The Compensatory Response of Photosystem II Photochemistry to Short-Term Insect Herbivory Is Suppressed Under Water Deficit
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Tuta Absoluta
2.3. Experimental Design
2.4. Water Stress Treatments
2.5. Soil and Leaf Water Water Content Determination
2.6. Chlorophyll Fluorescence Analysis
2.7. Statistics
3. Results
3.1. Leaf Water Content and Soil Water Content
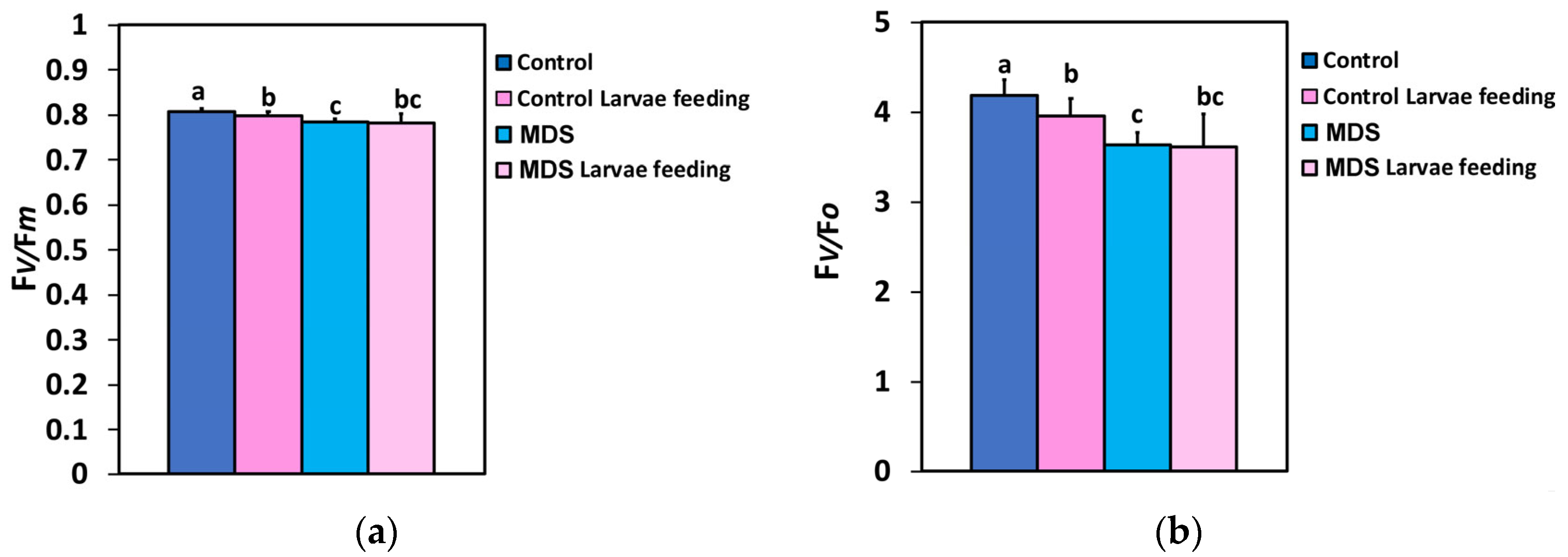
3.2. Impact of Herbivore Feeding on the Maximum Efficiency of Photosystem II Photochemistry and the Efficiency of the the Oxygen-Evolving Complex
