Evolution of Insect Pollination Before Angiosperms and Lessons for Modern Ecosystems
Simple Summary
Abstract
1. Introduction
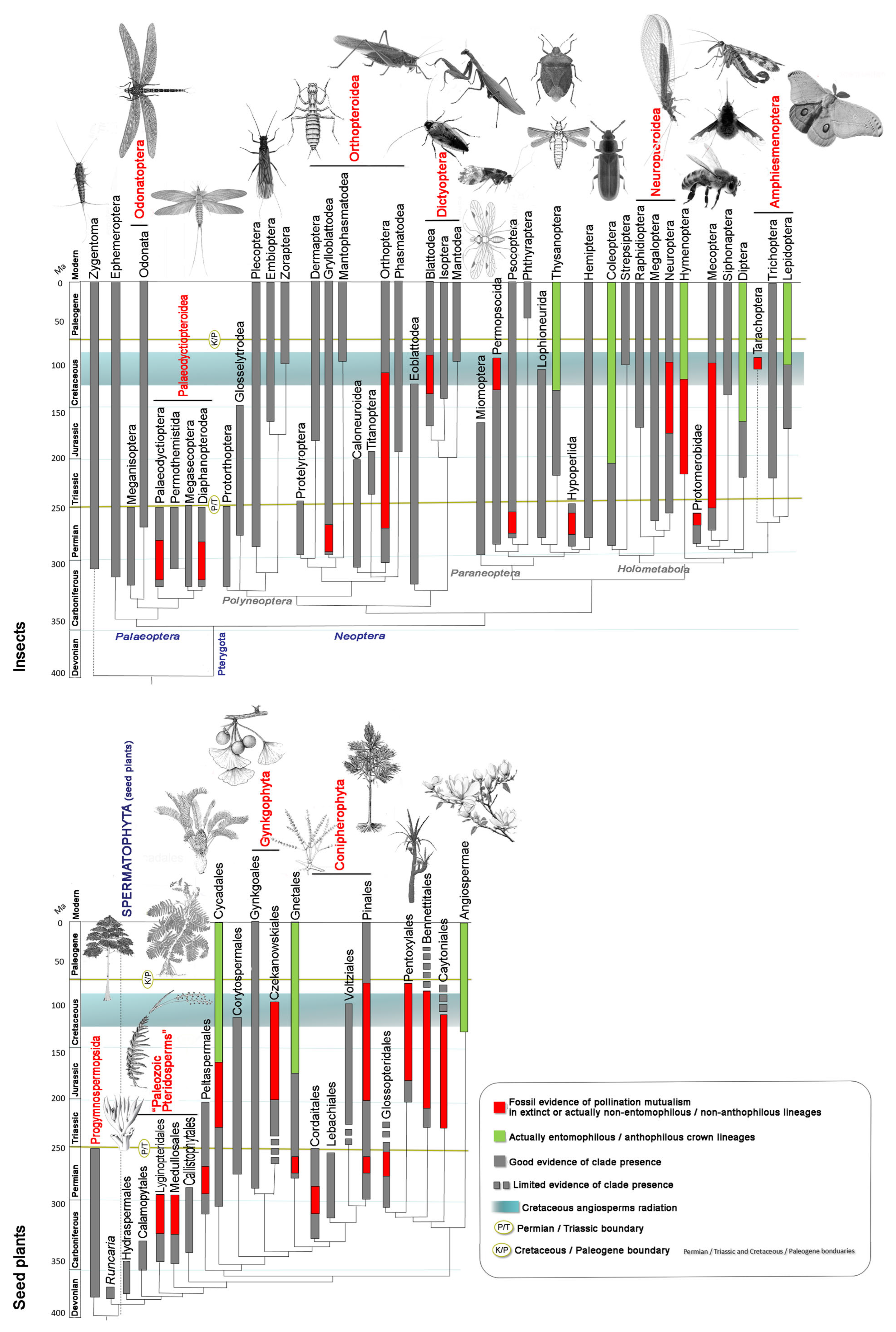
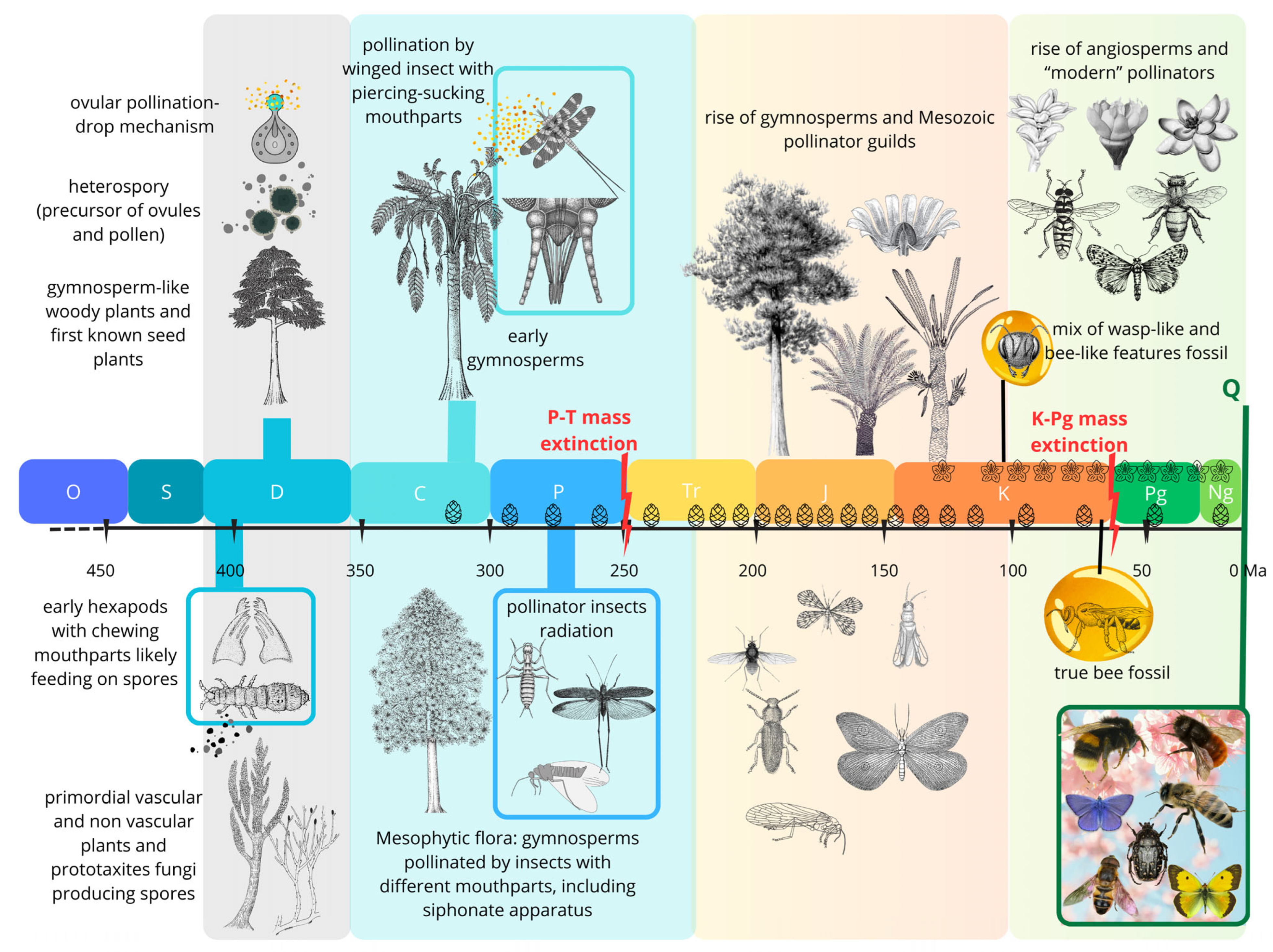
2. Fossil Evidence of Host-Plant and Insect Interactions and the Evolutionary Phases of Insect Pollination
- A Late Silurian to Late Devonian phase (about 60 million years) characterized by herbivorous arthropods, including apterygote hexapods (Entognathate and perhaps early Ectognathate), feeding on several clades of primitive vascular-plant hosts.
- A Late Mississippian to end-Permian phase (85 million years) involving principally apterygotes, paleopterans, non-holometabolan and (later) basal holometabolan neopterans, feeding on pteridophytes, basal and more advanced gymnosperm plant hosts.
- A Middle Triassic to Middle Cretaceous phase (ca. 130 million years) dominated by polyneopterans, paraneopterans and holometabolous, feeding mostly on gymnosperm plant hosts.
- A mid-Early Cretaceous to Recent phase (115 million years) featuring modern hemimetabolous and holometabolous, feeding principally on angiosperm plant hosts. This phase also witnessed the emergence of bees, establishing one of the most important modern pollinator lineages.
