The Molecular Drivers of Honey Robbing in Apis mellifera L.: Morphological Divergence and Oxidative-Immune Regulation Mechanisms Based on Proteomic Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Bee Samples
2.2. Morphometric Measurements
2.3. Survival Experiment
2.4. Protein Extraction and Digestion
2.5. LC-MS/MS Analysis
2.6. Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Differential Morphological Characteristics
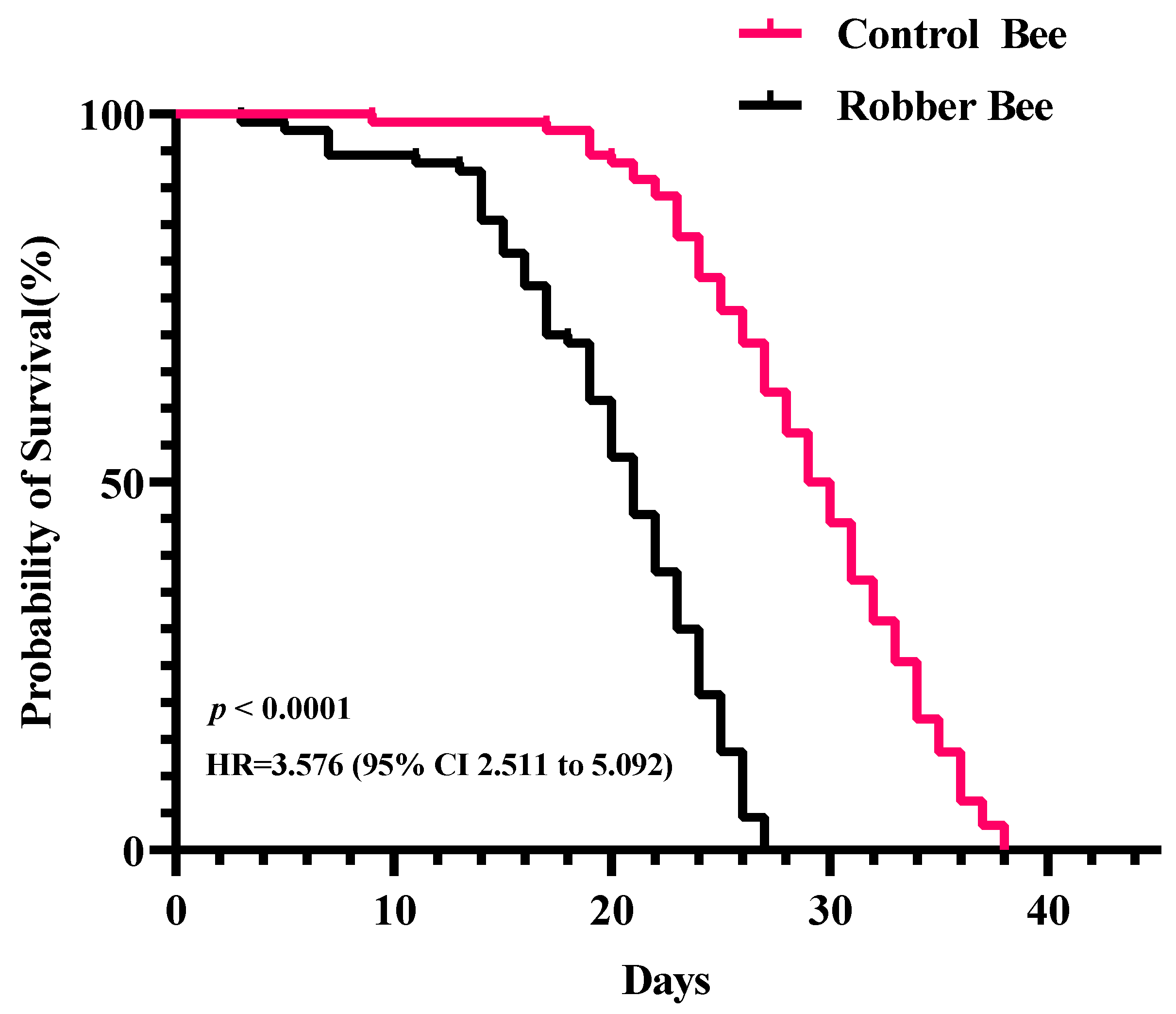
3.2. Survival Analysis
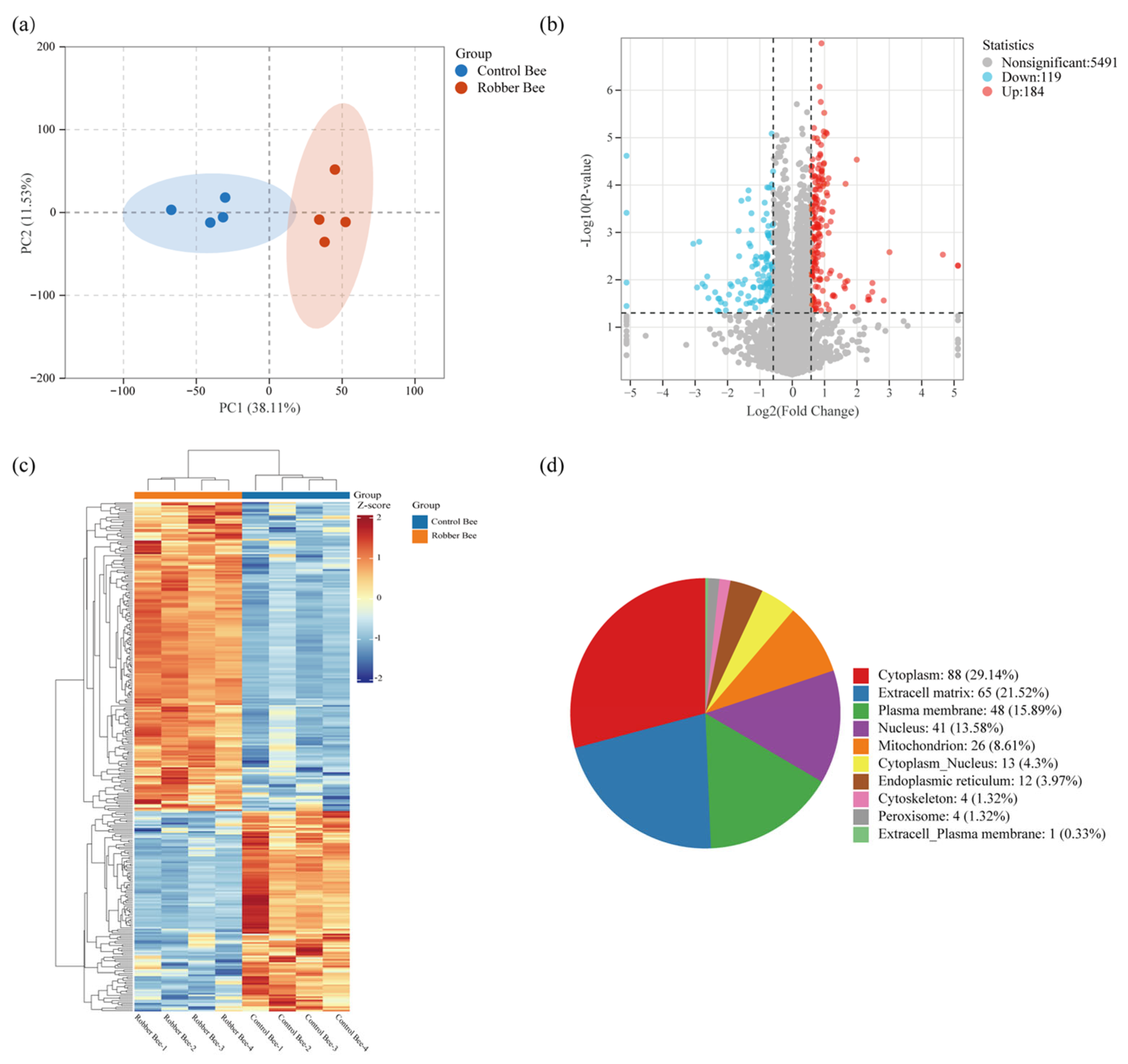
3.3. Identification of Proteins in the Heads
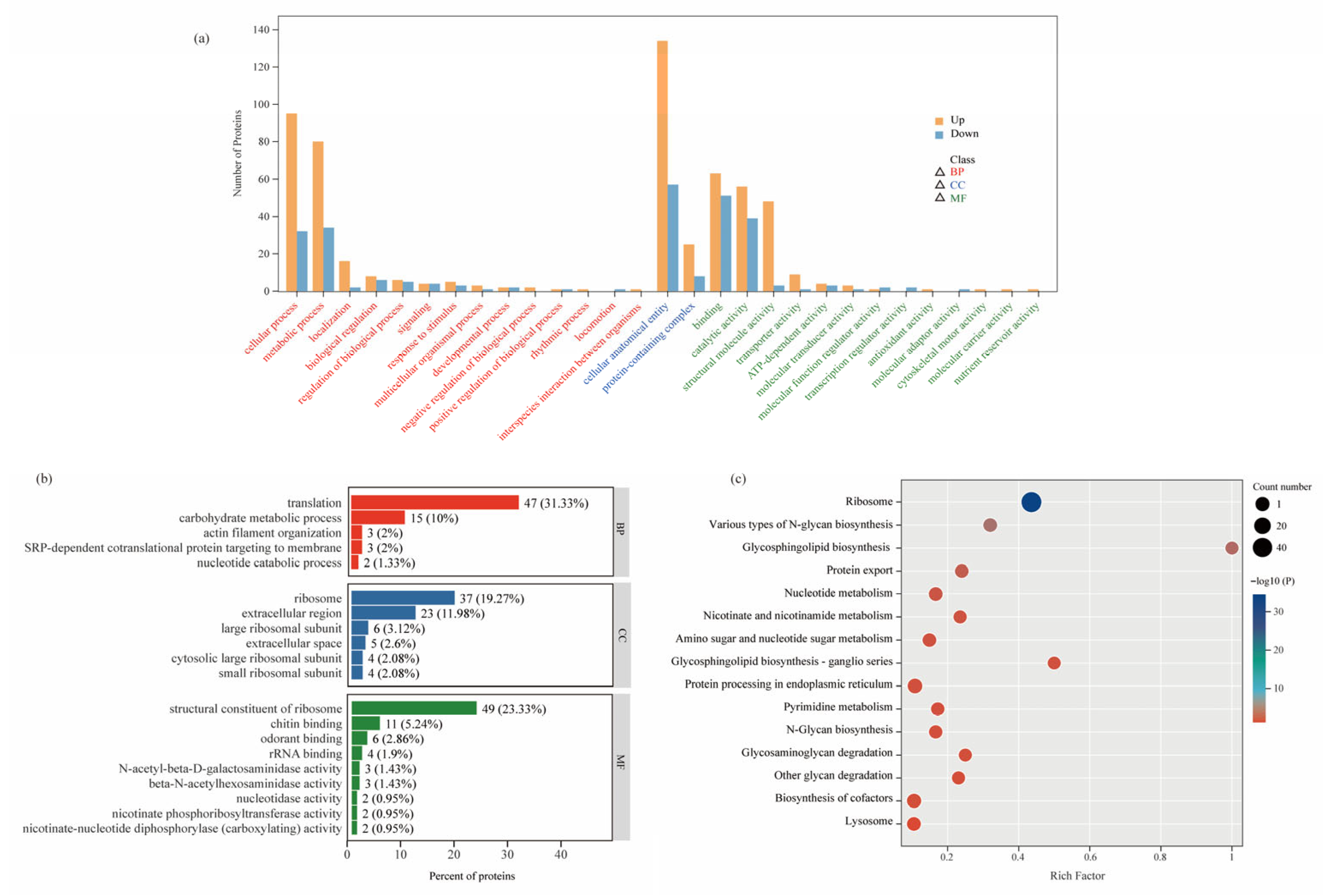
3.4. GO and KEGG Pathway Analysis of DEPs
4. Discussion
4.1. Laccase Upregulation Darkens Robber Bees’ Tergite Color
4.2. Oxidative Stress and Immunosuppression Mediate the Shortened Lifespan of Robber Bees
4.3. Metabolic and Proteomic Reprogramming in Honey Robbing Adaptation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DTT | Dithiothreitol |
| TFA | Trifluoroacetic acid |
| DIA | Data-independent acquisition |
| HCD | Higher-energy collisional dissociation |
| AGC | Automatic gain control |
| DEPs | Differentially expressed proteins |
| FC | Fold change |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| HR | Hazard Ratio |
| FMOs | Flavin-containing monooxygenases |
| FAD | Glucose dehydrogenase |
| HSP75 | Heat shock protein 75kDa |
| MF | Molecular Function |
| BP | Biological Process |
| CC | Cellular Component |
| GST | Glutathione transferase |
| CYP450 | CytochromeP450enzymesystem |
| Prx | Peroxiredoxin |
| MRJP | Main royal jelly proteins |
| VG | Vitellogenin |
References
- Halvorson, K.; Baumung, R.; Leroy, G.; Chen, C.; Boettcher, P. Protection of honeybees and other pollinators: One global study. Apidologie 2021, 52, 535–547. [Google Scholar] [CrossRef]
- Abrol, D.P. Decline in Pollinators. In Pollination Biology: Biodiversity Conservation and Agricultural Production; Springer: Dordrecht, The Netherlands, 2012; pp. 545–601. [Google Scholar]
