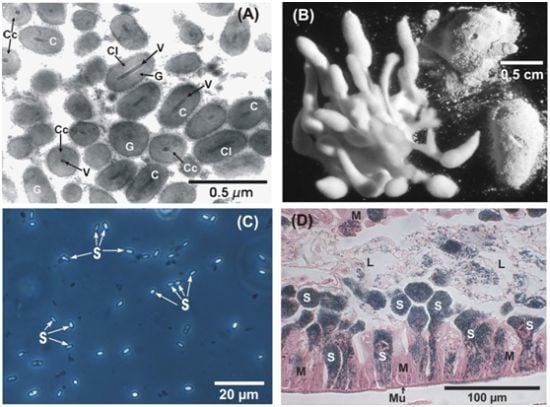
Occurrence and Prevalence of Insect Pathogens in Populations of the Codling Moth, Cydia pomonella L.: A Long-Term Diagnostic Survey
Abstract
:1. Introduction
2. Material and Methods
2.1. Examination and Identification
2.2. Experiments with Nosema carpocapsae
3. Results
3.1. General Information on Accessions
3.2. Pathogens, Other Microorganisms and Nematodes of C. pomonella (Larvae, Pupae, Adults) from Field Populations
| Pathogen-Group | Pathogens/Microorganisms/Nematodes | Origin (country) |
|---|---|---|
| Viruses | Granulovirus | CH, DE |
| Granulovirus + Nosema carpocapsae | CH, DE | |
| Bacteria | Bacteria, unidentified | DE |
| Bacteria (spore-formers), unidentified | DE | |
| Serratia liquefaciens | DE | |
| Serratia sp. | DE | |
| Hafnia alvei + Serratia sp. + Pseudomonas sp. | DE | |
| Serratia sp. + Nosema carpocapsae | DE | |
| Bacillus cereus + nematodes, unidentified | DE | |
| Fungi | Alternaria sp. | DE |
| Aspergillus flavus | DE | |
| Aspergillus sp. | AT, DE | |
| Beauveria bassiana | AT, DE | |
| Beauveria bassiana, hyperparasitized by Syspastospora parasitica (Melanospora parasitica) | DE | |
| Beauveria sp. | AT | |
| Cephalosporium sp. | AT, DE | |
| Cladosporium sp. | AT | |
| Fusarium avenaceum | AT | |
| Fusarium sp. | AT, DE | |
| Hirsutella gigantea | AT | |
| Hirsutella sp. | AT, CH, DE | |
| Hirsutella subulata | AT | |
| Isaria farinosa | AT, DE | |
| Isaria farinosa hyperparasitized by Syspastospora parasitica (Melanospora parasitica) | AT, DE | |
| Isaria fumosorosea | DE | |
| Lecanicillium sp. (Verticillium lecanii) | DE | |
| Metarhizium anisopliae | AT | |
| Mucor sp. | AT, DE | |
| Penicillium sp. | AT, DE | |
| Verticillium sp. | DE | |
| Mixed infections | ||
| Alternaria sp. + Cephalosporium sp. | DE | |
| Alternaria sp. + Fusarium sp. | DE | |
| Aspergillus sp. + Fusarium sp. | AT, DE | |
| Aspergillus sp. + Mucor sp. | AT | |
| Aspergillus sp. + Fusarium sp. + Penicillium sp. | AT | |
| Beauveria bassiana + bacteria, unidentified | AT | |
| Beauveria bassiana + Aspergillus sp. | AT | |
| Beauveria bassiana + Cephalosporium sp. | DE | |
| Beauveria bassiana + Cladosporium sp. | AT | |
| Beauveria bassiana + Hirsutella sp. | DE | |
| Beauveria bassiana + Mucor sp. | AT | |
| Beauveria bassiana + Isaria farinosa | DE | |
| Beauveria bassiana + Penicillium sp. | DE | |
| Beauveria bassiana + Aspergillus sp. + Hirsutella sp. | AT | |
| Beauveria bassiana + Mucor sp. + Penicillium sp. | AT | |
| Beauveria bassiana + Penicillium sp. + Aspergillus sp. | AT | |
| Hirsutella sp. + Aspergillus sp. | AT | |
| Hirsutella sp. + Mucor sp. | AT | |
| Isaria farinosa + Alternaria sp. | DE | |
| Isaria farinosa + Fusarium sp. | DE | |
| Isaria farinosa + Penicillium sp. | AT | |
| Isaria farinosa + Beauveria bassiana + Penicillium sp. | AT | |
| Isaria farinosa + nematodes, unidentified | DE | |
| Paecilomyces sp. + Alternaria sp. | DE | |
| Paecilomyces sp. + Beauveria sp. | DE | |