2.1. Early Mandibles on Early Spores: Silurian-Devonian First Evidence of Palynivory
2.2. Seed Plants and the Second Phase of Plants/Insect Associations: Late Paleozoic Pollination
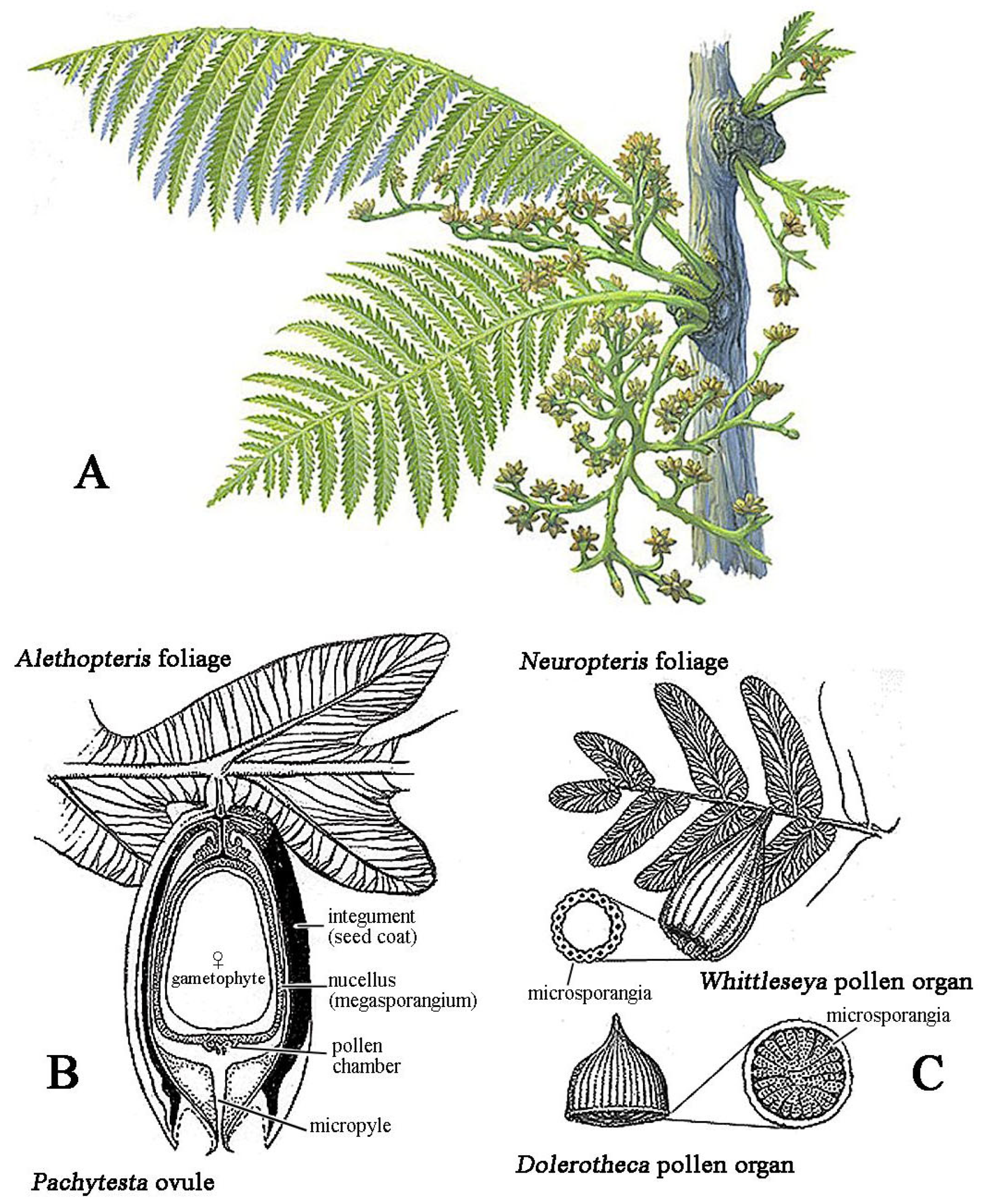
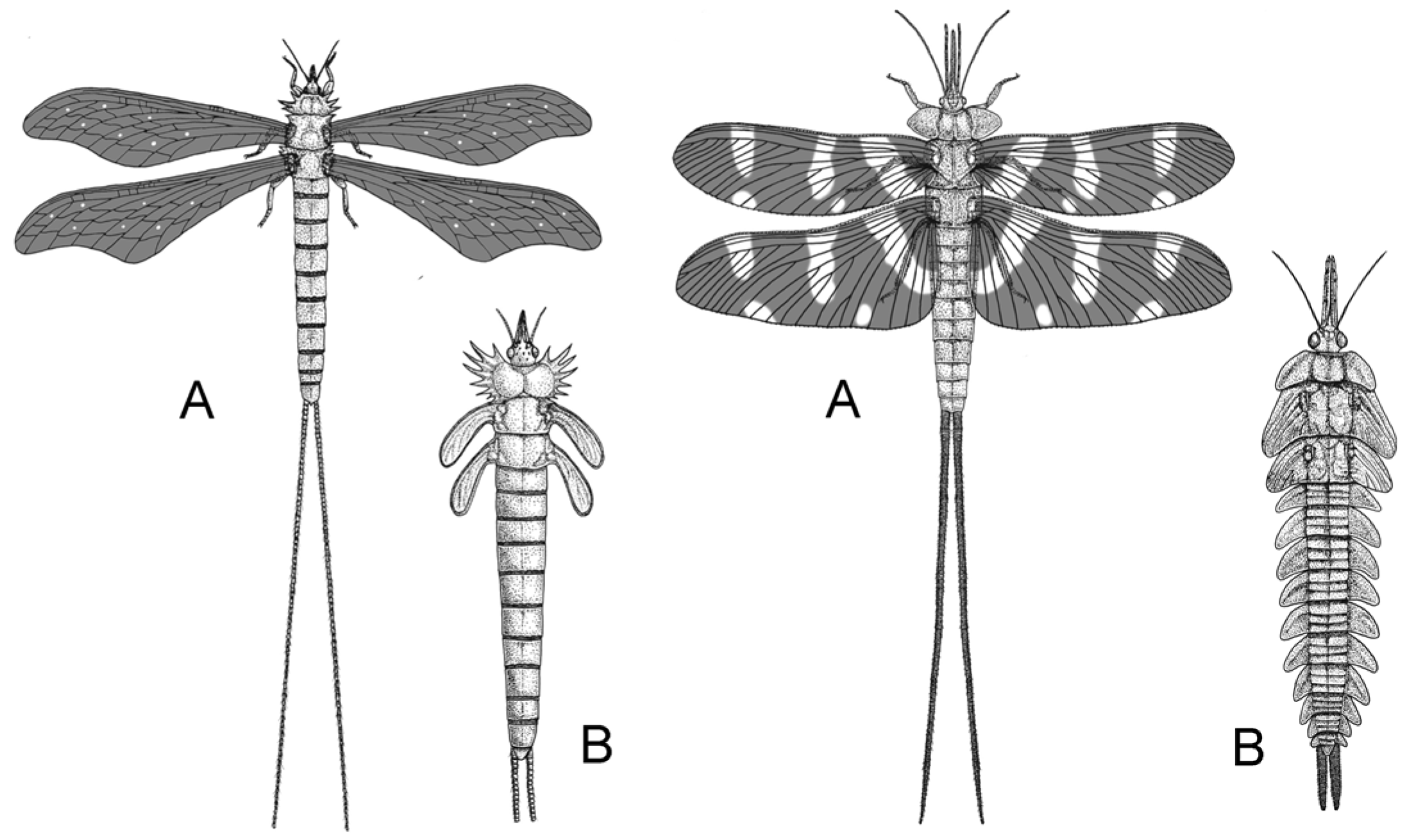
2.3. The Mesozoic Third Phase: Advanced Gymnosperms and Pollinator Guilds
2.3.1. The Permian-Triassic Crisis and Its Aftermath
2.3.2. Floristic Recovery and the Rise of Mesozoic Gymnosperms
- (a)
- The pollen and tissue reward systems primarily included plants with compact cones that produced abundant pollen and offered fleshy tissues as a reward. These structures were typically visited by small, mandibulate (e.g., beetles) or with piercing-sucking mouthparts (e.g., thrips), insects that lived in close association with the reproductive organs, often as larvae feeding within the cone tissues [6,9,75,76].
