
- Silva, R.B.; Cruz, I.; Figueiredo, M.L.C.; Tavares, W.S. Development of Coleomegilla maculata DeGeer (Coleoptera: Coccinellidae) with prey and artificial diet. Rev. Bras. Milho Sorgo 2010, 9, 13–26. [Google Scholar]
- Sighinolfi, L.; Febvay, G.; Dindo, M.L.; Rey, M.R.; Pageaux, J.F.; Grenier, S. Biochemical content in fatty acids and biological parameters of Harmonia axyridis reared on artificial diet. Bull. Insectol. 2013, 66, 283–290. [Google Scholar]
- Specty, O.; Febvay, G.; Grenier, S.; Delobel, B.; Piotte, C.; Pageaux, J.-F.; Ferran, A.; Guillaud, J. Nutritional plasticity of the predatory ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae): Comparison between natural and substitution prey. Arch. Insect Biochem. Physiol. 2003, 52, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.A.; Tirry, L.; de Clercq, P. Food consumption and immature growth of Adalia bipunctata (Coleoptera: Coccinellidae) on a natural prey and a factitious food. Eur. J. Entomol. 2009, 106, 193–198. [Google Scholar] [CrossRef]
- Cohen, A.C.; Smith, L.K. A new concept in artificial diets for Chrysoperla rufilabris: The efficacy of solid diets. Biol. Control 1998, 13, 49–54. [Google Scholar] [CrossRef]
- De Clercq, P.; Arijs, Y.; van Meir, T.; van Stappen, G.; Sorgeloos, P.; Dewettinck, K.; Rey, M.; Grenier, S.; Febvay, G. Nutritional value of brine shrimp cysts as a factitious food for Orius laevigatus (Heteroptera: Anthocoridae). Biocontrol Sci. Technol. 2005, 15, 467–479. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Vangansbeke, D.; de Clercq, P. Artificial and factitious foods support the development and reproduction of the predatory mite Amblyseius swirskii. Exp. Appl. Acarol. 2014, 62, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, R.; van Antwerpen, R. Lipid uptake by insect oocytes. Insect Biochem. Mol. Biol. 2006, 36, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Sayah, F. Changes in the lipid and fatty acid composition of hemolymph and ovaries during the reproductive cycle of Labidura riparia. Entomol. Sci. 2008, 11, 55–63. [Google Scholar] [CrossRef]
- Sloggett, J.J.; Lorenz, M.W. Egg composition and reproductive investment in aphidophagous ladybird beetles (Coccinellidae: Coccinellini): Egg development and interspecific variation. Physiol. Entomol. 2008, 33, 200–208. [Google Scholar] [CrossRef]
- Gilby, A.R. Lipids and their metabolism in insects. Annu. Rev. Entomol. 1965, 10, 141–160. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D.W.; Jurenka, R.A.; Cripps, C.; Blomquist, G.J.; Renobales, M. Fatty acids in insects: Composition, metabolism, and biological significance. Arch. Insect Biochem. Physiol. 1998, 9, 1–33. [Google Scholar] [CrossRef]
- Zar, J.H. The fatty acid composition of the ladybird beetle, Coleomegilla maculata (DeGeer) during hibernation. Comp. Biochem. Physiol. 1968, 26, 1127–1129. [Google Scholar] [CrossRef]
- Barlow, J.S. Fatty acids in some insect and spider fats. Can. J. Biochem. 1964, 42, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Callow, R.K.; Greenway, A.R.; Griffiths, D.C. Chemistry of the secretion from the cornicles of various species of aphids. J. Insect Physiol. 1973, 19, 737–748. [Google Scholar] [CrossRef]
- Greenway, A.R.; Griffiths, D.C.; Furk, C.; Prior, R.N.B. Composition of triglycerides from aphids of six different families and from different seasonal forms of Aphis evonymi. J. Insect Physiol. 1974, 20, 2423–2431. [Google Scholar] [CrossRef]
- Wu, X.-H.; Zhou, X.-R.; Pang, B.-P.; Li, Y.-Y.; Hao, J.-X. Changes in major body constituents of Aphis gossypii Glover (Homoptera: Aphididae) on different host plants. Acta Entomol. Sin. 2009, 52, 1249–1254. (In Chinese) [Google Scholar]
- Giles, K.L.; Stockland, R.; Madden, R.D.; Payton, M.E.; Dillwith, J.W. Preimaginal survival and development of Coleomegilla maculata and Hippodamia convergens (Coleoptera: Coccinellidae) reared on Acyrthosiphon pisum: Effects of host plants. Environ. Entomol. 2001, 30, 964–971. [Google Scholar] [CrossRef]
- Giles, K.L.; Madden, R.D.; Stockland, R.; Payton, M.E.; Dillwith, J.W. Host plants affect predator fitness via the nutritional value of herbivore prey: Investigation of a plant-aphid-ladybeetle system. BioControl 2002, 47, 1–21. [Google Scholar] [CrossRef]
- Du, L; Ge, F.; Zhu, S.; Parajulee, M.N. Effect of cotton cultivar on development and reproduction of Aphis gossypii (Homoptera: Aphididae) and its predator Propylaea japonica (Coleoptera: Coccinellidae). J. Econ. Entomol. 2004, 97, 1278–1283. [Google Scholar]
- Bashir, M.O. Effect of Nutrition on Development and Reproduction of Aphidophagous Coccinellids with Special Reference to Olla abdominalis (Say). Ph.D. Thesis, University of California, Berkeley, CA, USA, 1973. [Google Scholar]
- Haulotte, E.; Laurent, P.; Braekman, J.-C. Biosynthesis of defensive Coccinellidae alkaloids: Incorporation of fatty acids in adaline, coccinelline, and harmonine. Eur. J. Org. Chem. 2012. [Google Scholar] [CrossRef]
- Riddick, E.W.; Wu, Z.; Jamie Whitten Delta States Research Center, ARS-USDA, Stoneville, MS, USA. Unpublished data. 2015.
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: New Jersey, NJ, USA, 1999. [Google Scholar]
- Dixon, A.F.G. Insect Predator-Prey Dynamics: Ladybird Beetles and Biological Control; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Robertson, J.G. Ovariole numbers in Coleoptera. Can. J. Zool. 1961, 39, 245–263. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riddick, E.W.; Wu, Z. Does a Change from Whole to Powdered Food (Artemia franciscana eggs) Increase Oviposition in the Ladybird Coleomegilla maculata? Insects 2015, 6, 815-826. https://doi.org/10.3390/insects6040815
Riddick EW, Wu Z. Does a Change from Whole to Powdered Food (Artemia franciscana eggs) Increase Oviposition in the Ladybird Coleomegilla maculata? Insects. 2015; 6(4):815-826. https://doi.org/10.3390/insects6040815
Chicago/Turabian StyleRiddick, Eric W., and Zhixin Wu. 2015. "Does a Change from Whole to Powdered Food (Artemia franciscana eggs) Increase Oviposition in the Ladybird Coleomegilla maculata?" Insects 6, no. 4: 815-826. https://doi.org/10.3390/insects6040815
APA StyleRiddick, E. W., & Wu, Z. (2015). Does a Change from Whole to Powdered Food (Artemia franciscana eggs) Increase Oviposition in the Ladybird Coleomegilla maculata? Insects, 6(4), 815-826. https://doi.org/10.3390/insects6040815