| Paecilomyces sp. + Mucor sp. | DE | |
| Paecilomyces sp. + Verticillium sp. | DE | |
| Penicillium sp. + Alternaria sp. | DE | |
| Penicillium sp. +Aspergillus sp. | DE | |
| Penicillium sp. +Cephalosporium sp. | DE | |
| Penicillium sp. + Mucor sp. | AT, DE | |
| Verticillium sp. + Alternaria sp. | DE | |
| Verticillium sp. + Penicillium sp. | DE | |
| Fungi/Microsporidia | Nosema carpocapsae | CH, DE |
| Nosema carpocapsae + nematodes, unidentified | DE | |
| Nosema carpocapsae + fungi, unidentified | DE | |
| Microsporidia, unidentified + bacteria, unidentified | CH | |
| Nematodes | Nematodes, unidentified | DE |
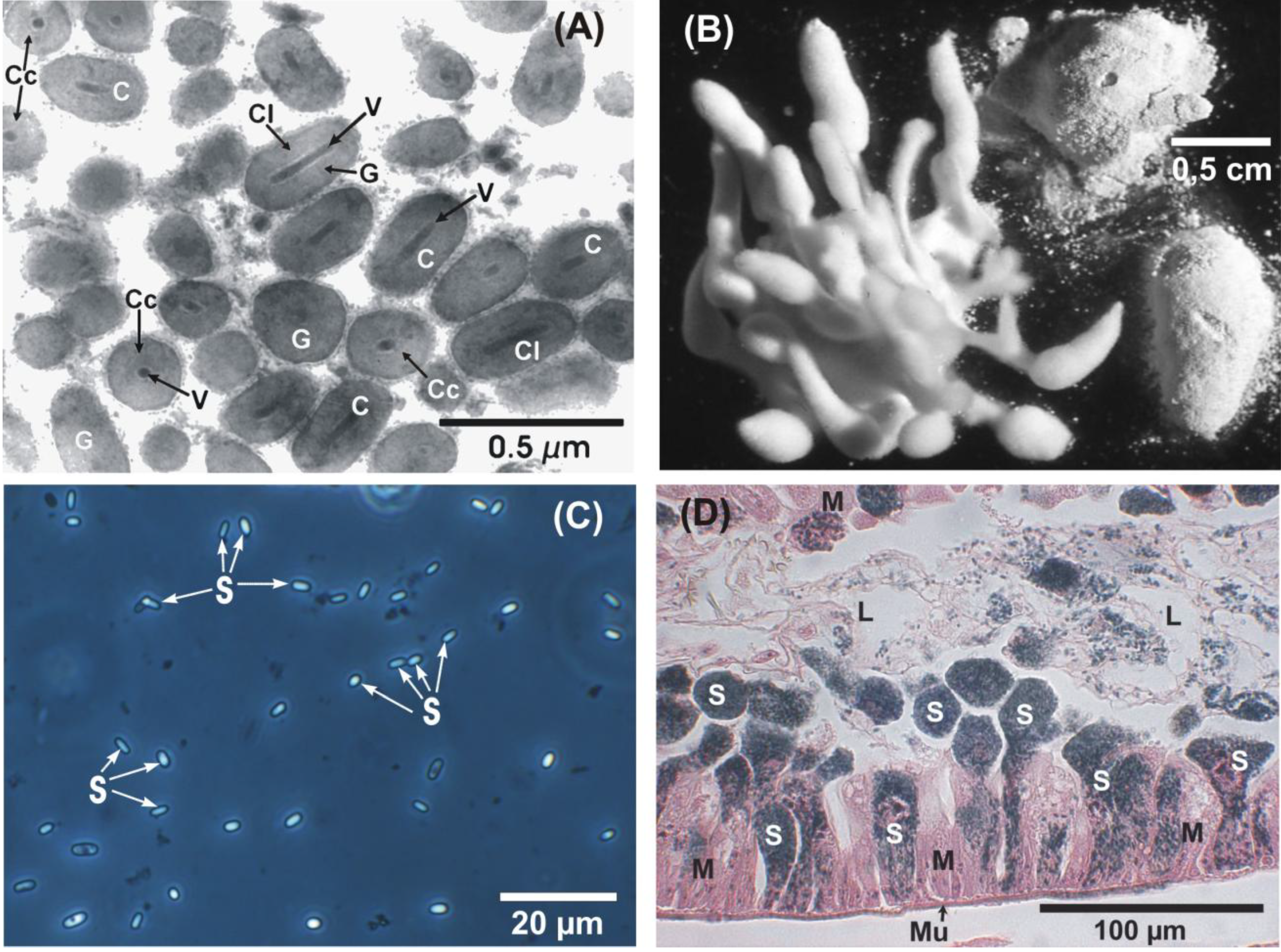
3.3. Pathogens and Other Microorganisms of C. pomonella (Larvae, Pupae, Adults) from Laboratory Colonies
| Pathogen-Group | Pathogens/Microorganisms | Accessions (n) |
|---|---|---|
| Viruses | Granulovirus | 4 |
| Granulovirus + Nosema carpocapsae | 1 | |
| Granulovirus + microsporidia, unidentified | 3 | |
| Bacteria | Bacteria, unidentified | 4 |
| Serratia liquefaciens | 1 | |
| Bacteria, unidentified+ Serratia sp. | 1 | |
| Bacteria, unidentified + microsporidia, unidentified | 1 | |
| Serratia sp. + Pseudomonas sp. | 1 | |
| Fungi | Beauveria bassiana | 1 |
| Isaria farinosa | 1 | |
| Fungi/Microsporidia | Nosema carpocapsae | 6 |
| Nosema carpocapsae + Serratia sp. | 1 |
3.4. Occurrence and Prevalence of the Most Frequent Pathogens
3.4.1. Cydia pomonella Granulovirus (CpGV)
3.4.2. Beauveria bassiana
| Origin | Year | Results | Infestation in % and total number of individuals examined (n) |
|---|---|---|---|
| Austria | |||
| Kronberg | 1971 | B.ba. | 36.9 (111) |
| B.ba. + Aspergillus sp. | 1.8 | ||
| B.ba. + Aspergillus sp. + Hirsutella sp. | 0.9 | ||
| B.ba. + bacteria, unidentified | 0.9 | ||
| B.ba. + Cladosporium sp. | 1.8 | ||
| B.ba. + I. farinosa + Penicillium sp. | 1.8 | ||
| B.ba. + Mucor sp. | 1.8 | ||
| B.ba. + Mucor sp. + Penicillium sp. | 0.9 | ||
| B.ba. + Penicillium sp. + Aspergillus sp. | 1.8 | ||
| 1972 | B.ba. | 59.9 (2,355) | |
| Germany | |||
| Dossenheim | 1983 | B.ba. | 0.9 (1,954) |
| B.ba. + Paecilomyces sp. | 0.3 | ||
| Frankfurt | 1955 | B.ba. | 88.2 (17) |
| 1973 | B.ba. | 87.5 (24) | |
| B.ba. + I. farinosa | 4.2 | ||
| B.ba. + Penicillium sp. | 4.2 | ||
| 1974 | B.ba. | 87.8 (74) | |
| B.ba. + Syspastospora parasitica | 5.4 | ||
| 1975 | B.ba. | - | |
| B.ba. + Cephalosporium sp. | |||
| B.ba. + I. farinosa | |||
| Heidelberg | 1957 | B.ba. | 42.8 (14) |
| Mainz | 1967 | B.ba. | - |
| Neustadt/Meckenheim | 1955/1 | B.ba. | ca. 20 (ca. 25) |
| 1955/2 | B.ba. | ca . 75 (ca. 52) | |
| Mucor sp. on B.ba. | ca. 25 | ||
| Neustadt/Weisenheim | 1955 | B.ba. | 53.3 (75) |
| Offenbach | 1973 | B.ba. | 100 (23) |
| Stuttgart | 1957 | B.ba. | 29.4 (17) |
| 1973 | B.ba. | 8.2 (49) | |
| 1975 | B.ba. | 30.0 (40) | |
| B.ba. + I. farinosa | 2.5 | ||
| 2002 | B.ba. | 19.2 (52) | |
| B.ba. + Hirsutella sp. | 1.9 | ||
| 2004 | B.ba. | 9.2 (488) | |
| 2005 | B.ba. | 2.4 (454) | |