- (b)
2.3.3. Pre-Angiosperm Complex Mutualist Balances
2.4. The Cretaceous Terrestrial Revolution: Angiosperm Radiation and the Evolution of Bees
3. The Resilience of Plant–Pollinator Interactions: Lessons from Deep Time for the Anthropocene
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005; p. 755. [Google Scholar]
- Misof, B.; Liu, S.; Meusemann, K.; Peters, R.S.; Donath, A.; Mayer, C.; Frandsen, P.B.; Ware, J.; Flouri, T.; Beutel, R.G.; et al. Phylogenomics Resolves the Timing and Pattern of Insect Evolution. Science 2014, 346, 763–767. [Google Scholar] [CrossRef]
- Labandeira, C.C. The Fossil Record of Insect Mouthparts: Innovation, Functional Convergence, and Associations with Other Organisms. In Insect Mouthparts; Krenn, H., Ed.; Zoological Monographs; Springer: Cham, Switzerland, 2019; Volume 5, pp. 567–671. [Google Scholar] [CrossRef]
- Labandeira, C.C. The History of associations between Plants and Animals. In Plant-Animal Interactions: An Evolutionary Approach; Herrera, C.M., Pellmyr, O., Eds.; Blackwell: London, UK, 2002; pp. 26–74. [Google Scholar]
- Labandeira, C.C. Silurian to Triassic Plant and Hexapod Clades and Their Associations: New Data, a Review, and Interpretations. Arthropod Syst. Phylogeny 2006, 64, 53–94. [Google Scholar] [CrossRef]
- Labandeira, C.C. The Pollination of Mid Mesozoic Seed Plants and the Early History of Long-Proboscid Insects. Ann. Mo. Bot. Gard. 2010, 97, 469–513. [Google Scholar] [CrossRef]
- Labandeira, C.C. The Paleobiology of Pollination and Its Precursors. Paleontol. Soc. Pap. 2000, 6, 233–270. [Google Scholar] [CrossRef]
- Rothwell, G.W.; Grauvogel-Stamm, L.; Mapes, G. An Herbaceous Fossil Conifer: Gymnospermous Ruderals in the Evolution of Mesozoic Vegetation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 156, 139–145. [Google Scholar] [CrossRef]
- Labandeira, C.C.; Kvaček, J.; Mostovski, M.B. Pollination Drops, Pollen, and Insect Pollination of Mesozoic Gymnosperms. Taxon 2007, 56, 663–695. [Google Scholar] [CrossRef]
- Krassilov, V.A. Diversity of Mesozoic Gnetophytes and the First Angiosperms. Paleontol. J. 2009, 43, 1272–1280. [Google Scholar] [CrossRef]
- McLoughlin, S. History of life: Plants: Gymnosperms. In Encyclopedia of Geology, 2nd ed.; Alderton, D., Elias, S.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 3, pp. 476–500. [Google Scholar] [CrossRef]
- Labandeira, C.C.; Sepkoski, J.J. Insect Diversity in the Fossil Record. Science 1993, 261, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Friis, E.M.; Crane, P.R.; Pedersen, K.R. Early Flowers and Angiosperm Evolution, 1st ed.; Cambridge University Press: Cambridge, UK, 2011; p. 585. [Google Scholar] [CrossRef]
- Schachat, S.R.; Labandeira, C.C. Are Insects Heading Toward Their First Mass Extinction? Distinguishing Turnover From Crises in Their Fossil Record. Ann. Entomol. Soc. Am. 2020, 114, 99–118. [Google Scholar] [CrossRef]
- Peris, D.; Condamine, F.L. The Angiosperm Radiation Played a Dual Role in the Diversification of Insects and Insect Pollinators. Nat. Commun. 2024, 15, 552. [Google Scholar] [CrossRef]
- Cardinal, S.; Danforth, B.N. Bees Diversified in the Age of Eudicots. Proc. R. Soc. B 2013, 280, 20122686. [Google Scholar] [CrossRef]
- Almeida, E.A.B.; Bossert, S.; Danforth, B.N.; Porto, D.S.; Freitas, F.V.; Davis, C.C.; Murray, E.A.; Blaimer, B.B.; Spasojevic, T.; Ströher, P.R.; et al. The Evolutionary History of Bees in Time and Space. Curr. Biol. 2023, 33, 3409–3422.e6. [Google Scholar] [CrossRef] [PubMed]
- Labandeira, C.C. Why Did Terrestrial Insect Diversity Not Increase During the Angiosperm Radiation? Mid-Mesozoic, Plant-Associated Insect Lineages Harbor Clues. In Evolutionary Biology: Genome Evolution, Speciation, Coevolution and Origin of Life; Pontarotti, P., Ed.; Springer: Cham, Switzerland, 2014; pp. 261–299. [Google Scholar] [CrossRef]
- Labandeira, C.C. The Four Phases of Plant-Arthropod Associations in Deep Time. Geol. Acta 2006, 4, 409–438. [Google Scholar] [CrossRef]
- Ren, D.; Labandeira, C.C.; Santiago-Blay, J.A.; Rasnitsyn, A.; Shih, C.; Bashkuev, A.; Logan, M.A.V.; Hotton, C.L.; Dilcher, D. A Probable Pollination Mode Before Angiosperms: Eurasian, Long-Proboscid Scorpionflies. Science 2009, 326, 840–847. [Google Scholar] [CrossRef]
- Hu, S.; Dilcher, D.L.; Taylor, D.W. Pollen Evidence for the Pollination Biology of Early Flowering Plants. In Evolution of Plant-Pollinator Relationships; Patiny, S., Ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 165–236. [Google Scholar] [CrossRef]
- Peris, D.; Labandeira, C.C.; Peñalver, E.; Delclòs, X.; Barrón, E.; Pérez-de la Fuente, R. The Case of Darwinylus marcosi (Insecta: Coleoptera: Oedemeridae): A Cretaceous Shift from a Gymnosperm to an Angiosperm Pollinator Mutualism. Commun. Integr. Biol. 2017, 10, e1325048. [Google Scholar] [CrossRef][Green Version]
- Cai, C.; Escalona, H.E.; Li, L.; Yin, Z.; Huang, D.; Engel, M.S. Beetle Pollination of Cycads in the Mesozoic. Curr. Biol. 2018, 28, 2806–2812.e1. [Google Scholar] [CrossRef]
- Khramov, A.V.; Bashkuev, A.S.; Lukashevich, E.D. The Fossil Record of Long-Proboscid Nectarivorous Insects. Entomol. Rev. 2020, 100, 881–968. [Google Scholar] [CrossRef]
- Khramov, A.V.; Naugolnykh, S.V.; Węgierek, P. Possible Long-Proboscid Insect Pollinators from the Early Permian of Russia. Curr. Biol. 2022, 32, 3815–3820.e2. [Google Scholar] [CrossRef]
- Tihelka, E.; Li, L.; Fu, Y.; Su, Y.; Huang, D.; Cai, C. Angiosperm Pollinivory in a Cretaceous Beetle. Nat. Plants 2021, 7, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Peña-Kairath, C.; Delclòs, X.; Álvarez-Parra, S.; Peñalver, E.; Engel, M.S.; Ollerton, J.; Peris, D. Insect Pollination in Deep Time. Trends Ecol. Evol. 2023, 38, 749–759. [Google Scholar] [CrossRef]
- Engel, M.S. A Monograph of the Baltic Amber Bees and Evolution of the Apoidea (Hymenoptera). Bull. Am. Mus. Nat. Hist. 2001, 259, 1–192. [Google Scholar] [CrossRef]
- Vajda, V.; McLoughlin, S.; Slater, S.M.; Gustafsson, O.; Rasmusson, A.G. The ‘Seed-Fern’ Lepidopteris Mass-Produced the Abnormal Pollen Ricciisporites during the End-Triassic Biotic Crisis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 627, 111723. [Google Scholar] [CrossRef]
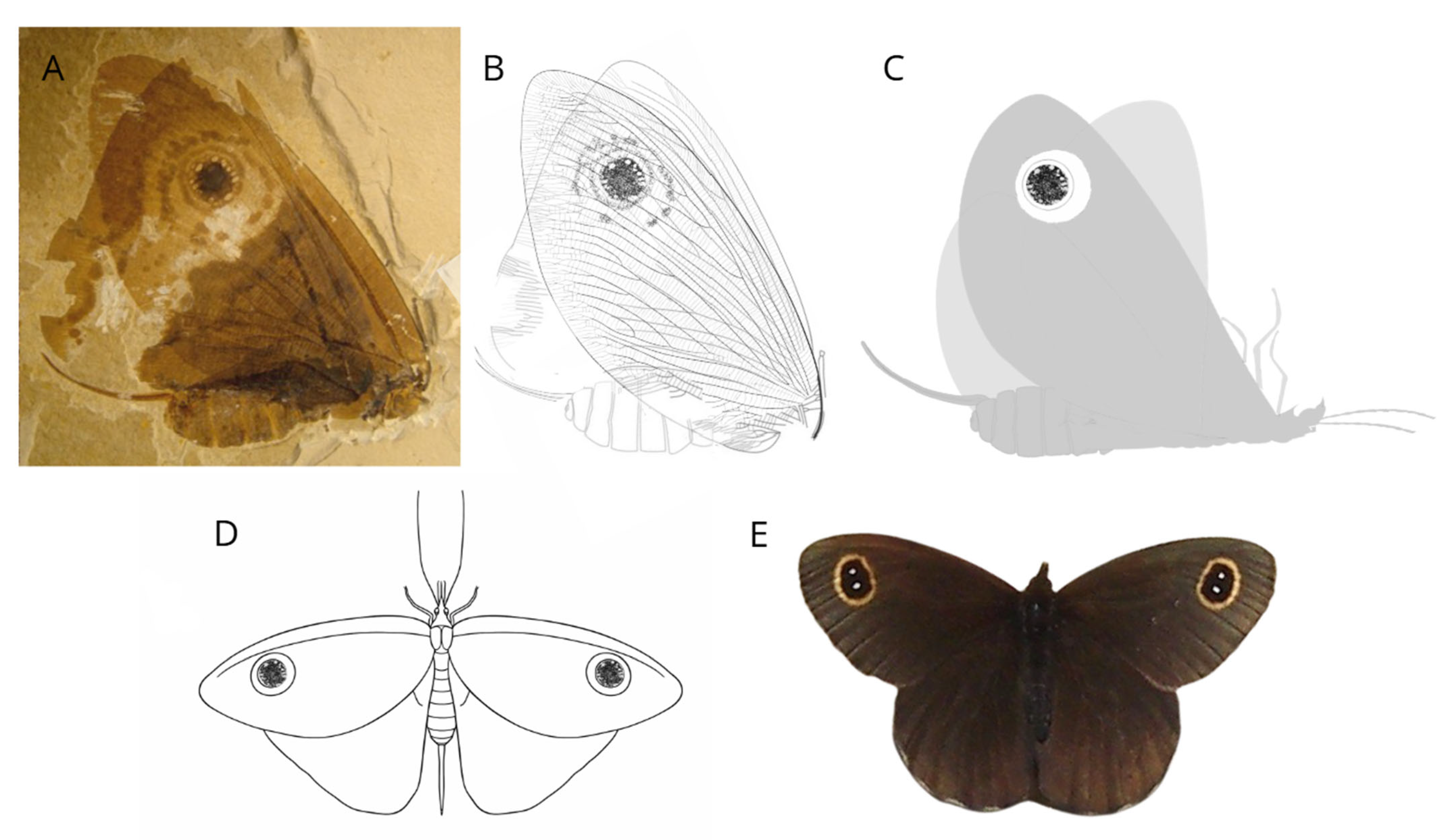
- Labandeira, C.C.; Yang, Q.; Santiago-Blay, J.A.; Hotton, C.L.; Monteiro, A.; Wang, Y.-J.; Goreva, Y.; Shih, C.; Siljeström, S.; Rose, T.R.; et al. The Evolutionary Convergence of Mid-Mesozoic Lacewings and Cenozoic Butterflies. Proc. R. Soc. B 2016, 283, 20152893. [Google Scholar] [CrossRef]
- Gorelick, R. Did Insect Pollination Cause Increased Seed Plant Diversity? Biol. J. Linn. Soc. 2001, 74, 407–427. [Google Scholar] [CrossRef]
- Toon, A.; Terry, L.I.; Tang, W.; Walter, G.H.; Cook, L.G. Insect Pollination of Cycads. Austral Ecol. 2020, 45, 1033–1058. [Google Scholar] [CrossRef]
- Hsiao, Y.; Oberprieler, R.G.; Zwick, A.; Zhou, Y.-L.; Ślipiński, A. Museomics Unveil Systematics, Diversity and Evolution of Australian Cycad-Pollinating Weevils. Proc. R. Soc. B 2023, 290, 20231385. [Google Scholar] [CrossRef]
- Pirozynski, K.A.; Malloch, D.W. The Origin of Land Plants: A Matter of Mycotrophism. BioSystems 1975, 6, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.N.; Osborn, J.M. The Importance of Fungi in Shaping the Paleoecosystem. Rev. Palaeobot. Palynol. 1996, 90, 249–262. [Google Scholar] [CrossRef]
- Dunlop, J.A.; Garwood, R.J. Terrestrial Invertebrates in the Rhynie Chert Ecosystem. Philos. Trans. R. Soc. B 2017, 373, 20160493. [Google Scholar] [CrossRef]
- Edwards, D.; Selden, P.A.; Richardson, J.B.; Axe, L. Coprolites as Evidence for Plant–Animal Interaction in Siluro–Devonian Terrestrial Ecosystems. Nature 1995, 377, 329–331. [Google Scholar] [CrossRef]
- Beck, C.B. On the Origin of Gymnosperms. Taxon 1966, 15, 337–339. [Google Scholar] [CrossRef]