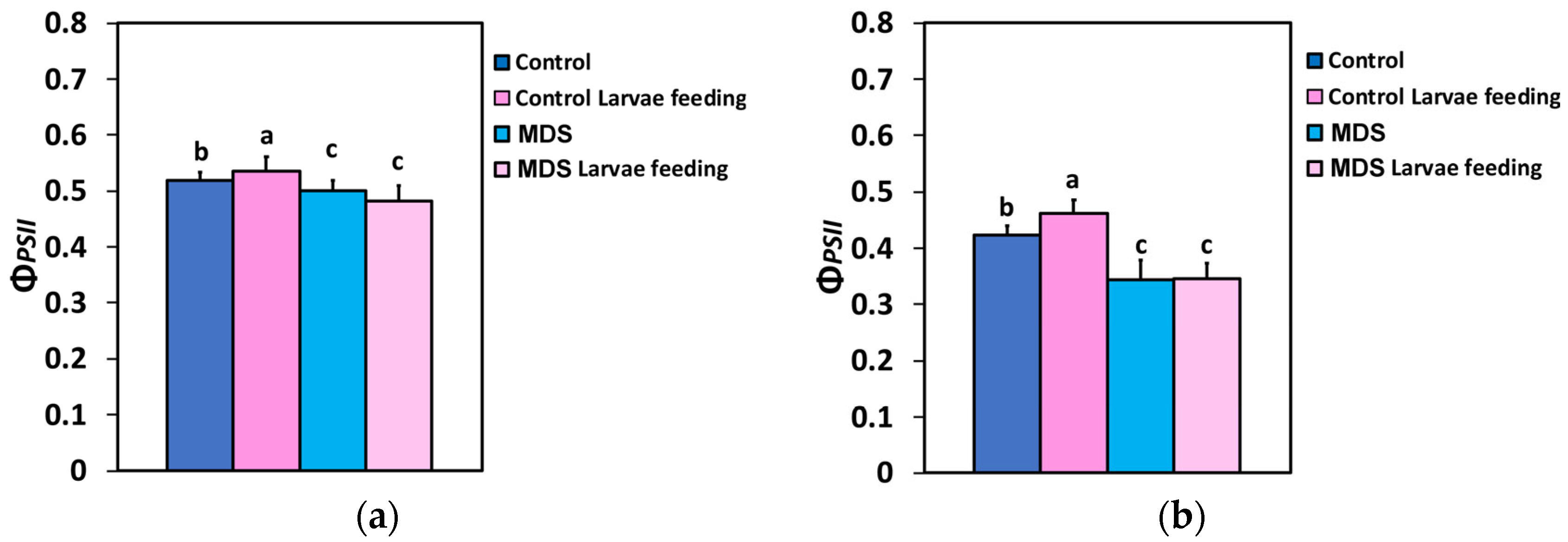
3.3. Impact of Herbivore Feeding on Light Energy Use Efficiency
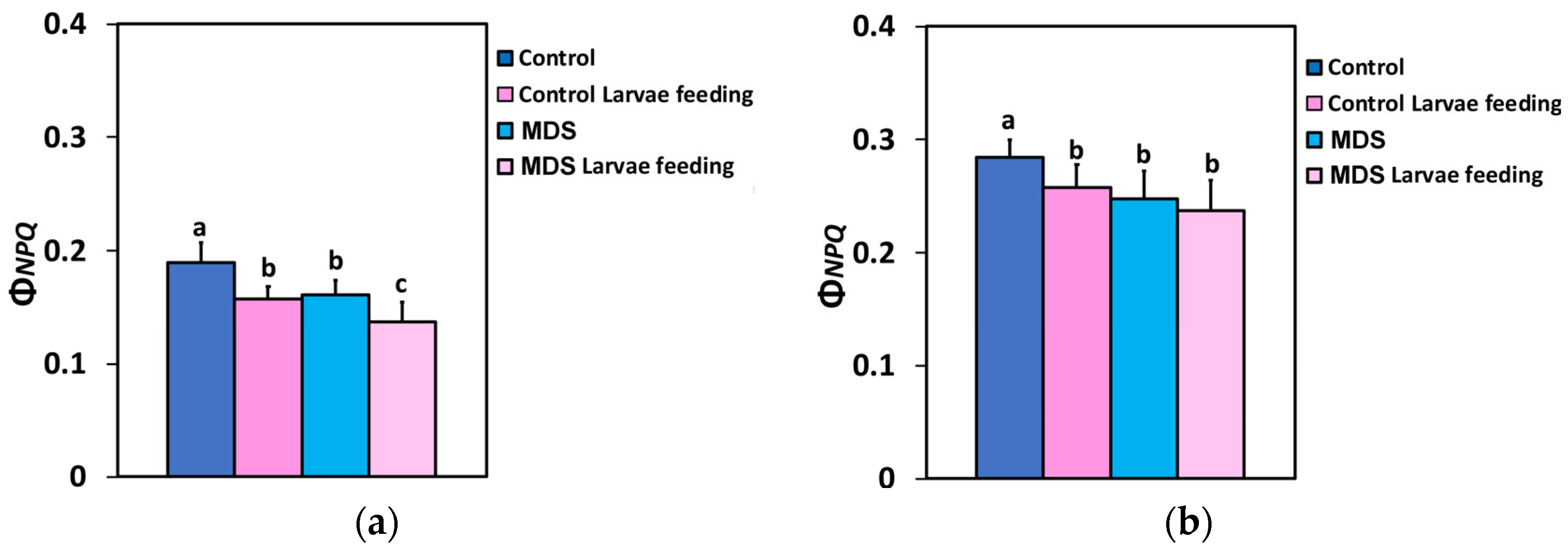
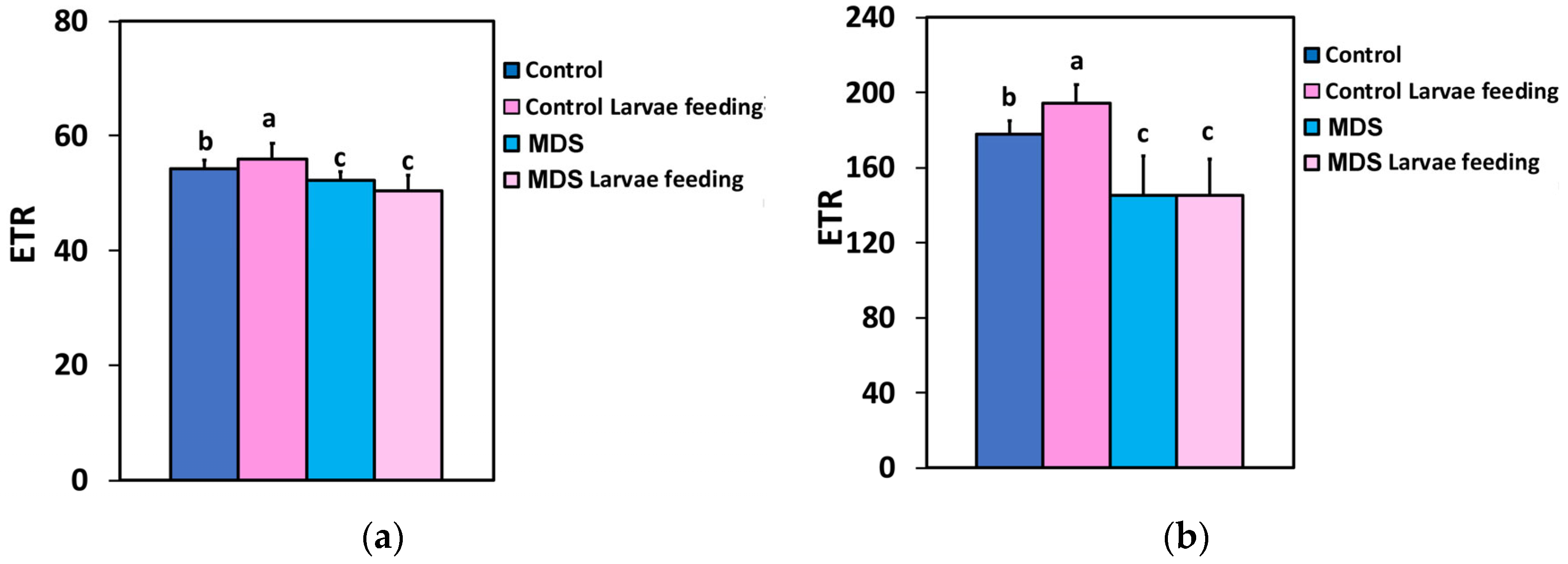