- Johnson, B.R. Division of labor in honeybees: Form, function, and proximate mechanisms. Behav. Ecol. Sociobiol. 2010, 64, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Arenas, A.; Kohlmaier, M.G. Nectar source profitability influences individual foraging preferences for pollen and pollen-foraging activity of honeybee colonies. Behav. Ecol. Sociobiol. 2019, 73, 34. [Google Scholar] [CrossRef]
- Sharma, G.; Malthankar, P.A.; Mathur, V. Insect-Plant Interactions: A Multilayered Relationship. Ann. Entomol. Soc. Am. 2021, 114, 1–16. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; Abd El-Wahed, A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. [Google Scholar] [CrossRef]
- Ryan, W.; Jeanette, K.; James, E. Robbing Behavior in Honey Bees. EDIS 2021, 2015, 163. [Google Scholar] [CrossRef]
- Free, J.B. The behaviour of robber honeybees. Behaviour 1954, 7, 233–240. [Google Scholar] [CrossRef]
- Kuszewska, K.; Woyciechowski, M. Risky robbing is a job for short-lived and infected worker honeybees. Apidologie 2014, 45, 537–544. [Google Scholar] [CrossRef]
- Peck, D.T.; Seeley, T.D. Mite bombs or robber lures? The roles of drifting and robbing in transmission from collapsing honey bee colonies to their neighbors. PLoS ONE 2019, 14, e0218392. [Google Scholar] [CrossRef]
- Lindström, A.; Korpela, S.; Fries, I. Horizontal transmission of Paenibacillus larvae spores between honey bee (Apis mellifera) colonies through robbing. Apidologie 2008, 39, 515–522. [Google Scholar] [CrossRef]
- Rittschof, C.C.; Nieh, J.C. Honey robbing: Could human changes to the environment transform a rare foraging tactic into a maladaptive behavior? Curr. Opin. Insect Sci. 2021, 45, 84–90. [Google Scholar] [CrossRef]
- Li, G.L.; Zhao, H.; Liu, Z.G.; Wang, H.F.; Xu, B.H.; Guo, X.Q. The Wisdom of Honeybee Defenses Against Environmental Stresses. Front. Microbiol. 2018, 9, 722. [Google Scholar] [CrossRef]