- Bateman, R.M.; DiMichele, W.A. Heterospory: The Most Iterative Key Innovation in the Evolutionary History of the Plant Kingdom. Biol. Rev. 1994, 69, 345–417. [Google Scholar] [CrossRef]
- Bonacorsi, N.K.; Leslie, A.B. Sporangium Position, Branching Architecture, and the Evolution of Reproductive Morphology in Devonian Plants. Int. J. Plant Sci. 2019, 180, 493–503. [Google Scholar] [CrossRef]
- von Aderkas, P.; Prior, N.A.; Little, S.A. The Evolution of Sexual Fluids in Gymnosperms From Pollination Drops to Nectar. Front. Plant Sci. 2018, 9, 1844. [Google Scholar] [CrossRef]
- Rothwell, G.W. Evidence for a Pollination-Drop Mechanism in Paleozoic Pteridosperms. Science 1977, 198, 1251–1252. [Google Scholar] [CrossRef]
- DiMichele, W.A.; Pfefferkorn, H.W.; Gastaldo, R.A. Response of Late Carboniferous and Early Permian Plant Communities to Climate Change. Annu. Rev. Earth Planet. Sci. 2001, 29, 461–487. [Google Scholar] [CrossRef]
- Beutel, R.G.; Xu, C.; Jarzembowski, E.; Kundrata, R.; Boudinot, B.E.; McKenna, D.D.; Goczał, J. The Evolutionary History of Coleoptera (Insecta) in the Late Palaeozoic and the Mesozoic. Syst. Entomol. 2024, 49, 355–388. [Google Scholar] [CrossRef]
- Dos Santos, T.B.; De Souza Pinheiro, E.R.; Iannuzzi, R. First Evidence of Seed Predation by Arthropods from Gondwana and its Early Paleozoic History (Rio Bonito Formation, Paraná Basin, Brazil). Palaios 2020, 35, 292–301. [Google Scholar] [CrossRef]
- McLoughlin, S.; Prevec, R. The Reproductive Biology of Glossopterid Gymnosperms—A Review. Rev. Palaeobot. Palynol. 2021, 295, 104527. [Google Scholar] [CrossRef]
- Retallack, G.J.; Dilcher, D.L. Reconstructions of Selected Seed Ferns. Ann. Mo. Bot. Gard. 1988, 75, 1010. [Google Scholar] [CrossRef]
- Jarzembowski, E.A.; Ross, A.J. Insect Origination and Extinction in the Phanerozoic. Geol. Soc. Spec. Publ. 1996, 102, 65–78. [Google Scholar] [CrossRef]
- Prokop, J.; Krzemińska, E.; Krzemiński, W.; Rosová, K.; Pecharová, M.; Nel, A.; Engel, M.S. Ecomorphological diversification of the Late Palaeozoic Palaeodictyopterida reveals different larval strategies and amphibious lifestyle in adults. R Soc Open Sci. 2019, 6, 190460. [Google Scholar] [CrossRef]
- Shcherbakov, D.E. On Permian and Triassic Insect Faunas in Relation to Biogeography and the Permian-Triassic Crisis. Paleontol. J. 2008, 42, 15–31. [Google Scholar] [CrossRef]
- Aristov, D.S.; Bashkuev, A.S.; Golubev, V.K.; Gorochov, A.V.; Karasev, E.V.; Kopylov, D.S.; Ponomarenko, A.G.; Rasnitsyn, A.P.; Rasnitsyn, D.A.; Sinitshenkova, N.D.; et al. Fossil Insects of the Middle and Upper Permian of European Russia. Paleontol. J. 2013, 47, 641–832. [Google Scholar] [CrossRef]
- Huang, D.-Y.; Bechly, G.; Nel, P.; Engel, M.S.; Prokop, J.; Azar, D.; Cai, C.-Y.; van de Kamp, T.; Staniczek, A.H.; Garrouste, R.; et al. New Fossil Insect Order Permopsocida Elucidates Major Radiation and Evolution of Suction Feeding in Hemimetabolous Insects (Hexapoda: Acercaria). Sci. Rep. 2016, 6, 23004. [Google Scholar] [CrossRef]
- Wootton, R.J. Palaeozoic Insects. Annu. Rev. Entomol. 1981, 26, 319–344. [Google Scholar] [CrossRef]
- Zhuzhgova, L.V.; Ponomareva, G.Y.; Aristov, D.S.; Naugolnykh, S.V. Chekarda is a location of fossil insects and plants from the Permian period. In Monograph on the Geology, Paleobotany and Paleoentomology of Chekarda; ZAO Tipographia: Perm, Russia, 2015; p. 160. ISBN 978-5-7944-2545-1. [Google Scholar]
- Novokshonov, V.G. New Insects (Insecta: Hypoperlida, Mischopterida, Jurinida) from the Lower Permian of the Middle Urals. Paleontol. J. 1998, 32, 46–53. [Google Scholar]
- Poschmann, M.J.; Nel, A. The First Permian Scorpionfly from Germany (Insecta, Panorpida: Protomeropidae). Palaeoentomology 2021, 4, 231–236. [Google Scholar] [CrossRef]
- Dmitriev, V.Y.; Aristov, D.S.; Bashkuev, A.S.; Vasilenko, D.V.; Vřsanský, P.; Gorochov, A.V.; Lukashevitch, E.D.; Mostovski, M.B.; Ponomarenko, A.G.; Popov, Y.A.; et al. Insect Diversity from the Carboniferous to Recent. Paleontol. J. 2018, 52, 610–619. [Google Scholar] [CrossRef]
- Erwin, D.H. The End-Permian Mass Extinction: What Really Happened and Did It Matter? Trends Ecol. Evol. 1989, 4, 225–229. [Google Scholar] [CrossRef]
- Benton, M.J.; Twitchett, R.J. How to Kill (Almost) All Life: The End-Permian Extinction Event. Trends Ecol. Evol. 2003, 18, 358–365. [Google Scholar] [CrossRef]
- Looy, C.V.; Brugman, W.A.; Dilcher, D.L.; Visscher, H. The Delayed Resurgence of Equatorial Forests after the Permian–Triassic Ecologic Crisis. Proc. Natl. Acad. Sci. USA 1999, 96, 13857–13862. [Google Scholar] [CrossRef]
- Galfetti, T.; Hochuli, P.A.; Brayard, A.; Bucher, H.; Weissert, H.; Vigran, J.O. Smithian-Spathian Boundary Event: Evidence for Global Climatic Change in the Wake of the End-Permian Biotic Crisis. Geology 2007, 35, 291–294. [Google Scholar] [CrossRef]
- McElwain, J.C.; Punyasena, S.W. Mass Extinction Events and the Plant Fossil Record. Trends Ecol. Evol. 2007, 22, 548–557. [Google Scholar] [CrossRef]
- Vajda, V.; McLoughlin, S.; Mays, C.; Frank, T.D.; Fielding, C.R.; Tevyaw, A.; Lehsten, V.; Bocking, M.; Nicoll, R.S. End-Permian (252 Mya) Deforestation, Wildfires and Flooding—An Ancient Biotic Crisis with Lessons for the Present. Earth Planet Sci. Lett. 2020, 529, 115875. [Google Scholar] [CrossRef]
- Bodnar, J.; Coturel, E.P.; Falco, J.I.; Beltrán, M. An Updated Scenario for the End-Permian Crisis and the Recovery of Triassic Land Flora in Argentina. Hist. Biol. 2021, 33, 3654–3672. [Google Scholar] [CrossRef]
- Peng, H.; Yang, W.; Wan, M.; Liu, J.; Liu, F. Refugium amidst Ruins: Unearthing the Lost Flora That Escaped the End-Permian Mass Extinction. Sci. Adv. 2025, 11, eads5614. [Google Scholar] [CrossRef]
- Nowak, H.; Schneebeli-Hermann, E.; Kustatscher, E. No Mass Extinction for Land Plants at the Permian–Triassic Transition. Nat. Commun. 2019, 10, 384. [Google Scholar] [CrossRef]
- Nowak, H.; Vérard, C.; Kustatscher, E. Palaeophytogeographical Patterns Across the Permian–Triassic Boundary. Front. Earth Sci. 2020, 8, 613350. [Google Scholar] [CrossRef]
- Montagna, M.; Tong, K.J.; Magoga, G.; Strada, L.; Tintori, A.; Ho, S.Y.W.; Lo, N. Recalibration of the Insect Evolutionary Time Scale Using Monte San Giorgio Fossils Suggests Survival of Key Lineages through the End-Permian Extinction. Proc. R. Soc. B 2019, 286, 20191854. [Google Scholar] [CrossRef] [PubMed]
- Montagna, M.; Magoga, G.; Stockar, R.; Magnani, F. The Contribution of the Middle Triassic Fossil Assemblage of Monte San Giorgio to Insect Evolution. Commun. Biol. 2024, 7, 1023. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Che, L.-H.; Li, Y.; Liang, D.; Pang, H.; Ślipiński, A.; Zhang, P. Evolutionary History of Coleoptera Revealed by Extensive Sampling of Genes and Species. Nat. Commun. 2018, 9, 205. [Google Scholar] [CrossRef]
- Shcherbakov, D.E. Insect Recovery after the Permian/Triassic Crisis. Alavesia 2008, 2, 125–131. [Google Scholar]
- Taylor, T.N. Paleobotany: The Biology and Evolution of Fossil Plants, 2nd ed.; Elsevier Science & Technology: Oxford, UK, 2009; ISBN 978-0-08-055783-0. [Google Scholar]
- Zhang, Y.; Zheng, S.; Singh, K.J.; Wang, Y.; Zhang, S.; Saxena, A. Glossopterids Survived End-Permian Mass Extinction in North Hemisphere. Glob. Geol. 2022, 25, 214–254. [Google Scholar] [CrossRef]
- Kustatscher, E.; Visscher, H.; Van Konijnenburg-van Cittert, J.H.A. Did the Czekanowskiales Already Exist in the Late Permian? PalZ 2019, 93, 465–477. [Google Scholar] [CrossRef]
- Crepet, W.L. Investigations of North American Cycadeoids: Pollination Mechanisms in Cycadeoidea. Am. J. Bot. 1972, 59, 1048–1056. [Google Scholar] [CrossRef]
- Klavins, S.D. Coprolites in a Middle Triassic Cycad Pollen Cone: Evidence for Insect Pollination in Early Cycads? Evol. Ecol. Res. 2005, 7, 479–488. Available online: http://hdl.handle.net/1808/16782 (accessed on 25 April 2025).