| Wiesbaden | 1958 | B.ba. | 100 (5) |
3.4.3. Nosema carpocapsae and Its Effects on Fecundity and Fertility of C. pomonella
| Origin | Year—Infestation in % and number of individuals (n) | ||||||
|---|---|---|---|---|---|---|---|
| Hessen (Rhein-Main area) | 1972 | 1973 | 1974 | 1975 | 1976 | 1977 | 1978 |
| Billings | 0 (20) | ||||||
| Darmstadt | 57.1 (7) | ||||||
| Frankfurt-Liederbach | 62.0 (50) | 41.5 (94) | |||||
| Kriftel | 57.5 (80) | 37.0 * (309) | 30.8 * (100) | 17.9 * (140) | 36.8 * (36) | ||
| Niederhofheim | 35.3 (122) | 43.4 * (237) | 40.0 (30) | 22.9 (70) | |||
| Bayern | |||||||
| Pittersberg | 29.8 (94) | ||||||
| Baden-Württemberg | |||||||
| Dossenheim | 26.3 * (103) | 18.1 * (791) | 17.3 (110) | ||||
| Heidelberg-Kirchheim | 74.0 (49) | 50.3 * (79) | 33.3 (30) | ||||
| Karlsruhe-Augustenberg | 3.0 (30) | 20.0 (35) | |||||
| Weinheim | 23.3 (30) | ||||||
| Origin | Year–Infestation in % and number of individuals (n) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hessen (Rhein-Main area) | 1972 | 1973 | 1974 | 1975 | 1976 | 1977 | 1978 | 1979 | 1980 | 1981 | 1982 | 1983 | 1984 | 1985 | 1986 | 1987 | 1989 | 1990 |
| Darmstadt (light trap) | 23.9 (117) | 27.9 (147) | 26.0 (100) | |||||||||||||||
| Frankfurt (light trap) | 48.2 (170) | 55.5 (321) | 44.0 (275) | 59.7 (139) | 34.4 (206) | 44.4 (187) | 38.5 (377) | 46.6 (253) | 33.0 (264) | 42.4 (184) | 46.8 (171) | 37.2 (204) | 34.4 (90) | 43.5 * (361) | 33.6 (235) | |||
| Geisenheim (light trap) | 49.8 * (253) | 59.9 * (372) | 55.4 (186) | 45.5 (110) | 51.7 (29) | |||||||||||||
| Kriftel (hibernation cages) | 50.0 (12) | 50.0 (28) | 45.0 (20) | 51.5 (33) | 0 (1) | |||||||||||||
| Kriftel (light trap) | 30.4 * (303) | 31.7 (202) | 45.4 (77) | 38.1 (189) | 22.4 (112) | 29.5 (61) | 31.7 (63) | 29.0 (48) | 22.3 (94) | 16.8 (125) | 18.7 (112) | 22.9 (135) | 57.3 (75) | 25.8 (124) | 30.8 (39) | |||
| Kriftel (pheromone trap) | 30.7 (319) | 35.5 (284) | 18.1 (343) | 25.3 (162) | 25.5 (153) | 23.7 (156) | ||||||||||||
| Langensel-bold (pheromone trap) | 38.0 (50) | |||||||||||||||||
| Nordenstadt (light trap) | 0 (13) | |||||||||||||||||
| Bayern | ||||||||||||||||||
| Deutenhofen (light trap) | 20.5 * (259) | 24.0 (50) | 23.5 (51) | |||||||||||||||
| Erlabrunn (light trap) | 6.3 (16) | 20.0 (15) | 11.9 (76) | 11.7 (170) | ||||||||||||||
| Neuhaus (light trap) | 0 (18) | 0 (11) | 0 (15) | 33.0 (3) | ||||||||||||||
| Igensdorf (light trap) | 0 (3) | |||||||||||||||||
| Eichelsdorf (light trap) | 0 (35) | 10.0 (10) | ||||||||||||||||
| Mallersdorf (light trap) | 27.3 (11) | 33.3 (3) | ||||||||||||||||
| Uffenheim (light trap) | 33.3 (3) | 14.3 (21) | 19.0 (21) | |||||||||||||||
| Weihen-stephan (light trap) | 100 (5) | |||||||||||||||||
| Baden-Württemberg | ||||||||||||||||||
| Dossenheim (light trap) | 28.4 * (123) | 24.1 * (112) | ||||||||||||||||
| Dossenheim (hibernation cages) | 20.8 * (120) | |||||||||||||||||
| Heidelberg (pheromone trap) | 50.6 (16) | |||||||||||||||||