- Grume, G.J.; Biedenbender, S.P.; Rittschof, C.C.; Foraging, Q.; Robbing, S. Honey robbing causes coordinated changes in foraging and nest defence in the honey bee, Apis mellifera. Anim. Behav. 2021, 173, 53–65. [Google Scholar] [CrossRef]
- Wolschin, F.; Amdam, G.V. Plasticity and robustness of protein patterns during reversible development in the honey bee (Apis mellifera). Anal. Bioanal. Chem. 2007, 389, 1095–1100. [Google Scholar] [CrossRef]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. 2014, 89, 255–269. [Google Scholar] [CrossRef]
- Fang, Y.; Feng, M.; Ma, C.; Rueppell, O.; Li, J.K. Major royal jelly proteins influence the neurobiological regulation of the division of labor among honey bee workers. Int. J. Biol. Macromol. 2023, 225, 848–860. [Google Scholar] [CrossRef]
- Guo, Y.; Fu, B.C.; Qin, G.J.; Song, H.L.; Wu, W.Q.; Shao, Y.Q.; Altaye, S.Z.; Yu, L.S. Proteome analysis reveals a strong correlation between olfaction and pollen foraging preference in honeybees. Int. J. Biol. Macromol. 2019, 121, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.Y.; Zhou, X.R.; Tan, Y.; Pang, B.P. Proteomic analysis of adult Galeruca daurica (Coleoptera: Chrysomelidae) at different stages during summer diapause. Comp. Biochem. Phys. D 2019, 29, 351–357. [Google Scholar] [CrossRef]
- Wang, S.; Minter, M.; Homem, R.A.; Michaelson, L.V.; Venthur, H.; Lim, K.S.; Withers, A.; Xi, J.H.; Jones, C.M.; Zhou, J.J. Odorant binding proteins promote flight activity in the migratory insect, Helicoverpa armigera. Mol. Ecol. 2020, 29, 3795–3808. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.M.; Wang, X.H.; Zhang, Y.; Zhang, L.P.; Chen, Q.M.; Zhang, X.L.; Zhao, P.; Xia, Q.Y. Proteome profiling reveals tissue-specific protein expression in male and female accessory glands of the silkworm, Bombyx mori. Amino Acids 2016, 48, 1173–1183. [Google Scholar] [CrossRef]