- Procheş, Ş.; Johnson, S.D. Beetle Pollination of the Fruit-scented Cones of the South African Cycad Stangeria eriopus. Am. J. Bot. 2009, 96, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Hayashi, N. Cretaceous Coleopteran Larva Fed on a Female Fructification of Extinct Gymnosperm. J. Plant Res. 1996, 109, 327–330. [Google Scholar] [CrossRef]
- Terry, I.; Walter, G.H.; Moore, C.; Roemer, R.; Hull, C. Odor-Mediated Push-Pull Pollination in Cycads. Science 2007, 318, 70. [Google Scholar] [CrossRef]
- Terry, I.; Tang, W.; Taylor Blake, A.S.; Donaldson, J.S.; Singh, R.; Vovides, A.P.; Cibrián Jaramillo, A. An Overview of Cycad Pollination Studies. In Proceedings of the 8th International Conference on Cycad Biology; Panama City, Panama, 13–15 January 2008, Stevenson, D.W., Osborne, R., Blake, A.S.T., Eds.; The New York Botanical Garden Press: New York, NY, USA, 2012; Volume 106, pp. 352–394. [Google Scholar]
- Schneider, D.; Wink, M.; Sporer, F.; Lounibos, P. Cycads: Their Evolution, Toxins, Herbivores and Insect Pollinators. Sci. Nat. 2002, 89, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Oberprieler, R.G. “Evil Weevils”—The Key to Cycad Survival and Diversification? In Proceedings of the 6th International Conference on Cycad Biology, Chonburi, Thailand, 29 July–3 August 2002; pp. 170–194. [Google Scholar]
- Liu, Z.; Ślipiński, A.; Lawrence, J.F.; Ren, D.; Pang, H. Palaeoboganium gen. nov. from the Middle Jurassic of China (Coleoptera: Cucujoidea: Boganiidae): The First Cycad Pollinators? J. Syst. Palaeontol. 2017, 16, 351–360. [Google Scholar] [CrossRef]
- Downie, D.A.; Donaldson, J.S.; Oberprieler, R.G. Molecular Systematics and Evolution in an African Cycad-Weevil Interaction: Amorphocerini (Coleoptera: Curculionidae: Molytinae) Weevils on Encephalartos. Mol. Phylogenet. Evol. 2008, 47, 102–116. [Google Scholar] [CrossRef]
- Mound, L. The First Thrips Species (Insecta, Thysanoptera) from Cycad Male Cones, and Its Family Level Significance. J. Nat. Hist. 1991, 25, 647–652. [Google Scholar] [CrossRef]
- Mound, L.A.; Terry, I. Thrips Pollination of the Central Australian Cycad, Macrozamia macdonnellii (Cycadales). Int. J. Plant Sci. 2001, 162, 147–154. [Google Scholar] [CrossRef]
- Grimaldi, D.; Shmakov, A.; Fraser, N. Mesozoic Trhips and Early Evolution of the Order Thysanoptera (Insecta). J. Paleontol. 2004, 78, 941–952. [Google Scholar] [CrossRef]
- Peñalver, E.; Labandeira, C.C.; Barrón, E.; Delclòs, X.; Nel, P.; Nel, A.; Tafforeau, P.; Soriano, C. Thrips Pollination of Mesozoic Gymnosperms. Proc. Natl. Acad. Sci. USA 2012, 109, 8623–8628. [Google Scholar] [CrossRef] [PubMed]
- Brookes, D.R.; Hereward, J.P.; Terry, L.I.; Walter, G.H. Evolutionary Dynamics of a Cycad Obligate Pollination Mutualism—Pattern and Process in Extant Macrozamia Cycads and Their Specialist Thrips Pollinators. Mol. Phylogenet. Evol. 2015, 93, 83–93. [Google Scholar] [CrossRef]
- Crane, P.R.; Friis, E.M.; Pedersen, K.R. The Origin and Early Diversification of Angiosperms. Nature 1995, 374, 27–33. [Google Scholar] [CrossRef]
- Crepet, W.L. Investigations of North American Cycadeoids: The Reproductive Biology of Cycadeoidea. Palaeontogr. Abt. B 1974, 148, 144–169. [Google Scholar]
- Popa, M.E. Review of the Bennettitalean Genus Weltrichia. J. Palaeogeogr. 2019, 8, 12. [Google Scholar] [CrossRef]
- Gottsberger, G. The Reproductive Biology of Primitive Angiosperms. Taxon 1988, 37, 630–643. [Google Scholar] [CrossRef]
- Watson, J.; Henderson, C.M.B.; Sincock, C.A. Bennettitales of the English Wealden. Palaeontogr. Soc. Monogr. 1991, 145, 2–224. [Google Scholar] [CrossRef]
- Pott, C. A Revision of Wielandiella angustifolia, a Shrub-Sized Bennettite from the Rhaetian-Hettangian of Scania, Sweden, and Jameson Land, Greenland. Int. J. Plant Sci. 2014, 175, 467–499. [Google Scholar] [CrossRef]
- Roemer, R.; Terry, I.; Chockley, C.; Jacobsen, J. Experimental Evaluation and Thermo-Physical Analysis of Thermogenesis in Male and Female Cycad Cones. Oecologia 2005, 144, 88–97. [Google Scholar] [CrossRef]
- Dieringer, G.; Cabrera, R.L.; Lara, M.; Loya, L.; Reyes-Castillo, P. Beetle Pollination and Floral Thermogenicity in Magnolia tamaulipana (Magnoliaceae). Int. J. Plant Sci. 1999, 160, 64–71. [Google Scholar] [CrossRef]
- Bernhardt, P. Convergent Evolution and Adaptive Radiation of Beetle-Pollinated Angiosperms. Plant Syst. Evol. 2000, 222, 293–320. [Google Scholar] [CrossRef]
- Seymour, R.S.; Matthews, P.G.D. The Role of Thermogenesis in the Pollination Biology of the Amazon Waterlily Victoria amazonica. Ann. Bot. 2006, 98, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Kubitzki, K. Welwitschiaceae. In Pteridophytes and Gymnosperms; Kramer, K.U., Green, P.S., Eds.; Springer Sciences & Business: Berlin/Heidelberg, Germany, 1990; Volume 3, pp. 387–391. [Google Scholar] [CrossRef]
- Kato, M.; Inoue, T. Origin of Insect Pollination. Nature 1994, 368, 195. [Google Scholar] [CrossRef]
- Kato, M.; Inoue, T.; Nagamitsu, T. Pollination Biology of Gnetum (Gnetaceae) in a Lowland Mixed Dipterocarp Forest in Sarawak. Am. J. Bot. 1995, 82, 862–868. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, M.; Vamosi, J.C.; Yang, H.; Mu, W.; Li, J.; Wan, T. Wind or Insect Pollination? Ambophily in a Subtropical Gymnosperm Gnetum parvifolium (Gnetales). Plant Species Biol. 2015, 31, 272–279. [Google Scholar] [CrossRef]
- Rydin, C.; Bolinder, K. Moonlight Pollination in the Gymnosperm Ephedra (Gnetales). Biol. Lett. 2015, 11, 20140993. [Google Scholar] [CrossRef]
- Balme, B.E. Fossil in Situ Spores and Pollen Grains: An Annotated Catalogue. Rev. Palaeobot. Palynol. 1995, 87, 81–323. [Google Scholar] [CrossRef]
- Krassilov, V.A.; Rasnitsyn, A.P.; Afonin, S.A. Pollen Eaters and Pollen Morphology: Co-Evolution through the Permian and Mesozoic. Afr. Invertebr. 2007, 48, 3–11. Available online: https://hdl.handle.net/10520/EJC84595 (accessed on 25 April 2025).