| Ludwigsburg (pheromone trap) | 0 (27) | |||||||||||||||||
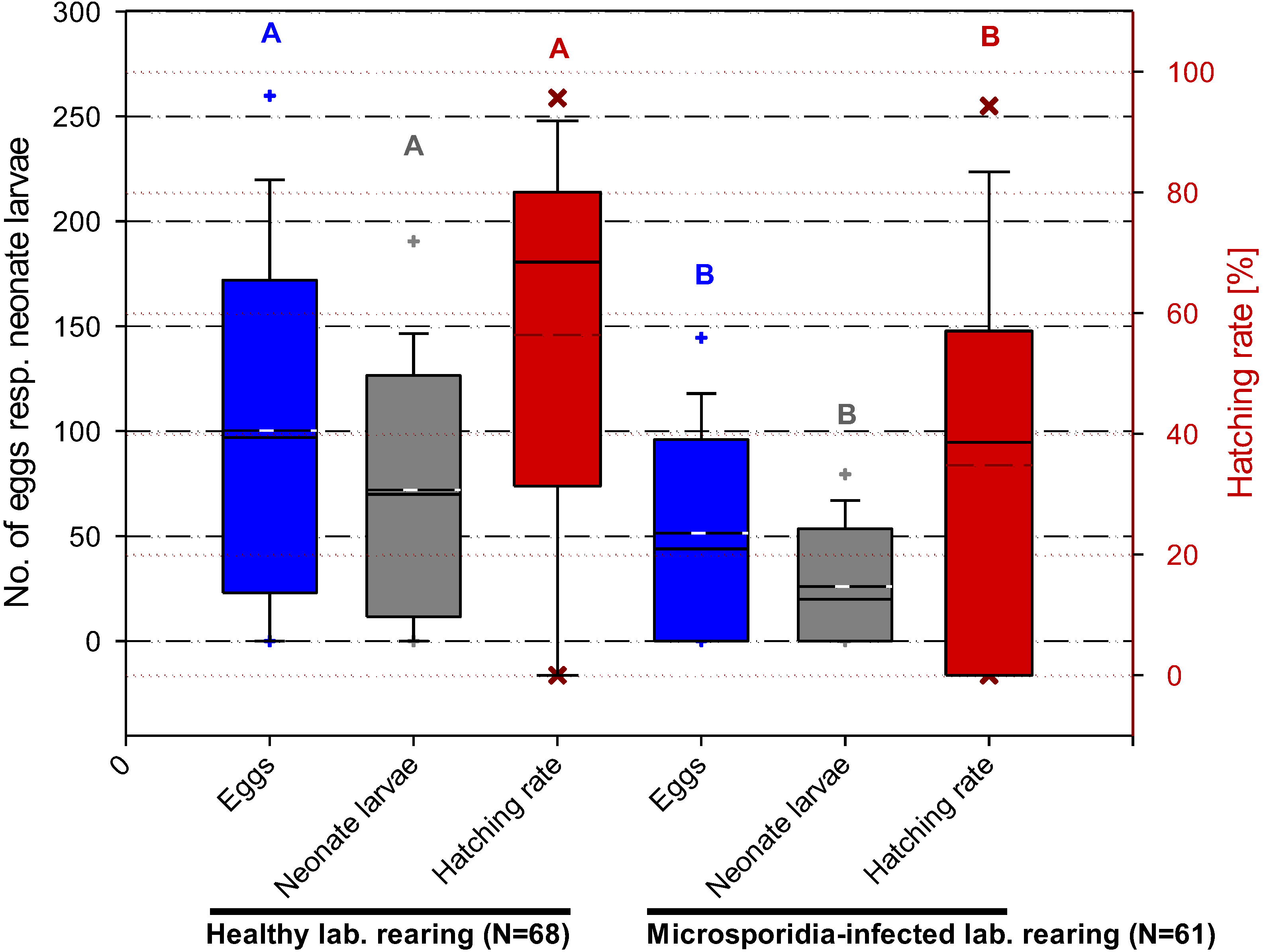
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Alford, D.V. Farbatlas der Obstschädlinge: Erkennung, Lebensweise und Bekämpfung; Publisher F. Enke: Stuttgart, Germany, 1987; pp. 170–171. [Google Scholar]
- Galli, P.; Epp, P. Pheromonfallen zur Flugüberwachung des Apfelwicklers. Obstbau 2006, 31, 280–282. [Google Scholar]
- Kienzle, J.; Gernoth, H.; Litterst, M.; Huber, J.; Zebitz, C.P.W.; Jehle, J.A. Biologie und Wirkungsweise des Apfelwickler-Granulovirus. Obstbau 2006, 31, 284–286. [Google Scholar]
- Litterst, M.; Gernoth, H.; Fried, A. Pflanzenschutzmittel zur Bekämpfung des Apfelwicklers. Obstbau 2006, 31, 282–283. [Google Scholar]
- Trautmann, M.; Scheer, C. Einsatz der Verwirrungsmethode zur biotechnischen Bekämpfung des Apfelwicklers am Bodensee (1996–2005). Obstbau 2006, 31, 287–290. [Google Scholar]
- Witzgall, P.; Stelinski, L.; Gut, L.; Thomson, D. Codling moth management and chemical ecology. Ann. Rev. Entomol. 2008, 53, 503–522. [Google Scholar] [CrossRef]
- Cross, J.V.; Solomon, M.G.; Chandler, D.; Jarrett, P.; Richardson, P.N.; Winstanley, D.; Bathon, H.; Huber, J.; Keller, B.; Langenbruch, G.-A.; et al. Biocontrol of pests of apples and pears in Northern and Central Europe: 1. Microbial agents and nematodes. Biocontrol Sci. Technol. 1999, 9, 125–149. [Google Scholar] [CrossRef]
- Siegel, J.P.; Lacey, L.A.; Vossbrinck, C.R. Impact of a North American isolate of the microsporidium Nosema carpocapsae on a laboratory population of the codling moth, Cydia pomonella. J. Invertebr. Pathol. 2001, 78, 244–250. [Google Scholar] [CrossRef]
- Charmillot, P.J.; Pasquier, D. Progression de la résistance du carpocapse Cydia pomonella aux insecticides. Rev. Suisse Vitic. Arboric. Hortic. 2002, 34, 95–100. [Google Scholar]
- Charmillot, P.J.; Pasquier, D. Combination of mating disruption (MD) technique and granulosis virus to control resistant strains of codling moth Cydia pomonella. Bull. OILB/SROP 2013, 26, 27–29. [Google Scholar]
- Schmitt, A.; Bisutti, I.L.; Ladurner, E.; Benuzzi, M.; Sauphanor, B.; Kienzle, J.; Zingg, D.; Undorf-Spahn, K.; Fritsch, E.; Huber, J.; et al. The occurrence and distribution of resistance of codling moth to Cydia pomonella granulovirus in Europe. J. Appl. Entomol. 2013. [Google Scholar] [CrossRef]
- Lacey, L.A.; Unruh, T.R. Biological control of codling moth (Cydia pomonella, Lepidoptera: Tortricidae) and its role in integrated pest management, with emphasis on entomopathogen. Vedalia 2005, 12, 33–60. [Google Scholar]
- Charmillot, P.J.; Pasquier, D.; Salamin, C.; Briand, F.; Ter-Hovannesyan, A.; Azizian, A.; Kutinkova, H.; Peeva, P.; Velcheva, N. Détection de la résistance du carpocapse Cydia pomonella. Tests d’insecticides sur les chénilles diapausantes de Suisse, d’Armenie et de Bulgarie. Rev. Suisse Vitic. Arboric. Hortic. 2007, 39, 385–389. [Google Scholar]