- Ruttner, F. Biogeography and Taxonomy of Honeybees, 1st ed.; Springer: Berlin, Heidelberg, 1988; pp. 66–78. [Google Scholar]
- Fang, Y.; Song, F.F.; Zhang, L.; Aleku, D.W.; Han, B.; Feng, M.; Li, J.K. Differential antennal proteome comparison of adult honeybee drone, worker and queen (Apis mellifera L.). J. Proteom. 2012, 75, 756–773. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Swiderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkolazka, A.; Paszczynski, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Asano, T.; Seto, Y.; Hashimoto, K.; Kurushima, H. Mini-review an insect-specific system for terrestrialization: Laccase-mediated cuticle formation. Insect Biochem. Mol. Biol. 2019, 108, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.I. The cuticular phenoloxidase in Drosophila virilis. J. Insect Physiol. 1969, 15, 2203–2211. [Google Scholar] [CrossRef]
- Barrett, F.M. Phenoloxidases from larval cuticle of the sheepblowfly, Lucilia cuprina: Characterization, developmental changes, and inhibition by antiphenoloxidase antibodies. Arch. Insect Biochem. Physiol. 1987, 5, 99–118. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S.; Beeman, R.W.; Kanost, M.R.; Kramer, K.J. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc. Natl. Acad. Sci. USA 2005, 102, 11337–11342. [Google Scholar] [CrossRef] [PubMed]
- Arakane, Y.; Lomakin, J.; Beeman, R.W.; Muthukrishnan, S.; Gehrke, S.H.; Kanost, M.R.; Kramer, K.J. Molecular and functional analyses of amino acid decarboxylases involved in cuticle tanning in Tribolium castaneum. J. Biol. Chem. 2009, 284, 16584–16594. [Google Scholar] [CrossRef]
- Xu, L.J.; Voloboueva, L.A.; Ouyang, Y.B.; Emery, J.F.; Giffard, R.G. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 365–374. [Google Scholar] [CrossRef]
- Dong, W.B.; Wang, C.Z.; Li, X.; Huang, T.B.; Li, F.; Wu, S.Y. Glutathione S-Transferase Contributes to the Resistance of Against Lambda-Cyhalothrin by Strengthening Its Antioxidant Defense Mechanisms. Arch. Insect Biochem. 2024, 117, e70010. [Google Scholar] [CrossRef]