- Chaudonneret, J. Les Piéces Buccales Des Insectes: Théme Et Variations; Éditions hors série du Bulletin scientifique de Bourgogne; Society of Natural Sciences of Burgundy: Dijon, France, 1990; p. 225. [Google Scholar]
- Labandeira, C.C. Insect Mouthparts: Ascertaining the Paleobiology of Insect Feeding Strategies. Annu. Rev. Ecol. Syst. 1997, 28, 153–193. [Google Scholar] [CrossRef]
- Krenn, H.W. Feeding Mechanisms of Adult Lepidoptera: Structure, Function, and Evolution of the Mouthparts. Annu. Rev. Entomol. 2010, 55, 307–327. [Google Scholar] [CrossRef]
- Krenn, H.W. Form and Function of Insect Mouthparts. In Insects Mouthparts; Krenn, H.W., Ed.; Zoological Monographs; Springer: Cham, Switzerland, 2019; Volume 5, pp. 9–46. [Google Scholar] [CrossRef]
- Krenn, H.W. Fluid-Feeding Mouthparts. In Insects Mouthparts; Krenn, H.W., Ed.; Zoological Monographs; Springer: Cham, Switzerland, 2019; Volume 5, pp. 47–99. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Daniel, T.L. Mechanics of Food Handling by Fluid-Feeding Insects. In Regulatory Mechanisms in Insect Feeding; Chapman, R.F., De Boer, G., Eds.; Chapman and Hall: New York, NY, USA; London, UK, 1995; pp. 32–73. [Google Scholar] [CrossRef]
- Grimaldi, D.A. Basal Cyclorrhapha in Amber from the Cretaceous and Tertiary(Insecta: Diptera), and Their Relationships: Brachycera in Cretaceous Amber Part IX. Bull. Am. Mus. Nat. Hist. 2018, 423, 1–97. [Google Scholar] [CrossRef]
- Gillung, J.P.; Winterton, S.L. Evolution of Fossil and Living Spider Flies Based on Morphological and Molecular Data (Diptera, Acroceridae). Syst. Entomol. 2019, 44, 820–841. [Google Scholar] [CrossRef]
- Szucsich, N.U.; Krenn, H.W. Morphology and Function of the Proboscis in Bombyliidae (Diptera, Brachycera) and Implications for Proboscis Evolution in Brachycera. Zoomorphology 2000, 120, 79–90. [Google Scholar] [CrossRef]
- Krenn, H.W.; Mauss, V.; Plant, J. Evolution of the Suctorial Proboscis in Pollen Wasps (Masarinae, Vespidae). Arth. Struct. Dev. 2002, 31, 103–120. [Google Scholar] [CrossRef]
- Krenn, H.W.; Plant, J.D.; Szucsich, N.U. Mouthparts of Flower-Visiting Insects. Arth. Struct. Dev. 2005, 34, 1–40. [Google Scholar] [CrossRef]
- Labandeira, C.C. Fossil History and Evolutionary Ecology of Diptera and Their Associations with Plants. In The Evolutionary Biology of Flies; Yeates, D.K., Wiegmann, B.M., Eds.; Columbia University Press: New York, NY, USA, 2005; pp. 217–272. [Google Scholar]
- Nilsson, L.A. The Evolution of Flowers with Deep Corolla Tubes. Nature 1988, 334, 147–149. [Google Scholar] [CrossRef]
- Pauw, A.; Stofberg, J.; Waterman, R.J. Flies and Flowers in Darwin’s Race. Evolution 2009, 63, 268–279. [Google Scholar] [CrossRef]
- Peris, D.; Pérez-de la Fuente, R.; Peñalver, E.; Delclòs, X.; Barrón, E.; Labandeira, C.C. False Blister Beetles and the Expansion of Gymnosperm-Insect Pollination Modes before Angiosperm Dominance. Curr. Biol. 2017, 27, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, C.; Jarzembowski, E.A. Ecological Radiations of Insects in the Mesozoic. Trends Ecol. Evol. 2022, 37, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Bicha, W.J. Biodiversity of Mecoptera. In Insect Biodiversity: Sciences and Society; Foottit, R.G., Adler, P.H., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; Volume 2, pp. 705–720. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, X.; Zhang, Q.; Chen, J.; Zheng, X.; Zhang, W.; Liu, X.; Wang, B. High Niche Diversity in Mesozoic Pollinating Lacewings. Nat. Commun. 2018, 9, 3793. [Google Scholar] [CrossRef]
- Lin, X.; Labandeira, C.C.; Shih, C.; Hotton, C.L.; Ren, D. Life Habits and Evolutionary Biology of New Two-Winged Long-Proboscid Scorpionflies from Mid-Cretaceous Myanmar Amber. Nat. Commun. 2019, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Y.; Labandeira, C.C.; Shih, C.; Ren, D. Mesozoic Lacewings from China Provide Phylogenetic Insight into Evolution of Kalligrammatidae (Neuroptera). BMC Evol. Biol. 2014, 14, 16. [Google Scholar] [CrossRef]
- Krassilov, V.A.; Rasnitsyn, A.P. Plant Remains from the Guts of Fossil Insects: Evolutionary and Paleoecological Inferences. In Proceedings of the First Palaeoentomological Conference; Moscow, Russia, 30 August–4 September 1998, Vršanský, P., Ed.; AMBA Projects: Bratislava, Slovakia, 1999; pp. 65–72. [Google Scholar]
- Gu, J.-J.; Qiao, G.-X.; Ren, D. Revision and New Taxa of Fossil Prophalangopsidae (Orthoptera: Ensifera). J. Orthopt. Res. 2010, 19, 41–56. [Google Scholar] [CrossRef]
- Rasnitsyn, A.P.; Krassilov, V.A. The First Documented Occurrence of Phyllophagy in Pre-Cretaceous Insects: Leaf Tissues in the Gut of Upper Jurassic Insects from Southern Kazakhstan. Paleontol. J. 2000, 34, 301–309. [Google Scholar]
- Sendi, H.; Hinkelman, J.; Vršanská, L.; Kúdelová, T.; Kúdela, M.; Zuber, M.; van de Kamp, T.; Vršanský, P. Roach Nectarivory, Gymnosperm and Earliest Flower Pollination Evidence from Cretaceous Ambers. Biologia 2020, 75, 1613–1630. [Google Scholar] [CrossRef]
- Vlasáková, B.; Kalinová, B.; Gustafsson, M.H.G.; Teichert, H. Cockroaches as Pollinators of Clusia aff. sellowiana (Clusiaceae) on Inselbergs in French Guiana. Ann. Bot. 2008, 102, 295–304. [Google Scholar] [CrossRef]
- Pérez-Gómez, Á.; León-Osper, M.; Pareja, D.; Robla, J. Flower Visits of Cockroaches (Insecta: Blattodea) in the Iberian Peninsula: Are They Neglected Pollinators? J. Appl. Entomol. 2023, 147, 565–576. [Google Scholar] [CrossRef]
- Gillot, C. The Plecopteroid, Blattoid, and Orthopteroid Orders. In Entomology, 3rd ed.; Gillot, C., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 147–197. ISBN 978-1-4020-3184-7. [Google Scholar]
- Prokop, J.; Nel, A.; Engel, M.S. Diversity, Form, and Postembryonic Development of Paleozoic Insects. Annu. Rev. Entomol. 2023, 68, 401–429. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Lienhard, C. Bridging the Gap between Chewing and Sucking in the Hemipteroid Insects: New Insights from Cretaceous Amber. Zootaxa 2016, 4079, 229–245. [Google Scholar] [CrossRef]
- Grimaldi, D. The Co-Radiations of Pollinating Insects and Angiosperms in the Cretaceous. Ann. Mo. Bot. Gard. 1999, 86, 373–406. [Google Scholar] [CrossRef]
- Mey, W.; Wichard, W.; Müller, P.; Wang, B. The Blueprint of the Amphiesmenoptera—Tarachoptera, a New Order of Insects from Burmese Amber (Insecta, Amphiesmenoptera). Foss. Rec. 2017, 20, 129–145. [Google Scholar] [CrossRef]
- Mey, W.; Wichard, W. Tarachoptera: The Extinct and Enigmatic Cousins of Trichoptera and Lepidoptera, with Descriptions of Two New Species. Contrib. Entomol. 2023, 73, 137–146. [Google Scholar] [CrossRef]
- Rasnitsyn, A.P. New Triassic Hymenoptera from Central Asia. Paleontol. J. 1964, 1, 88–96. (In Russian) [Google Scholar]
- Lara, M.B.; Rasnitsyn, A.P.; Zavattieri, A.M. Potrerilloxyela menendezi gen. et sp. nov. from the Late Triassic of Argentina: The Oldest Representative of Xyelidae (Hymenoptera: Symphyta) for Americas. Paleontol. J. 2014, 48, 182–190. [Google Scholar] [CrossRef]
- Denisova, E.A.; Kopylov, D.S.; Rasnitsyn, A.P. New Archexyelinae (Hymenoptera: Xyelidae) from the Triassic Madygen Formation of Kyrgyzstan. PalZ 2023, 98, 95–104. [Google Scholar] [CrossRef]
- Zhang, W.; Shih, C.; Labandeira, C.C.; Sohn, J.-C.; Davis, D.R.; Santiago-Blay, J.A.; Flint, O.; Ren, D. New Fossil Lepidoptera (Insecta: Amphiesmenoptera) from the Middle Jurassic Jiulongshan Formation of Northeastern China. PLoS ONE 2013, 8, e79500. [Google Scholar] [CrossRef]
- Mitter, C.; Davis, D.R.; Cummings, M.P. Phylogeny and Evolution of Lepidoptera. Annu. Rev. Entomol. 2017, 62, 265–283. [Google Scholar] [CrossRef] [PubMed]