- Asser-Kaiser, S.; Fritsch, E.; Undorf-Spahn, K.; Kienzle, J.; Eberle, K.E.; Gund, N.A.; Reineke, A.; Zebitz, C.P.W.; Heckel, D.G.; Huber, J.; et al. Rapid emergence of baculovirus resistance in codling moth due to dominant sex-linked inheritance. Science 2007, 317, 1916–1918. [Google Scholar] [CrossRef]
- Lacey, L.A.; Shapiro-Ilan, D.I. Microbial control of insect pests in temperate orchard systems: Potential for incorporation into IPM. Ann. Rev. Entomol. 2008, 53, 121–144. [Google Scholar] [CrossRef]
- Undorf-Spahn, K.; Fritsch, E.; Huber, J.; Kienzle, J.; Zebitz, C.P.; Jehle, J.A. High stability and no fitness costs of the resistance of codling moth to Cydia pomonella granulovirus (CpGV-M). J. Invertebr. Pathol. 2012, 111, 136–142. [Google Scholar] [CrossRef]
- Falcon, L.A.; Huber, J. Biological control of the codling moth. In Tortricid Pests, Their Biology, Natural Enemies and Control; van der Geest, L.P.S., Evenhuis, H.H., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; pp. 355–369. [Google Scholar]
- Zimmermann, G.; Weiser, J. Pathogens and diseases. In Tortricid Pests, Their Biology, Natural Enemies and Control; van der Geest, L.P.S., Evenhuis, H.H., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; Chapter 3.2; pp. 253–271. [Google Scholar]
- Lacey, L.A.; Shapiro-Ilan, D.I. The potential role for microbial control of orchard insect pests in sustainable agriculture. J. Food Agric. Environ. 2003, 1, 326–331. [Google Scholar]
- Konecka, E.; Kaznowski, A.; Ziemnicka, J.; Kiemnicki, K. Molecular and phenotypic characterisation of Bacillus thuringiensis isolated during epizootics in Cydia pomonella L. J. Invertebr. Pathol. 2007, 94, 56–63. [Google Scholar] [CrossRef]
- Müller-Kögler, E. Pilzkrankheiten bei Insekten. Anwendung zur Biologischen Schädlingsbekämpfung und Grundlagen der Insektenmykologie; Publisher P. Parey: Berlin, Germany, 1965. [Google Scholar]
- Burges, H.G.; Hussey, N.W. (Eds.) Microbial Control of Insects and Mites; Academic Press: London, UK, 1971.
- Ferron, P. Pest Control by the Fungi Beauveria and Metarhizium. In Microbial Control of Pests and Plant Diseases 1970–1980; Burges, H.D., Ed.; Academic Press: London, UK, 1971; pp. 465–482. [Google Scholar]
- Ferron, P.; Vincent, J.J. Preliminary experiments on the use of Beauveria bassiana against Carpocapsa pomonella. Mitt. Biologischen Bundesanstalt Land Forstwirtschaft Berlin Dahlem 1978, 180, 84–87. [Google Scholar]
- Huger, A.M. Influence of a Microsporidian Disease on Fecundity and Fertility on the Codling Moth; Annual Report of the Federal Biological Research Centre for Agriculture and Forestry: Braunschweig, Germany, 1978; pp. H90–H91. [Google Scholar]
- Audemard, H.; Ferron, P. Codling moth control with Beauveria bassiana in orchards. IOBC/WPRS Bull. 1980, 3, 55–56. [Google Scholar]
- Peters, A.; Katz, P.; Elias, E. Entomopathogenic nematodes for biological control of codling moth. In Proceedings of the 13th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing, Weinsberg, Germany, 18–20 February 2008; pp. 284–286.
