The Importance of Maintaining Protected Zone Status against Bemisia tabaci
Abstract
:1. Introduction
2. Economic Importance of Protected Zone Status
3. Bemisia tabaci Interceptions
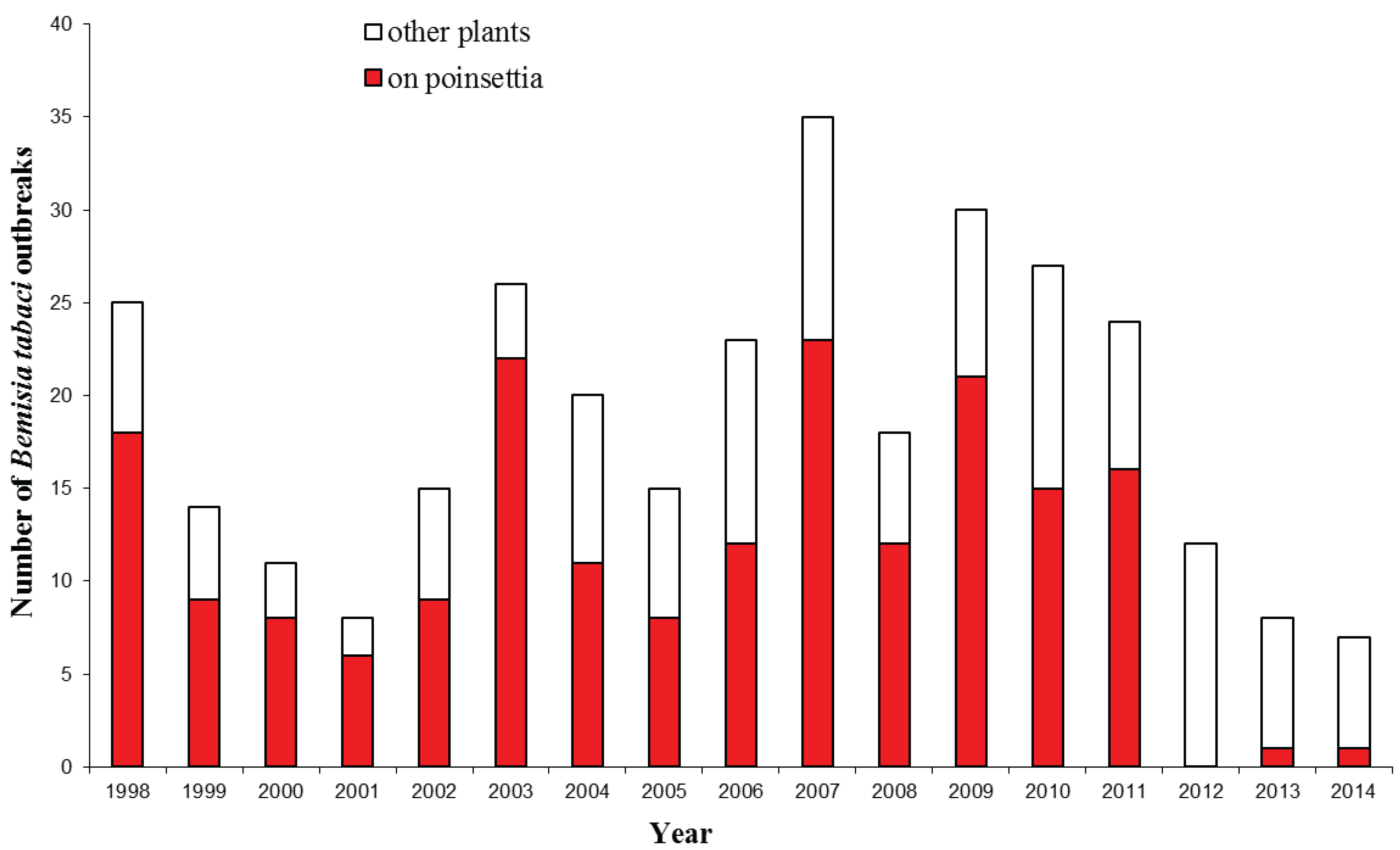
4. Impact of Bemisia tabaci Establishment
5. Maintaining Protected Zone Status
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Xu, C.; Qiu, B.-L.; Cuthbertson, A.G.S.; Zhang, Y.; Ren, S.-X. Adaptability of sweetpotato whitefly Bemisia tabaci (Hemipetera: Aleyrodidae) on seven marginal host plants. Int. J. Pest Manage. 2012, 58, 297–301. [Google Scholar] [CrossRef]
- Oliveira, M.R.V.; Henneberry, T.J.; Anderson, P. History, current status and collaborative research projects for Bemisia tabaci. Crop Prot. 2002, 20, 709–723. [Google Scholar] [CrossRef]
- Perring, T.M. The Bemisia tabaci species complex. Crop Prot. 2001, 20, 725–737. [Google Scholar] [CrossRef]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.M.; De Barro, P. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Ann. Rev. Entomol 2011, 56, 1–19. [Google Scholar]
- Powell, M.E.; Cuthbertson, A.G.S. Pest control: Distinguishing between different biotypes of Bemisia tabaci in the UK. The Biologist 2013, 60, 18–21. [Google Scholar]
- Boykin, L.M.; De Barro, P.J. A practical guide to identifying members of the Bemisia tabaci species complex: And other morphologically identical species. Front. Ecol. Evol. 2014, 45, 1–5. [Google Scholar]
- Shatters, R.G.; Powell, C.A.; Boykin, L.; Liansheng, H.; McKenzie, C.L. Improved DNA barcoding method for Bemisia tabaci and related Aleyrodidae: Development of universal and Bemisia tabaci biotype-specific mitochondrial cytochrome c oxidase I polymerase chain reaction primers. J. Econ. Entomol. 2009, 102, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Bethke, J.A.; Byrne, F.J.; Hodges, G.S.; McKenzie, C.L.; Shatters, R.G. First record of the Q biotype of the sweetpotato whitefly, Bemisia tabaci, in Guatemala. Phytoparasitica 2009, 37, 61–64. [Google Scholar] [CrossRef]
- Qiu, B.-L.; Dang, F.; Li, S.-J.; Ahmed, M.Z.; Jin, F.-L.; Ren, S.-X.; Cuthbertson, A.G.S. Comparison of biological parameters between the invasive B biotype and a new defined Cv biotype of Bemisia tabaci (Hemiptera: Aleyradidae) in China. J. Pest Sci. 2011, 84, 419–427. [Google Scholar] [CrossRef]