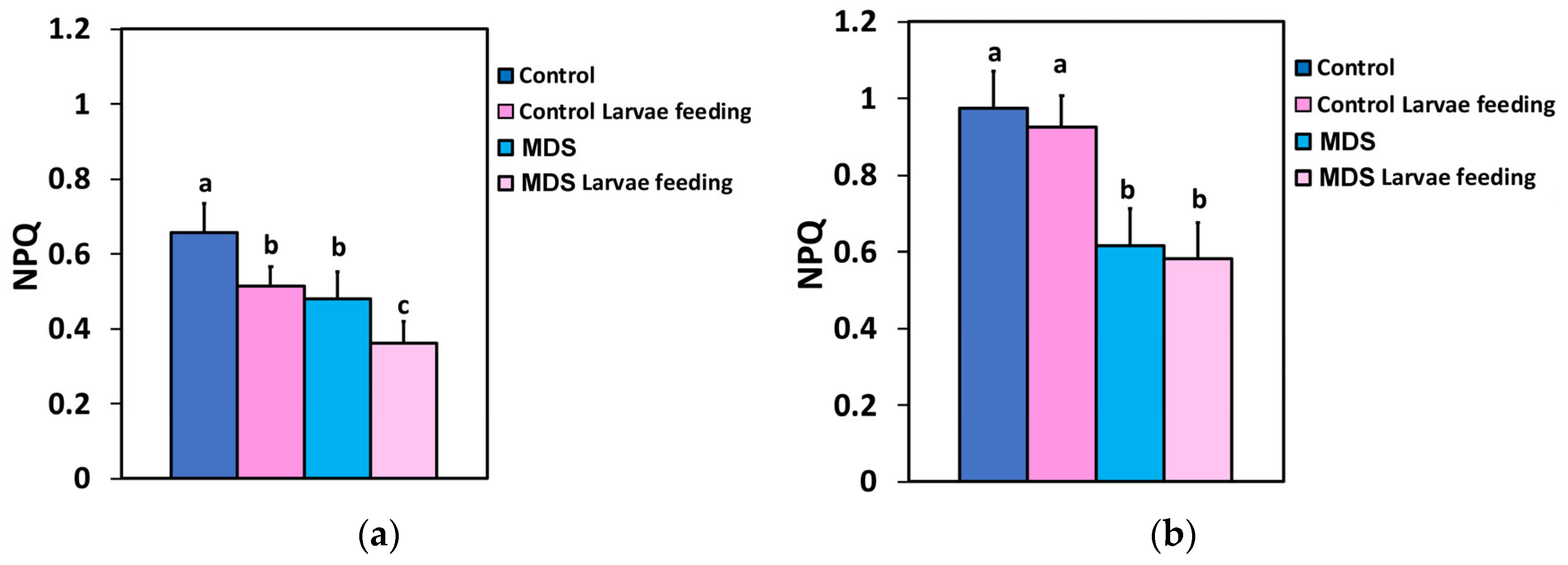
3.4. Impact of Herbivore Feeding on the Electron Transport Rate and the Photoprotective Mechanism
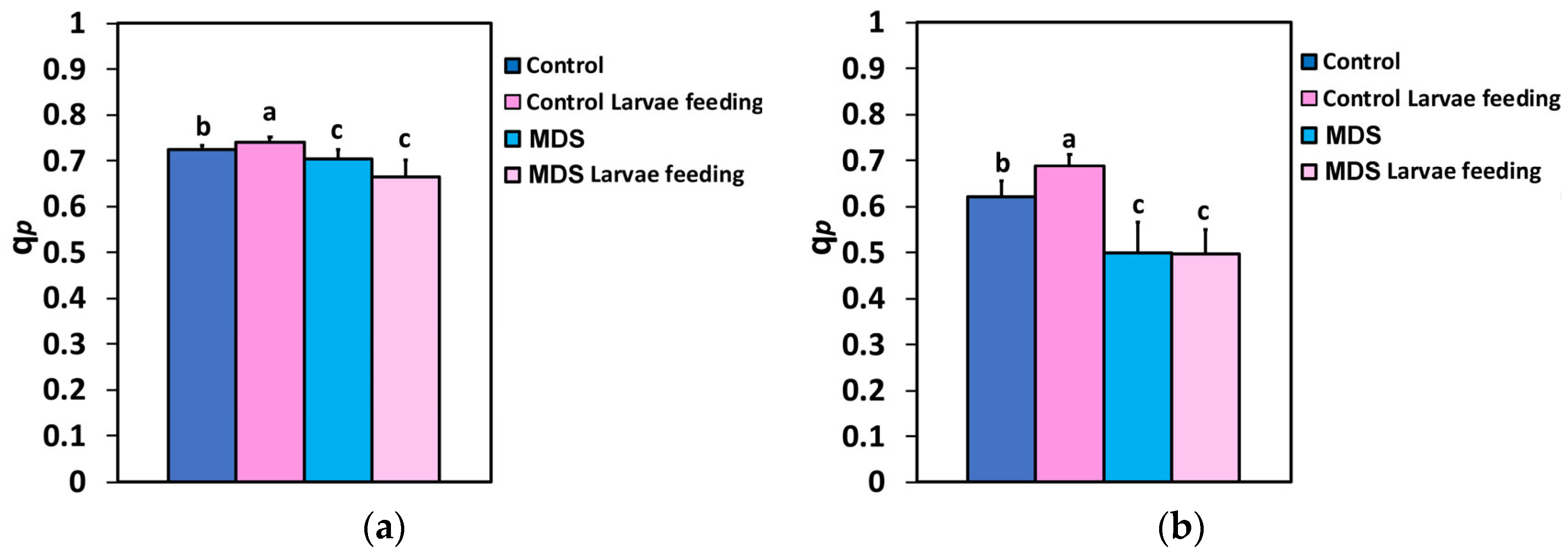