- Kienzle, J.; Heinisch, D.; Kiefer, J.; Trautmann, M.; Volk, F.; Zimmer, J.; Zebitz, C.P.W. Three years experience with entomopathogenic nematodes for the control of overwintering codling moth larvae in different regions of Germany. In Proceedings of the 14th International Conference on Organic Fruit-Growing, Hohenheim, Germany, 22–24 February 2010; pp. 163–168.
- Lacey, L.A.; Shapiro-Ilan, D.I.; Glenn, G.M. Post-application of anti-desiccant agents improves efficacy of entomopathogenic nematodes in formulated host cadavers or aqueous suspension against diapausing codling moth larvae (Lepidoptera: Tortricidae). Biocontrol Sci. Technol. 2010, 20, 909–921. [Google Scholar] [CrossRef]
- Michelbacher, A.E.; Middlekauff, W.W.; Hansen, C. Occurrence of a fungus disease in overwintering stages of the codling moth. J. Econ. Entomol. 1950, 43, 955–956. [Google Scholar]
- Russ, K. Über ein bemerkenswertes Auftreten von Beauveria bassiana (Bals.) Vuill. an Carpocapsa pomonella (L.). Pflanzenschutzberichte 1964, 31, 105–108. [Google Scholar]
- Müller-Kögler, E. Insektenpathogene Pilze von Apfelwicklerraupen; Annual Report of the Federal Biological Research Centre for Agriculture and Forestry: Braunschweig, Germany, 1971; p. 79. [Google Scholar]
- Huger, A.M. Diagnostische Untersuchungen über das Auftreten von Krankheiten in Freilandpopulationen Wichtiger Schadinsekten; Annual Report of the Federal Biological Research Centre for Agriculture and Forestry: Braunschweig, Germany, 1976; pp. H79–H80. [Google Scholar]
- Subinprasert, S. Natural enemies and their impact on overwintering codling moth populations (Laspeyresia pomonella L.) (Lep., Tortricidae) in South Sweden. J. Appl. Entomol. 1987, 103, 46–55. [Google Scholar] [CrossRef]
- Glen, D.M.; Milsom, N.F. Survival of mature larvae of codling moth (Cydia pomonella) on apple trees and ground. Ann. Appl. Biol. 1978, 90, 133–146. [Google Scholar] [CrossRef]
- Zelger, R.; Harzer, U.; Epp, P.; Trautmann, M. Untersuchungen zur Überwinterung des Apfelwicklers. Obstbau 2006, 31, 262–264. [Google Scholar]
- Kleespies, R.G.; Huger, A.M.; Zimmermann, G. Diseases of insects and other arthropods: Results of diagnostic research over 55 years. Biocontrol. Sci. Technol. 2008, 18, 439–484. [Google Scholar] [CrossRef]
- Kleespies, R.G.; Huger, A.M.; Zimmermann, G. Database on Arthropod Diseases. Available online: http://arthropodenkrankheiten.jki.bund.de/ (accessed on 18 March 2009).
- Huger, A.M. Histologie und Diagnose als praxisbezogene Grundlagenforschung im biologischen Pflanzenschutz. Gesunde Pflanzen 1970, 22, 36–40. [Google Scholar]
- Huger, A.M. Methoden und Bedeutung der Diagnosen von Insektenkrankheiten. Z. Pflanzenkr. Pflanzenschutz 1974, 81, 372–388. [Google Scholar]
- Poinar, G.O., Jr.; Thomas, G.M. Diagnostic Manual for the Identification of Insect Pathogens; Plenum Press: New York, NY, USA, London, UK, 1978. [Google Scholar]
- Burges, H.D. (Ed.) Microbial Control of Pests and Plant Diseases 1970–1980; Academic Press: London, UK, 1981.
- Plattner, H.; Zingsheim, H.-P. Elektronenmikroskopische Methodik in der Zell- und Molekularbiologie; Publisher G. Fischer: Stuttgart, Germany, 1987. [Google Scholar]
- Romeis, B. Mikroskopische Technik; Publisher Urban & Schwarzenberg: München, Germany, 1989. [Google Scholar]
- Tanada, Y.; Kaya, H.K. Insect Pathology; Academic Press, Inc.: San Diego, CA, USA, 1993. [Google Scholar]
- Lacey, L.A. (Ed.) Manual of Techniques in Insect Pathology; Academic Press: San Diego, CA, USA, 1997.
- Lacey, L.A. (Ed.) Field Manual of Techniques in Invertebrate Pathology; Publisher Springer: Berlin, Heidelberg, Germany, 2000.
- Kleespies, R.G.; Vossbrinck, C.R.; Lange, M.; Jehle, J.A. Morphological and molecular investigations of a microsporidium infecting the European grape vine moth, Lobesia botrana Den. et Schiff., and its taxonomic determination as Cystosporogenes legeri nov. comb. J. Invertebr. Pathol. 2003, 83, 240–248. [Google Scholar] [CrossRef]
- Lacey, L.A.; Kaya, H.K. (Eds.) Field Manual of Techniques in Insect Pathology, 2nd ed. ed.; Publisher Springer: Berlin, Heidelberg, Germany, 2007.