- Jones, C.M.; Gorman, K.; Denholm, I.; Williamson, M.S. High-throughput allelic discrimination of B and Q biotypes of the whitefly, Bemisia tabaci, using TaqMan allele-selective PCR. Pest Manag. Sci. 2008, 64, 12–15. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.L.; Hodges, G.; Osborne, L.; Byrne, F.J.; Shatters, R.G. Distribution of Bemisia tabaci (Hemiptera: Aleyrodidae) biotypes in Florida - investigating the Q invasion. J. Econ. Entomol. 2009, 102, 670–676. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Scientific opinion on the risks to plant health posed by Bemisia tabaci species complex and viruses it transmits for the EU territory. EFSA J. 2013, 11. [CrossRef]
- Cuthbertson, A.G.S. Update on the status of Bemisia tabaci in the UK and the use of entomopathogenic fungi within eradication programmes. Insects 2013, 4, 198–205. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Blackburn, L.F.; Eyre, D.P.; Cannon, R.J.C.; Millar, J.; Northing, P. Bemisia tabaci: The current situation in the UK and the prospect of developing strategies for eradication using entomopathogens. Insect Sci. 2011, 18, 1–10. [Google Scholar] [CrossRef]
- Cathrin, P.B.; Ghanim, M. Recent advances on interactions between the whitefly Bemisia tabaci and begomovirus, with emphasis on tomato yellow leaf curl virus. In Plant Virus-Host Interaction: Molecular Approaches and Viral Evolution; Elsevier: Amsterdam, The Netherlands, 2014; pp. 79–103. [Google Scholar]
- Morgan, D.; MacLeod, A. Assessing the economic threat of Bemisia tabaci and tomato yellow leaf curl virus to the tomato industry in England and Wales. Proc. BCPC Conf. 1996, 3, 1077–1082. [Google Scholar]
- Gilioli, G.; Pasquali, S.; Parisi, S.; Winter, S. Modelling the potential distribution of Bemisia tabaci in Europe in light of the climate change scenario. Pest Manag. Sci. 2014, 70, 1611–1623. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.E.; Cuthbertson, A.G.S.; Boonham, N.; Morris, J.; Bell, H.A.; Northing, P. First record of the Q Biotype of the sweetpotato whitefly, Bemisia tabaci, intercepted in the UK. Eur. J. Plant Pathol. 2012, 133, 797–801. [Google Scholar] [CrossRef]
- Nauen, R. Insecticide resistance in European agriculture: Research instead of rumours. In Proceedings BCPC International Congress—Crop Science & Technology, BCPC, Alton, Hants, UK; 2005; Vol. 3A-1, pp. 123–130. [Google Scholar]