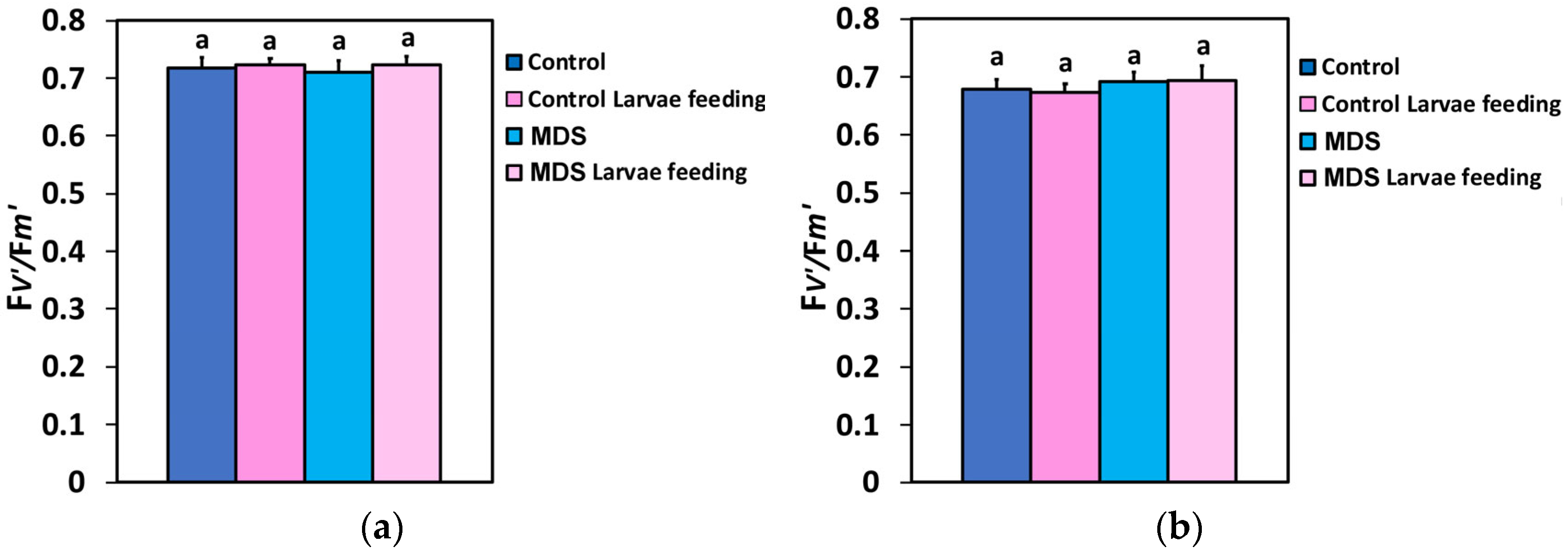
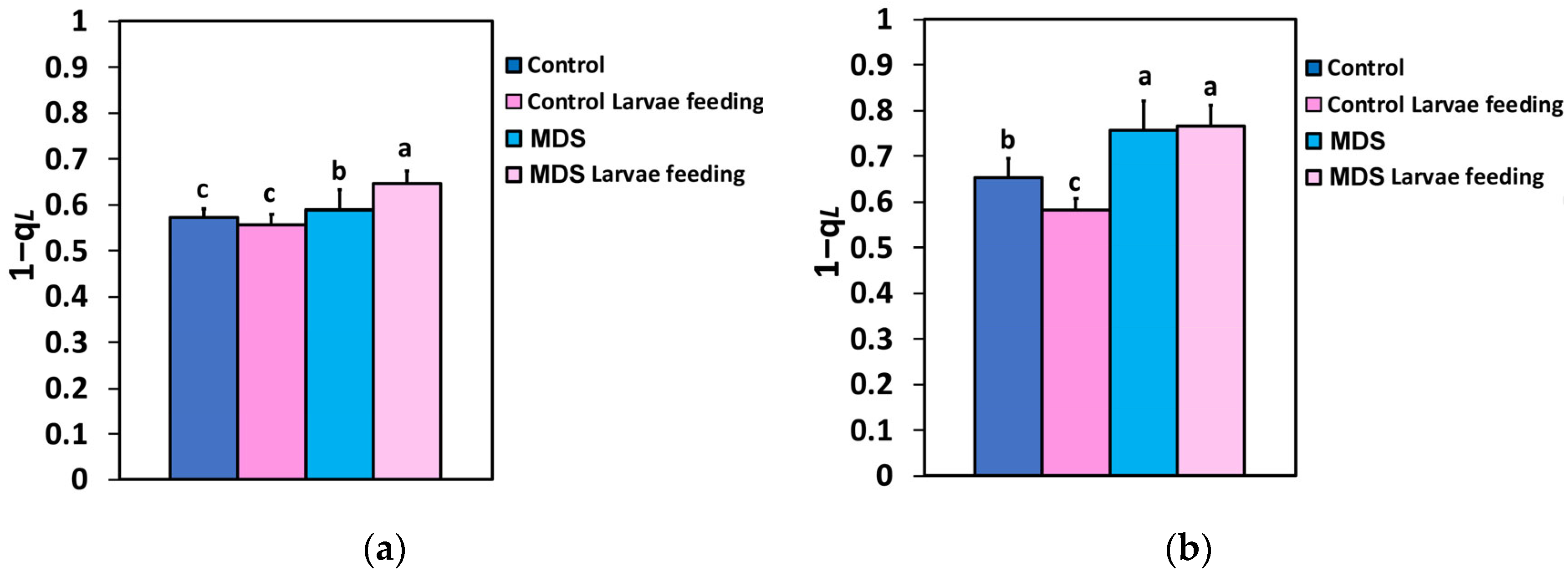
3.5. The Fraction of Open PSII Reaction Centers and Their Efficiency Before and After Herbivore Feeding