- Kleespies, R.G.; Marshall, S.D.G.; Schuster, C.; Townsend, R.J.; Jackson, T.; Leclerque, A. Genetic and electron-microscopic characterization of Rickettsiella bacteria from the manuka beetle, Pyronota setosa (Coleoptera: Scarabaeidae). J. Invertebr. Pathol. 2011, 107, 206–211. [Google Scholar] [CrossRef]
- Barnett, H.L. Illustrated Genera of Imperfect Fungi, 2nd ed. ed.; Burgess Publishing Company: Minneapolis, MN, USA, 1960. [Google Scholar]
- Barron, G.L. The Genera of Hyphomycetes from Soil; The Williams & Wilkins Company: Baltimore, MD, USA, 1968. [Google Scholar]
- Humber, R.A. Fungi: Identification. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 153–185. [Google Scholar]
- Humber, R.A. Identification of entomopathogenic fungi. In Manual of Techniques in Invertebrate Pathology, 2nd ed. ed.; Lacey, L.A., Ed.; Academic Press: London, UK, 2012; pp. 151–187. [Google Scholar]
- Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Manoch, L.; Samson, R.A. On the relationships of Paecilomyces sect. Isarioidea species. Mycol. Res. 2005, 109, 581–589. [Google Scholar] [CrossRef]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef]
- Huger, A.M. Erhebungen über infektiöse Erkrankungen in Freilandpopulationen des Apfelwicklers; Annual Report of the Federal Biological Research Centre for Agriculture and Forestry: Braunschweig, Germany, 1973; p. 90. [Google Scholar]
- Huger, A.M.; Federal Biological Research Centre for Agriculture and Forestry, Darmstadt, Germany. Unpublished work; 1972–1990.
- Weiss, L.M.; Edlind, T.D.; Vossbrinck, C.R.; Hashimoto, T. Microsporidian molecular phylogeny: The fungal connection. J. Eukaryot. Microbiol. 1999, 46, 17S–18S. [Google Scholar] [CrossRef]
- Corradi, N.; Slamovits, C.H. The intriguing nature of microsporidian genomes. Brief. Funct. Genomics 2011, 10, 115–124. [Google Scholar] [CrossRef]
- Posada, F.; Vega, F.E.; Rehner, S.A.; Blackwell, M.; Weber, D.; Suh, S.O.; Humber, R.A. Syspastospora parasitica, a mycoparasite of the fungus Beauveria bassiana attacking the Colorado potato beetle Leptinotarsa decemlineata: A tritrophic association. J. Insect Sci. 2004, 4, 24. [Google Scholar]
- Szalay-Marzso, L.; Vago, C. Transmission of baculovirus by mites. Study of granulosis virus of codling moth (Laspeyresia pomonella L.). Acta Phytopathol. Acad. Sci. Hung. 1975, 10, 113–122. [Google Scholar]
- Sheppard, R.F.; Stairs, G.R. Effects of dissemination of low dosage levels of a granulosis virus in populations of the codling moth. J. Econ. Entomol. 1976, 69, 583–586. [Google Scholar]
- Pultar, O.; Kocourek, F.; Berankova, J.; Stara, J.; Kuldova, J.; Hrdy, I. Codling moth management by means of pheromone stations with Cydia pomonella granulosis virus. In Proceedings of the International Conference on Integrated Fruit Production, Leuven, Belgium, 27 July–1 August 1998; pp. 477–480.
- Tanada, Y. A granulosis virus of the codling moth, Carpocapsa pomonella (Linnaeus) (Olethreutidae, Lepidoptera). J. Insect Pathol. 1964, 6, 378–380. [Google Scholar]
- Jaques, R.P.; MacLellan, C.R. Fungal mortality of overwintering larvae of the codling moth in apple orchards in Nova Scotia. J. Invertebr. Pathol. 1965, 7, 291–296. [Google Scholar] [CrossRef]
- Huger, A.M.; Federal Biological Research Centre for Agriculture and Forestry, Darmstadt, Germany. Unpublished work; 1973–1974.
- Andreadis, T.G. Epizootiology of Nosema pyrausta in field populations of the European corn borer (Lepidoptera, Pyralidae). Environ. Entomol. 1984, 13, 882–887. [Google Scholar]
- Lewis, L.C.; Sumerford, D.V.; Bing, L.A.; Gunnarson, R.D. Dynamics of Nosema pyrausta in natural populations of the European corn borer, Ostrinia nubilalis: A six-year study. BioControl 2006, 51, 627–642. [Google Scholar] [CrossRef]
- Bathon, H. Zur Chemotherapie einer Mikrosporidiose an Apfelwicklern; Annual Report of the Federal Biological Research Centre for Agriculture and Forestry: Braunschweig, Germany, 1974; pp. H88–H89. [Google Scholar]
- Huger, A.M. Effects of a persistent microsporidiosis on the reproduction rate of the European corn borer (in German); Annual Report of the Federal Biological Research Centre for Agriculture and Forestry: Berlin, Braunschweig, Germany, 1980; pp. H84–H85. [Google Scholar]
- Linde, A.; Richardt, K.; Bartsch, D.; Seidel, C. Evaluation of the potential of microsporidia for the regulation of gypsy moth populations (Lymantriadispar L.). Mitt. Dtsch. Ges. Allg. Ang. 2000, 12, 127–131. [Google Scholar]
- Goertz, D.; Pilarska, D.; Kereselidze, M.; Solter, L.F.; Linde, A. Studies on the impact of two Nosema isolates from Bulgaria on the gypsy moth (Lymantria dispar L.). J. Invertebr. Pathol. 2004, 87, 105–113. [Google Scholar]
- Goertz, D.; Solter, L.F.; Linde, A. Horizontal and vertical transmission of a Nosema sp. (Microsporidia) from Lymantria dispar (L.) (Lepidoptera: Lymantriidae). J. Invertebr. Pathol. 2007, 95, 9–16. [Google Scholar] [CrossRef]