- Fernandez, E.; Gravalos, C.; Haro, P.J.; Cifuentes, D.; Bielza, P. Insecticide resistance status of Bemisia tabaci Q biotype in south-eastern Spain. Pest Manag. Sci. 2009, 65, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, H.; Yang, Y.; Wu, Y. Biotype and insecticide resistance status of the whitefly Bemisia tabaci in China. Pest Manag. Sci. 2010, 66, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Lemmetty, A.; Vänninen, I. Bemisia tabaci biotype Q determined for the first time on poinsettia crops in Finland and Sweden. Ann. Zool. Fennici 2014, 51, 501–506. [Google Scholar] [CrossRef]
- The Finnish Food Safety Authority (Evira). New occurrences of regulated pest species 2009‒2013. Available online: http://www.evira.fi/files/attachments/fi/kasvit/kasvinterveys/tuhoojatilasto_2009-2013.pdf (accessed on 31 January 2015).
- The Finnish Food Safety Authority (Evira). Kasvinterveysyksikön valvontaraportti 2013. (Monitoring report for 2013 by the Plant Health Unit of the Finnish Food Safety Authority). Available online: http://www.evira.fi/portal/fi/tietoa%2Bevirasta/julkaisut/?a=view&productId=377 (accessed on 31 January 2015). (in Finnish).
- Muñiz, M.; Nombela, G. Differential variation in development of the B- and Q-biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae) on sweet pepper at constant temperatures. Environ. Entomol. 2001, 30, 720–727. [Google Scholar] [CrossRef]
- Hannunen, S.; Heikkilä, J.; Tuomola, J. A model for ranking plant pests and diseases (FinnPRIO). Available online: http://ageconsearch.umn.edu/bitstream/182970/2/EAAE2014_Prio_fullpaper_24062014.pdf (accessed on 31 January 2015).
- Cohen, J.; Gera, A.; Ecker, R.; Ben Joseph, R.; Perlsman, M.; Gokkes, M.; Lachman, O.; Antignus, Y. Lisianthus leaf curl a new disease of Lisianthus caused by tomato yellow leaf curl virus. Plant Dis. 1995, 79, 416–420. [Google Scholar] [CrossRef]
- Ioannou, N.; Kyriakou, A.; Hadjinicolis, A. Host range and natural reservoirs of tomato yellow leaf curl virus. Agricultural Research Institute, Ministry of Agriculture and Natural Resources, Finland. Tech. Bull. 1987, 85, 1–7. [Google Scholar]
- Polston, J.E.; Cohen, L.; Sherwood, T.A.; Ben-Joseph, R.; Lapidot, M. Capsicum species: Symptomless hosts and reservoirs of tomato yellow leaf curl virus. Phytopathology 2006, 96, 447–452. [Google Scholar] [CrossRef] [PubMed]
- NPPS (Netherlands Plant Protection Service), Tomato Yellow Leaf Curl Virus (TYLCV-Alm) on Solanum lycopersicum (Tomato) Plants; Ministry of Agriculture, Nature and Food Quality: Wageningen, The Netherlands, Pest Report 2007; p. 2.