- Haas, J.; Hayward, A.; Buer, B.; Maiwald, F.; Nebelsiek, B.; Glaubitz, J.; Bass, C.; Nauen, R. Phylogenomic and functional characterization of an evolutionary conserved cytochrome P450-based insecticide detoxification mechanism in bees. Proc. Natl. Acad. Sci. USA 2022, 119, e2205850119. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, W.X.; Liu, F.; Chen, X.B.; Li, H.; Xu, B.H. Characterization of an cytochrome P450 gene (AccCYP336A1) and its roles in oxidative stresses responses. Gene 2016, 584, 120–128. [Google Scholar] [CrossRef]
- Abbas, M.N.; Gul, I.; Khosravi, Z.; Amarchi, J.I.; Ye, X.; Yu, L.; Siyuan, W.; Cui, H. Molecular characterization, immune functions and DNA protective effects of peroxiredoxin-1 gene in Antheraea pernyi. Mol. Immunol. 2024, 170, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Ikeda, Y. Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep. 2002, 7, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.; Li-Byarlay, H.; Huang, M.H.; Strand, M.K.; Rueppell, O.; Tarpy, D.R. Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci. Rep. 2016, 6, 32023. [Google Scholar] [CrossRef] [PubMed]
- Olgun, T.; Dayioglu, M.; Ozsoy, N. Pesticide and pathogen induced oxidative stress in honey bees (Apis mellifera L.). Mellifera 2020, 20, 32–52. [Google Scholar]
- Belsky, J.; Joshi, N.K. Impact of Biotic and Abiotic Stressors on Managed and Feral Bees. Insects 2019, 10, 233. [Google Scholar] [CrossRef]
- Steinmann, N.; Corona, M.; Neumann, P.; Dainat, B. Overwintering Is Associated with Reduced Expression of Immune Genes and Higher Susceptibility to Virus Infection in Honey Bees. PLoS ONE 2015, 10, e0129956. [Google Scholar] [CrossRef]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive Markers of Honey Bee Colony Collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Gaifullina, L.R.; Saltykova, E.S.; Poskryakov, A.V.; Nikolenko, A.G. Review of the Expression of Antimicrobial Peptide Defensin in Honey Bees Apis mellifera L. J. Apic. Sci. 2012, 56, 115–124. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Gaifullina, L.R.; Saltykova, E.S.; Poskryakov, A.V.; Nikolaenko, A.G. Defensins in the Honeybee Antiinfectious Protection. J. Evol. Biochem. Phys. 2013, 49, 1–9. [Google Scholar] [CrossRef]
- Sun, Y.X.; Zhang, B.X.; Liu, F.F.; Rao, X.J. Functional characterization of Bombyx mori (Lepidoptera: Bombycidae) C-type lectin 5. J. Econ. Entomol. 2023, 116, 1862–1875. [Google Scholar] [CrossRef]
- Danihlík, J.; Aronstein, K.; Petrivalsky, M. Antimicrobial peptides: A key component of honey bee innate immunity. Physiology, biochemistry, and chemical ecology. J. Apic. Res. 2015, 54, 123–136. [Google Scholar] [CrossRef]
- Amici, A.; Grolla, A.A.; Del Grosso, E.; Bellini, R.; Bianchi, M.; Travelli, C.; Garavaglia, S.; Sorci, L.; Raffaelli, N.; Ruggieri, S.; et al. Synthesis and Degradation of Adenosine 5′-Tetraphosphate by Nicotinamide and Nicotinate Phosphoribosyltransferases. Cell Chem. Biol. 2017, 24, 553–564. [Google Scholar] [CrossRef][Green Version]
- Zu, C.F.; Wu, H.C.; Jiang, Y.Y.; Sheng, Y.C.; Wan, X.R.; Wang, J.; Wang, Y. Physiological Role of Nicotinamide Coenzyme I in Cellular Metabolism. In Biology of Nicotinamide Coenzymes: From Basic Science to Clinical Applications; Qin, Z.-H., Ed.; Springer Nature: Singapore, 2025; pp. 217–238. [Google Scholar][Green Version]
- Noma, T. Dynamics of nucleotide metabolism as a supporter of life phenomena. J. Med. Investig. 2005, 52, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit. Rev. Biochem. Mol. 2014, 49, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Genuth, N.R.; Barna, M. The Discovery of Ribosome Heterogeneity and Its Implications for Gene Regulation and Organismal Life. Mol. Cell 2018, 71, 364–374. [Google Scholar] [CrossRef]