- Goertz, D.; Golldack, J.; Linde, A. Two different and sublethal isolates of Nosema lymantriae (Microsporidia) reduce the reproductive success of their host, Lymantria dispar. Biocontrol Sci. Technol. 2008, 18, 419–430. [Google Scholar] [CrossRef]
- Goertz, D.; Hoch, G. Horizontal transmission pathways of terrestrial microsporidia: A quantitative comparison of three pathogens infecting different organs in Lymantria dispar L. (Lep.: Lymantriidae) larvae. Biol. Control 2008, 44, 196–206. [Google Scholar] [CrossRef]
- Huger, A.M. Effects of a Persistent Microsporidiosis on the Survival Rate of Overwintering Populations of the European Corn Borer (in German); Annual Report of the Federal Biological Research Centre for Agriculture and Forestry: Berlin, Braunschweig, Germany, 1980; pp. H83–H84. [Google Scholar]
- Huber, J. Wechselwirkung Zwischen der Granulose und einer Mikrosporidiose des Apfelwicklers; Annual Report of the Federal Biological Research Centre for Agriculture and Forestry: Berlin, Braunschweig, Germany, 1975; p. H82. [Google Scholar]
- Huger, A.M.; Neuffer, G. Das Mißlingen einer Apfelwickler-Parasitenzucht; Annual Report of the Federal Biological Research Centre for Agriculture and Forestry: Berlin, Braunschweig, Germany, 1977; pp. H87–H88. [Google Scholar]
- Huger, A.M.; Neuffer, G. Infection of the braconid parasite Ascogaster quadridentata (Hymenoptera: Braconidae) by a microsporidian of its host, Laspeyresia pomonella. (in German). Mitt. Biologischen Bundesanstalt Land Forstwirtschaft 1978, 180, 105–106. [Google Scholar]
- Huger, A.M. Investigations on the Effect of Microsporidium Nosema carpocapsae on Parasitizing Capacity of the Egg Parasitoid Trichogramma evanescens; (in German). Annual Report of the Federal Biological Research Centre for Agriculture and Forestry: Berlin, Braunschweig, Germany, 1983; p. H81. [Google Scholar]
- Reinecke, P.; Andersch, W.; Stenzel, K.; Hartwig, J. Bio-1020 A new microbial insecticide for use in horticultural crops. In Brighton Crop Protection Conference: Pests and Diseases; British Crop Protection Council: Farnham, England, UK, 1990; Volumn 1, pp. 49–54. [Google Scholar]
- Pernfuss, B.; Zelger, R.; Kron-Morelli, R.; Strasser, H. Control of the garden chafer Phyllopertha horticola with GranMet-P, a new product made of Metarhizium anisopliae. IOBC/WPRS Bull. 2001, 28, 48–56. [Google Scholar]
- Unruh, T.R.; Lacey, L.A. Control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae), with Steinernema carpocapsae: Effects of sublemental wetting in pupation site on infection rate. Biol. Control 2001, 20, 48–56. [Google Scholar] [CrossRef]
- Curto, G.; Reggiani, A.; Vergnani, S.; Caruso, S.; Ladurner, E. Effectiveness of entomopathogenic nematodes in the control of Cydia pomonella larvae in Northern Italy. In Proceedings of the 13th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing, Weinsberg, Germany, 18–20 February 2008; pp. 271–276.
- Huger, A.M. Versuche zur Künstlichen Infektion von Gesunden Freilandpopulationen Wichtiger Schadinsekten, vor Allem mit Mikrosporidien; Annual Report of the Federal Biological Research Centre for Agriculture and Forestry: Berlin, Braunschweig, Germany, 1979; p. 84. [Google Scholar]
- Kurtti, T.J.; Munderloh, U.G. Issues in the use of microsporidia for biological control of European corn borer. In Ecological Interactions and Biological Control; Andow, D.A., Ragsdale, D.W., Nyvall, R.F., Eds.; Westview Press: Boulder, CO, USA, 1997; pp. 195–214. [Google Scholar]
- Lewis, L.C.; Bruck, D.J.; Prasifka, J.R.; Raun, E.S. Nosema pyrausta: Its biology, history, and potential role in a landscape of transgenic insecticidal crops. Biol. Control 2009, 48, 223–231. [Google Scholar] [CrossRef]
- Jeffords, M.R.; Maddox, J.V.; McManus, M.L.; Webb, R.E.; Wieber, A. Egg contamination as a method for the inoculative release of exotic microsporidia of the gypsy moth. J. Invertebr. Pathol. 1988, 51, 190–196. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zimmermann, G.; Huger, A.M.; Kleespies, R.G. Occurrence and Prevalence of Insect Pathogens in Populations of the Codling Moth, Cydia pomonella L.: A Long-Term Diagnostic Survey. Insects 2013, 4, 425-446. https://doi.org/10.3390/insects4030425
Zimmermann G, Huger AM, Kleespies RG. Occurrence and Prevalence of Insect Pathogens in Populations of the Codling Moth, Cydia pomonella L.: A Long-Term Diagnostic Survey. Insects. 2013; 4(3):425-446. https://doi.org/10.3390/insects4030425
Chicago/Turabian StyleZimmermann, Gisbert, Alois M. Huger, and Regina G. Kleespies. 2013. "Occurrence and Prevalence of Insect Pathogens in Populations of the Codling Moth, Cydia pomonella L.: A Long-Term Diagnostic Survey" Insects 4, no. 3: 425-446. https://doi.org/10.3390/insects4030425
APA StyleZimmermann, G., Huger, A. M., & Kleespies, R. G. (2013). Occurrence and Prevalence of Insect Pathogens in Populations of the Codling Moth, Cydia pomonella L.: A Long-Term Diagnostic Survey. Insects, 4(3), 425-446. https://doi.org/10.3390/insects4030425