- Just, K.; Leke, W.N.; Sattar, M.N.; Luik, A.; Kvarnheden, A. Detection of tomato yellow leaf curl virus in imported tomato fruit in northern Europe. Plant Pathol. 2014, 63, 1454–1460. [Google Scholar] [CrossRef]
- Delatte, H.; Dalmon, A.; Rist, D.; Soustrade, I.; Wuster, G.; Lett, J.M.; Reynaud, B. Tomato yellow leaf curl virus can be acquired and transmitted by Bemisia tabaci (Gennadius) from tomato fruit. Plant Dis. 2003, 87, 1297–1300. [Google Scholar] [CrossRef]
- Heikkilä, J; Vänninen, I. Impact Simulation of Bemisia tabaci. Natural Resources Institute Finland, Helsinki and Jokioinen, Finland. Unpublished data. 2015. [Google Scholar]
- Vänninen, I.; Pereira-Querol, M.; Engeström, Y. Generating transformative agency among horticultural producers: An activity-theoretical approach to transforming. Int. Pest Manag. Agric. Syst 2015, in press. [Google Scholar]
- Bonato, O.; Lurette, A.; Vidal, C.; Fargues, J. Modelling temperature-dependent bionomics of Bemisia tabaci (Q-biotype). Physiol. Entomol. 2007, 32, 50–55. [Google Scholar] [CrossRef]
- Blackmer, J.L.; Lee, L.L.; Henneberry, T.J. Factors affecting egg hatch, development, and survival of Bemisia argentifolii (Hemiptera: Aleyrodidae) reared on an artificial feeding system. Environ. Entomol. 2002, 31, 306–312. [Google Scholar] [CrossRef]
- Yiejiang, C.; Hongwei, L.; Jian, H.; Qingzhu, H.; Yidi, X. Effect of photoperiod on the experimental population of sweetpotato whitefly, Bemisia tabaci (Gennadius). Entomol. J. East China 2002, 12, 38–41. [Google Scholar]
- Ovčarenko, I.; Kapantaidaki, D.E.; Lindström, L.M.; Gauthier, N.; Tsagkarakou, A.; Knott, K.E.; Vänninen, I. Agroecosystems shape population genetic structure of the greenhouse whitefly in Northern and Southern Europe. BMC Evol. Biol. 2014, 14, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Ovčarenko, I.; Lindström, L.; Saikkonen, K.; Vänninen, I. Variation in mortality among populations is higher for pymetrozine, than for imidacloprid and spiromesifen in Trialeurodes vaporariorum in greenhouses in Finland. Pest Manag. Sci. 2014, 70, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- DEFRA (Department for Environment, Food and Rural Affairs). Consultation on the UK Protected Zone (PZ) status of Bemisia tabaci (sweetpotato whitefly). Available online: https://secure.fera.defra.gov.uk/website2009/plants/plantHealth/pestsDiseases/documents/consultProtectZone.pdf (accessed on 31 January 2015).
- Zhang, G.-F.; Li, D.-C.; Liu, T.-X.; Wan, F.-H.; Wang, J.-J. Interspecific interactions between Bemisia tabaci biotype B and Trialeurodes vaporariorum (Hemiptera:Aleyrodidae). Environ. Entomol. 2011, 40, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, J. Cost benefit analysis for the protected zone of Bemisia tabaci in Finland (in Finnish with English summary). Available online: http://www.mtt.fi/mtts/pdf/mtts170.pdf (accessed on 5 February 2015).
- Cuthbertson, A.G.S.; Buxton, J.H.; Blackburn, L.F.; Mathers, J.J.; Robinson, K.; Powell, M.E.; Fleming, D.A.; Bell, H.A. Eradicating Bemisia tabaci Q on poinsettia plants in the UK. Crop Prot. 2012, 42, 42–48. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Collins, D.A. Tri-Tek (petroleum horticultural oil) and Beauveria bassiana: Use in eradication strategies for Bemisia tabaci Mediterranean species in UK glasshouses. Insects 2015, 6, 133–140. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Fera, Sand Hutton, York, UK. Unpublished data. 2015.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuthbertson, A.G.S.; Vänninen, I. The Importance of Maintaining Protected Zone Status against Bemisia tabaci. Insects 2015, 6, 432-441. https://doi.org/10.3390/insects6020432
Cuthbertson AGS, Vänninen I. The Importance of Maintaining Protected Zone Status against Bemisia tabaci. Insects. 2015; 6(2):432-441. https://doi.org/10.3390/insects6020432
Chicago/Turabian StyleCuthbertson, Andrew G. S., and Irene Vänninen. 2015. "The Importance of Maintaining Protected Zone Status against Bemisia tabaci" Insects 6, no. 2: 432-441. https://doi.org/10.3390/insects6020432
APA StyleCuthbertson, A. G. S., & Vänninen, I. (2015). The Importance of Maintaining Protected Zone Status against Bemisia tabaci. Insects, 6(2), 432-441. https://doi.org/10.3390/insects6020432