- Li, J.H.; Heerman, M.C.; Evans, J.D.; Rose, R.; Li, W.F.; Rodriguez-Garcia, C.; DeGrandi-Hoffman, G.; Zhao, Y.Z.; Huang, S.K.; Li, Z.G.; et al. Pollen reverses decreased lifespan, altered nutritional metabolism and suppressed immunity in honey bees (Apis mellifera) treated with antibiotics. J. Exp. Biol. 2019, 222, jeb202077. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Hu, R.Y.; Li, N.K.; Li, N.N.; Wu, J.L.; Yu, H.M.; Tan, J.; Li, Z.H.; Xu, S.F. Autophagy Is Required to Sustain Increased Intestinal Cell Proliferation during Phenotypic Plasticity Changes in Honey Bee (Apis mellifera). Int. J. Mol. Sci. 2023, 24, 1926. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, X.; Yan, Z.; Hao, M.; Cui, X.; Liu, Z.; Guo, J.; Zhao, Y. The Molecular Drivers of Honey Robbing in Apis mellifera L.: Morphological Divergence and Oxidative-Immune Regulation Mechanisms Based on Proteomic Analysis. Insects 2025, 16, 987. https://doi.org/10.3390/insects16090987
Wang X, Li X, Yan Z, Hao M, Cui X, Liu Z, Guo J, Zhao Y. The Molecular Drivers of Honey Robbing in Apis mellifera L.: Morphological Divergence and Oxidative-Immune Regulation Mechanisms Based on Proteomic Analysis. Insects. 2025; 16(9):987. https://doi.org/10.3390/insects16090987
Chicago/Turabian StyleWang, Xinyu, Xijie Li, Zhanfeng Yan, Mengjuan Hao, Xiao Cui, Zhenxing Liu, Jun Guo, and Yazhou Zhao. 2025. "The Molecular Drivers of Honey Robbing in Apis mellifera L.: Morphological Divergence and Oxidative-Immune Regulation Mechanisms Based on Proteomic Analysis" Insects 16, no. 9: 987. https://doi.org/10.3390/insects16090987
APA StyleWang, X., Li, X., Yan, Z., Hao, M., Cui, X., Liu, Z., Guo, J., & Zhao, Y. (2025). The Molecular Drivers of Honey Robbing in Apis mellifera L.: Morphological Divergence and Oxidative-Immune Regulation Mechanisms Based on Proteomic Analysis. Insects, 16(9), 987. https://doi.org/10.3390/insects16090987