- van Eldijk, T.J.B.; Wappler, T.; Strother, P.K.; van der Weijst, C.M.H.; Rajaei, H.; Visscher, H.; van de Schootbrugge, B. A Triassic-Jurassic Window into the Evolution of Lepidoptera. Sci. Adv. 2018, 4, e1701568. [Google Scholar] [CrossRef]
- Sohn, J.-C.; Labandeira, C.C.; Davis, D.R. The Fossil Record and Taphonomy of Butterflies and Moths (Insecta, Lepidoptera): Implications for Evolutionary Diversity and Divergence-Time Estimates. BMC Evol. Biol. 2015, 15, 12. [Google Scholar] [CrossRef]
- Kalugina, N.S.; Kovalev, V.G. Dvukrylye Nasekomye Yury Sibiri (Diptera of the Jurassic of Siberia); Nauka: Moscow, Russia, 1985; p. 198. [Google Scholar]
- Gilbert, F.; Jervis, M. Functional, Evolutionary and Ecological Aspects of Feeding-Related Mouthpart Specializations in Parasitoid Flies. Biol. J. Linn. Soc. 1998, 63, 495–535. [Google Scholar] [CrossRef]
- Krzemiński, W.; Krzemińska, E. Triassic Diptera: Descriptions, Revisions and Phylogenetic Relations. Acta Zool. Cracov. 2003, 46, 153–184. [Google Scholar]
- Arillo, A.; Peñalver, E.; Pérez-de la Fuente, R.; Delclòs, X.; Criscione, J.; Barden, P.M.; Riccio, M.L.; Grimaldi, D.A. Long-proboscid Brachyceran Flies in Cretaceous Amber (Diptera: Stratiomyomorpha: Zhangsolvidae). Syst. Entomol. 2015, 40, 242–267. [Google Scholar] [CrossRef]
- Peñalver, E.; Arillo, A.; Pérez-de la Fuente, R.; Riccio, M.L.; Delclòs, X.; Barrón, E.; Grimaldi, D.A. Long-Proboscid Flies as Pollinators of Cretaceous Gymnosperms. Curr. Biol. 2015, 25, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Lukashevich, E.D.; Ribeiro, G.C. Mesozoic Fossils and the Phylogeny of Tipulomorpha (Insecta: Diptera). J. Syst. Palaeontol. 2018, 17, 635–652. [Google Scholar] [CrossRef]
- Ollerton, J. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Willmer, P.G.; Cunnold, H.; Ballantyne, G. Insights from Measuring Pollen Deposition: Quantifying the Pre-Eminence of Bees as Flower Visitors and Effective Pollinators. Arth.-Plant Int. 2017, 11, 411–425. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, B. Evolution of Lower Brachyceran Flies (Diptera) and Their Adaptive Radiation with Angiosperms. Front. Plant Sci. 2017, 8, 631. [Google Scholar] [CrossRef] [PubMed]
- Ren, D. Flower-Associated Brachycera Flies as Fossil Evidence for Jurassic Angiosperm Origins. Science 1998, 280, 85–88. [Google Scholar] [CrossRef]
- Labandeira, C.C. How Old Is the Flower and the Fly? Science 1998, 280, 57–59. [Google Scholar] [CrossRef]
- Goldblatt, P.; Manning, J.C. The Long-Proboscid Fly Pollination System in Southern Africa. Ann. Mo. Bot. Gard. 2000, 87, 146–170. [Google Scholar] [CrossRef]
- Herendeen, P.S.; Friis, E.M.; Pedersen, K.R.; Crane, P.R. Palaeobotanical Redux: Revisiting the Age of the Angiosperms. Nat. Plants 2017, 3, 17015. [Google Scholar] [CrossRef]
- Mostovski, M.B. A Revision of the Nemestrinid Flies (Diptera, Nemestrinidae) Described by Rohdendorf, and a Deascription of New Taxa of the Nemestrinidae from the Upper Jurassic of Kazakhstan. Paleontol. J. 1998, 32, 369–375. [Google Scholar]
- Arnol’di, L.V.; Zherikin, V.V.; Nikritin, L.M.; Ponomarenko, A.G. Mesozoic Coleoptera; Oxonian Press Pvt Ltd.: New Delhi, India; Kolkata, India, 1992; 285p. [Google Scholar]
- Ponomarenko, A.G. The Geological History of Beetles. In Biology, Phylogeny, and Classification of Coleoptera: Papers Celebrating the 80th Birthday of Roy A. Crowson; Pakaluk, J., Slipinski, S.A., Eds.; Muzeum i Instytut Zoologii PAN: Warszawa, Poland, 1995; pp. 155–171. [Google Scholar]
- Ponomarenko, A.G. Ecological Evolution of Beetles (Insecta: Coleoptera). Acta Zool. Cracov. 2003, 46, 319–328. [Google Scholar]
- McKenna, D.D.; Sequeira, A.S.; Marvaldi, A.E.; Farrell, B.D. Temporal Lags and Overlap in the Diversification of Weevils and Flowering Plants. Proc. Natl. Acad. Sci. USA 2009, 106, 7083–7088. [Google Scholar] [CrossRef] [PubMed]
- Peris, D.; Kundrata, R.; Delclòs, X.; Mähler, B.; Ivie, M.A.; Rust, J.; Labandeira, C.C. Unlocking the Mystery of the Mid-Cretaceous Mysteriomorphidae (Coleoptera: Elateroidea) and Modalities in Transiting from Gymnosperms to Angiosperms. Sci. Rep. 2020, 10, 16854. [Google Scholar] [CrossRef]
- Peris, D.; Labandeira, C.C.; Barrón, E.; Delclòs, X.; Rust, J.; Wang, B. Generalist Pollen-Feeding Beetles during the Mid-Cretaceous. iScience 2020, 23, 100913. [Google Scholar] [CrossRef] [PubMed]
- Peris, D.; Rust, J. Cretaceous Beetles (Insecta: Coleoptera) in Amber: The Palaeoecology of This Most Diverse Group of Insects. Zool. J. Linn. Soc. 2019, 189, 1085–1104. [Google Scholar] [CrossRef]
- Bao, T.; Wang, B.; Li, J.; Dilcher, D. Pollination of Cretaceous Flowers. Proc. Natl. Acad. Sci. USA 2019, 116, 24707–24711. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The Number of Known Plants Species in the World and Its Annual Increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Sauquet, H.; Ramírez-Barahona, S.; Magallón, S. What Is the Age of Flowering Plants? J. Exp. Bot. 2022, 73, 3840–3853. [Google Scholar] [CrossRef]
- Crane, P.R.; Herendeen, P.; Friis, E.M. Fossils and Plant Phylogeny. Am. J. Bot. 2004, 91, 1683–1699. [Google Scholar] [CrossRef]
- Clarke, J.T.; Warnock, R.C.M.; Donoghue, P.C.J. Establishing a Time-scale for Plant Evolution. New Phytol. 2011, 192, 266–301. [Google Scholar] [CrossRef] [PubMed]
- Barba-Montoya, J.; dos Reis, M.; Schneider, H.; Donoghue, P.C.J.; Yang, Z. Constraining Uncertainty in the Timescale of Angiosperm Evolution and the Veracity of a Cretaceous Terrestrial Revolution. New Phytol. 2018, 218, 819–834. [Google Scholar] [CrossRef]
- Silvestro, D.; Cascales-Miñana, B.; Bacon, C.D.; Antonelli, A. Revisiting the Origin and Diversification of Vascular Plants through a Comprehensive Bayesian Analysis of the Fossil Record. New Phytol. 2015, 207, 425–436. [Google Scholar] [CrossRef]
- van der Kooi, C.J.; Ollerton, J. The Origins of Flowering Plants and Pollinators. Science 2020, 368, 1306–1308. [Google Scholar] [CrossRef]
- Coiro, M.; Doyle, J.A.; Hilton, J. How Deep Is the Conflict between Molecular and Fossil Evidence on the Age of Angiosperms? New Phytol. 2019, 223, 83–99. [Google Scholar] [CrossRef]
- Meredith, R.W.; Janečka, J.E.; Gatesy, J.; Ryder, O.A.; Fisher, C.A.; Teeling, E.C.; Goodbla, A.; Eizirik, E.; Simão, T.L.L.; Stadler, T.; et al. Impacts of the Cretaceous Terrestrial Revolution and KPg Extinction on Mammal Diversification. Science 2011, 334, 521–524. [Google Scholar] [CrossRef]
- Augusto, L.; Davies, T.J.; Delzon, S.; De Schrijver, A. The Enigma of the Rise of Angiosperms: Can We Untie the Knot? Ecol. Lett. 2014, 17, 1326–1338. [Google Scholar] [CrossRef]
- Michener, C.D. Biogeography of the Bees. Ann. Mo. Bot. Gard. 1979, 66, 277–347. [Google Scholar] [CrossRef]
- Michener, C. The Bees of the World, 2nd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2007; p. 992. ISBN 978-0-8018-8573-0. [Google Scholar]
- Ollerton, J.; Winfree, R.; Tarrant, S. How Many Flowering Plants Are Pollinated by Animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Orr, M.C.; Hughes, A.C.; Chesters, D.; Pickering, J.; Zhu, C.-D.; Ascher, J.S. Global Patterns and Drivers of Bee Distribution. Curr. Biol. 2021, 31, 451–458.e4. [Google Scholar] [CrossRef] [PubMed]
- Sann, M.; Niehuis, O.; Peters, R.S.; Mayer, C.; Kozlov, A.; Podsiadlowski, L.; Bank, S.; Meusemann, K.; Misof, B.; Bleidorn, C.; et al. Phylogenomic Analysis of Apoidea Sheds New Light on the Sister Group of Bees. BMC Evol. Biol. 2018, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Sann, M.; Meusemann, K.; Niehuis, O.; Escalona, H.E.; Mokrousov, M.; Ohl, M.; Pauli, T.; Schmid-Egger, C. Reanalysis of the Apoid Wasp Phylogeny with Additional Taxa and Sequence Data Confirms the Placement of Ammoplanidae as Sister to Bees. Syst. Entomol. 2021, 46, 558–569. [Google Scholar] [CrossRef]
- Negri, I.; Mavris, C.; Di Prisco, G.; Caprio, E.; Pellecchia, M. Honey Bees (Apis mellifera, L.) as Active Samplers of Airborne Particulate Matter. PLoS ONE 2015, 10, e0132491. [Google Scholar] [CrossRef]
- Pellecchia, M.; Papa, G.; Barbato, M.; Capitani, G.; Negri, I. Origin of Non-Exhaust PM in Cities by Individual Analysis of Particles Collected by Honey Bees (Apis Mellifera). Environ. Pollut. 2023, 331, 121885. [Google Scholar] [CrossRef] [PubMed]
- Capitani, G.; Papa, G.; Pellecchia, M.; Negri, I. Disentangling Multiple PM Emission Sources in the Po Valley (Italy) Using Honey Bees. Heliyon 2021, 7, e06194. [Google Scholar] [CrossRef]
- Martins, A.C.; Melo, G.A.R.; Renner, S.S. The Corbiculate Bees Arose from New World Oil-Collecting Bees: Implications for the Origin of Pollen Baskets. Mol. Phylogenetics Evol. 2014, 80, 88–94. [Google Scholar] [CrossRef]
- Poinar, G.O.; Danforth, B.N. A Fossil Bee from Early Cretaceous Burmese Amber. Science 2006, 314, 614. [Google Scholar] [CrossRef]
- Danforth, B.N.; Poinar, G.O. Morphology, Classification, and Antiquity of Melittosphex burmensis (Apoidea: Melittosphecidae) and Implications for Early Bee Evolution. J. Paleontol. 2011, 85, 882–891. [Google Scholar] [CrossRef]
- Engel, M.S. A New Interpretation of the Oldest Fossil Bee (Hymenoptera: Apidae). Am. Mus. Novit. 2000, 3296, 1–11. [Google Scholar] [CrossRef]
- Michener, C.D.; Grimaldi, D. The Oldest Fossil Bee: Apoid History, Evolutionary Stasis, and Antiquity of Social Behavior. Proc. Natl. Acad. Sci. USA 1988, 85, 6424–6426. [Google Scholar] [CrossRef]
- Rehan, S.M.; Leys, R.; Schwarz, M.P. First Evidence for a Massive Extinction Event Affecting Bees Close to the K-T Boundary. PLoS ONE 2013, 8, e76683. [Google Scholar] [CrossRef]
- Engel, M.S. Monophyly and Extensive Extinction of Advanced Eusocial Bees: Insights from an Unexpected Eocene Diversity. Proc. Natl. Acad. Sci. USA 2001, 98, 1661–1664. [Google Scholar] [CrossRef]
- Rust, J.; Singh, H.; Rana, R.S.; McCann, T.; Singh, L.; Anderson, K.; Sarkar, N.; Nascimbene, P.C.; Stebner, F.; Thomas, J.C.; et al. Biogeographic and Evolutionary Implications of a Diverse Paleobiota in Amber from the Early Eocene of India. Proc. Natl. Acad. Sci. USA 2010, 107, 18360–18365. [Google Scholar] [CrossRef] [PubMed]
- Winfree, R.; Aguilar, R.; Vázquez, D.P.; LeBuhn, G.; Aizen, M.A. A Meta-analysis of Bees’ Responses to Anthropogenic Disturbance. Ecology 2009, 90, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Vanbergen, A.J.; Woodcock, B.A.; Gray, A.; Grant, F.; Telford, A.; Lambdon, P.; Chapman, D.S.; Pywell, R.F.; Heard, M.S.; Cavers, S. Grazing Alters Insect Visitation Networks and Plant Mating Systems. Funct. Ecol. 2013, 28, 178–189. [Google Scholar] [CrossRef]
- Kovács-Hostyánszki, A.; Espíndola, A.; Vanbergen, A.J.; Settele, J.; Kremen, C.; Dicks, L.V. Ecological Intensification to Mitigate Impacts of Conventional Intensive Land Use on Pollinators and Pollination. Ecol. Lett. 2017, 20, 673–689. [Google Scholar] [CrossRef]
- Yang, P.; Peng, Y.; Zhao, R.; Yang, D. Biological Characteristics, Threat Factors and Conservation Strategies for the Giant Honey Bee Apis dorsata. Biodivers. Sci. 2018, 26, 476–485. [Google Scholar] [CrossRef]
- LeBuhn, G.; Vargas Luna, J. Pollinator Decline: What Do We Know about the Drivers of Solitary Bee Declines? Curr. Opin. Insect Sci. 2021, 46, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Papa, G.; Di Prisco, G.; Spini, G.; Puglisi, E.; Negri, I. Acute and Chronic Effects of Titanium Dioxide (TiO2) PM1 on Honey Bee Gut Microbiota under Laboratory Conditions. Sci Rep. 2021, 11, 5946. [Google Scholar] [CrossRef] [PubMed]
- Plutino, M.; Bianchetto, E.; Durazzo, A.; Lucarini, M.; Lucini, L.; Negri, I. Rethinking the Connections between Ecosystem Services, Pollinators, Pollution, and Health: Focus on Air Pollution and Its Impacts. Int. J. Environ. Res. Public Health 2022, 19, 2997. [Google Scholar] [CrossRef]
- Toledo-Hernández, E.; Peña-Chora, G.; Hernández-Velázquez, V.M.; Lormendez, C.C.; Toribio-Jiménez, J.; Romero-Ramírez, Y.; León-Rodriguéz, R. The Stingless Bees (Hymenoptera: Apidae: Meliponini): A Review of the Current Threats to Their Survival. Apidologie 2022, 53, 8. [Google Scholar] [CrossRef]
- Forister, M.L.; Dyer, L.A.; Gompert, Z.; Smilanich, A.M. Editorial Overview: Global Change Biology (2023)—Novel Perspectives on Futures, Mechanisms, and the Human Element of Insect Conservation in the Anthropocene. Curr. Opin. Insect Sci. 2024, 62, 101175. [Google Scholar] [CrossRef]
- López-Vázquez, K.; Lara, C.; Corcuera, P.; Castillo-Guevara, C.; Cuautle, M. The Human Touch: A Meta-Analysis of Anthropogenic Effects on Plant-Pollinator Interaction Networks. PeerJ 2024, 12, e17647. [Google Scholar] [CrossRef]
- Margaoan, R.; Papa, G.; Nicolescu, A.; Cornea-Cipcigan, M.; Kösoğlu, M.; Topal, E.; Negri, I. Environmental Pollution Effect on Honey Bees and Their Derived Products: A Comprehensive Analysis. Environ. Sci. Pollut. Res. 2024, 32, 10370–10391. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.E.; Lees, A.C.; Grames, E.M. Understanding and Counteracting the Denial of Insect Biodiversity Loss. Curr. Opin. Insect Sci. 2025, 68, 101338. [Google Scholar] [CrossRef]
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-Pollinator Interactions over 120 Years: Loss of Species, Co-Occurrence, and Function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global Change and Species Interactions in Terrestrial Ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef]
- González-Varo, J.P.; Biesmeijer, J.C.; Bommarco, R.; Potts, S.G.; Schweiger, O.; Smith, H.G.; Steffan-Dewenter, I.; Szentgyörgyi, H.; Woyciechowski, M.; Vilà, M. Combined Effects of Global Change Pressures on Animal-Mediated Pollination. Trends Ecol. Evol. 2013, 28, 524–530. [Google Scholar] [CrossRef]
- Geslin, B.; Le Féon, V.; Folschweiller, M.; Flacher, F.; Carmignac, D.; Motard, E.; Perret, S.; Dajoz, I. The Proportion of Impervious Surfaces at the Landscape Scale Structures Wild Bee Assemblages in a Densely Populated Region. Ecol. Evol. 2016, 6, 6599–6615. [Google Scholar] [CrossRef] [PubMed]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Hegland, S.J.; Nielsen, A.; Lázaro, A.; Bjerknes, A.; Totland, Ø. How Does Climate Warming Affect Plant-pollinator Interactions? Ecol. Lett. 2009, 12, 184–195. [Google Scholar] [CrossRef]
- Thomson, J.D. Flowering Phenology, Fruiting Success and Progressive Deterioration of Pollination in an Early-Flowering Geophyte. Philos. Trans. R. Soc. B 2010, 365, 3187–3199. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Negri, I.; Toledo, M.E. Evolution of Insect Pollination Before Angiosperms and Lessons for Modern Ecosystems. Insects 2026, 17, 103. https://doi.org/10.3390/insects17010103
Negri I, Toledo ME. Evolution of Insect Pollination Before Angiosperms and Lessons for Modern Ecosystems. Insects. 2026; 17(1):103. https://doi.org/10.3390/insects17010103
Chicago/Turabian StyleNegri, Ilaria, and Mario E. Toledo. 2026. "Evolution of Insect Pollination Before Angiosperms and Lessons for Modern Ecosystems" Insects 17, no. 1: 103. https://doi.org/10.3390/insects17010103
APA StyleNegri, I., & Toledo, M. E. (2026). Evolution of Insect Pollination Before Angiosperms and Lessons for Modern Ecosystems. Insects, 17(1), 103. https://doi.org/10.3390/insects17010103