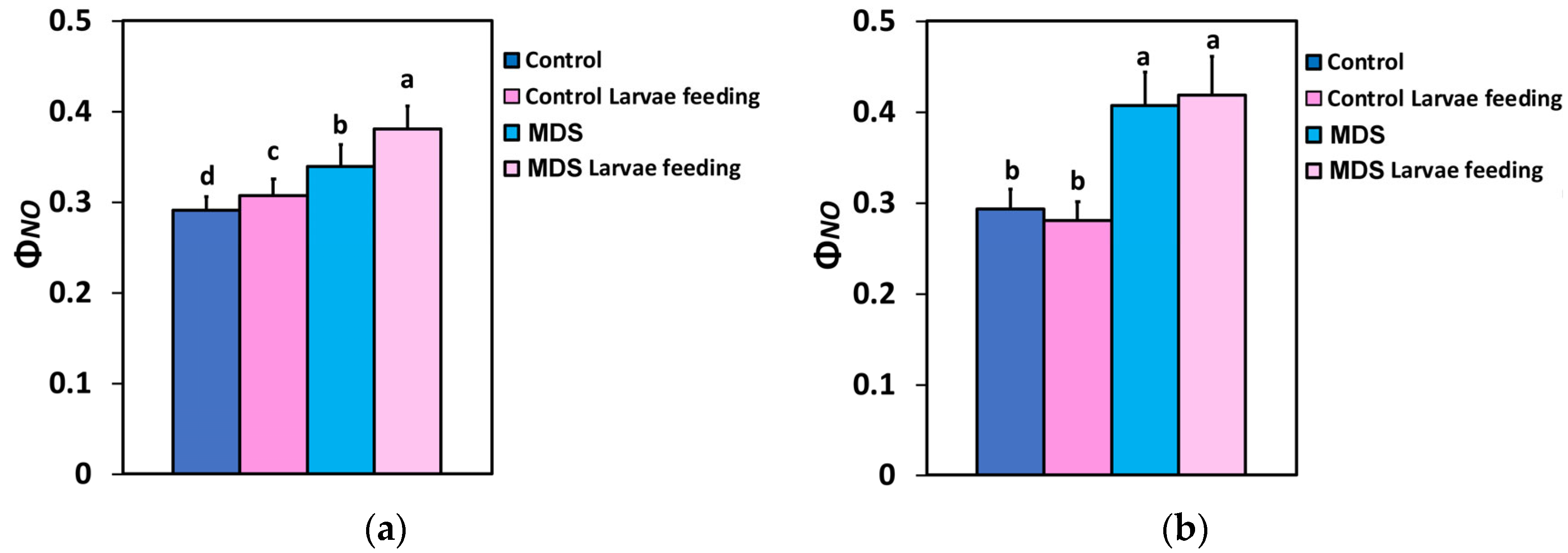
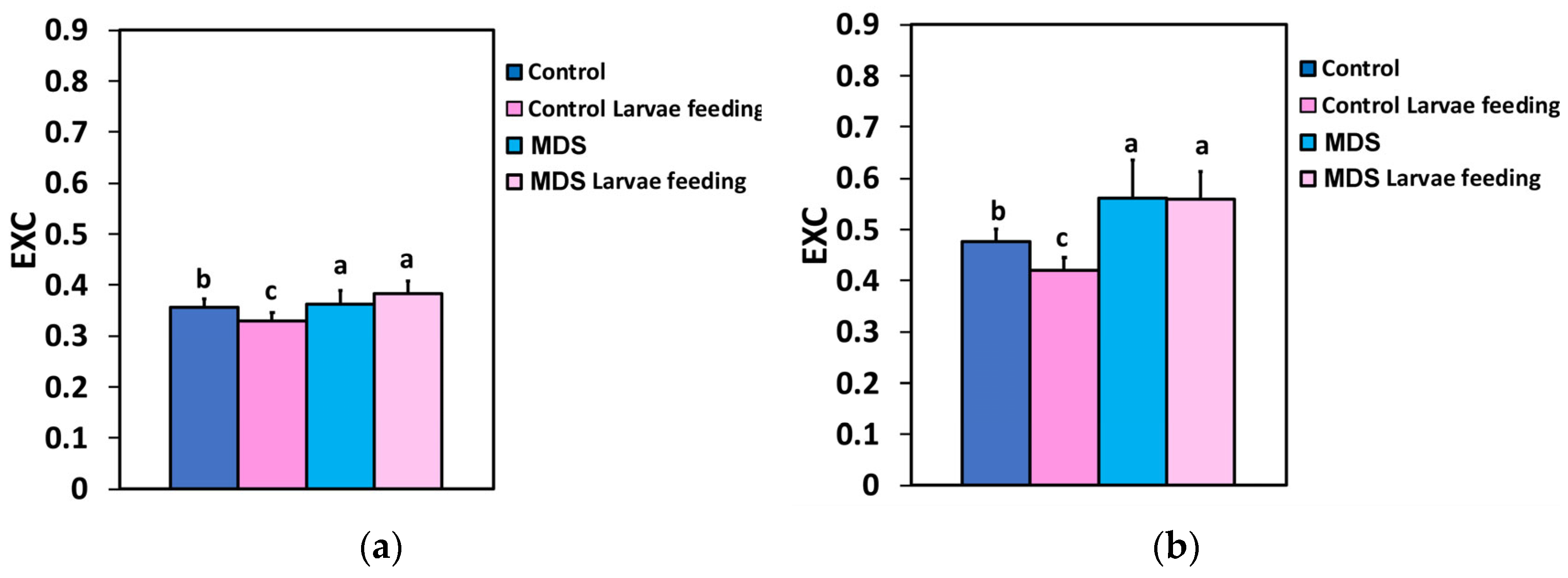
3.6. The Excess Excitation Energy and the Excitation Pressure at PSII Before and After Herbivore Feeding
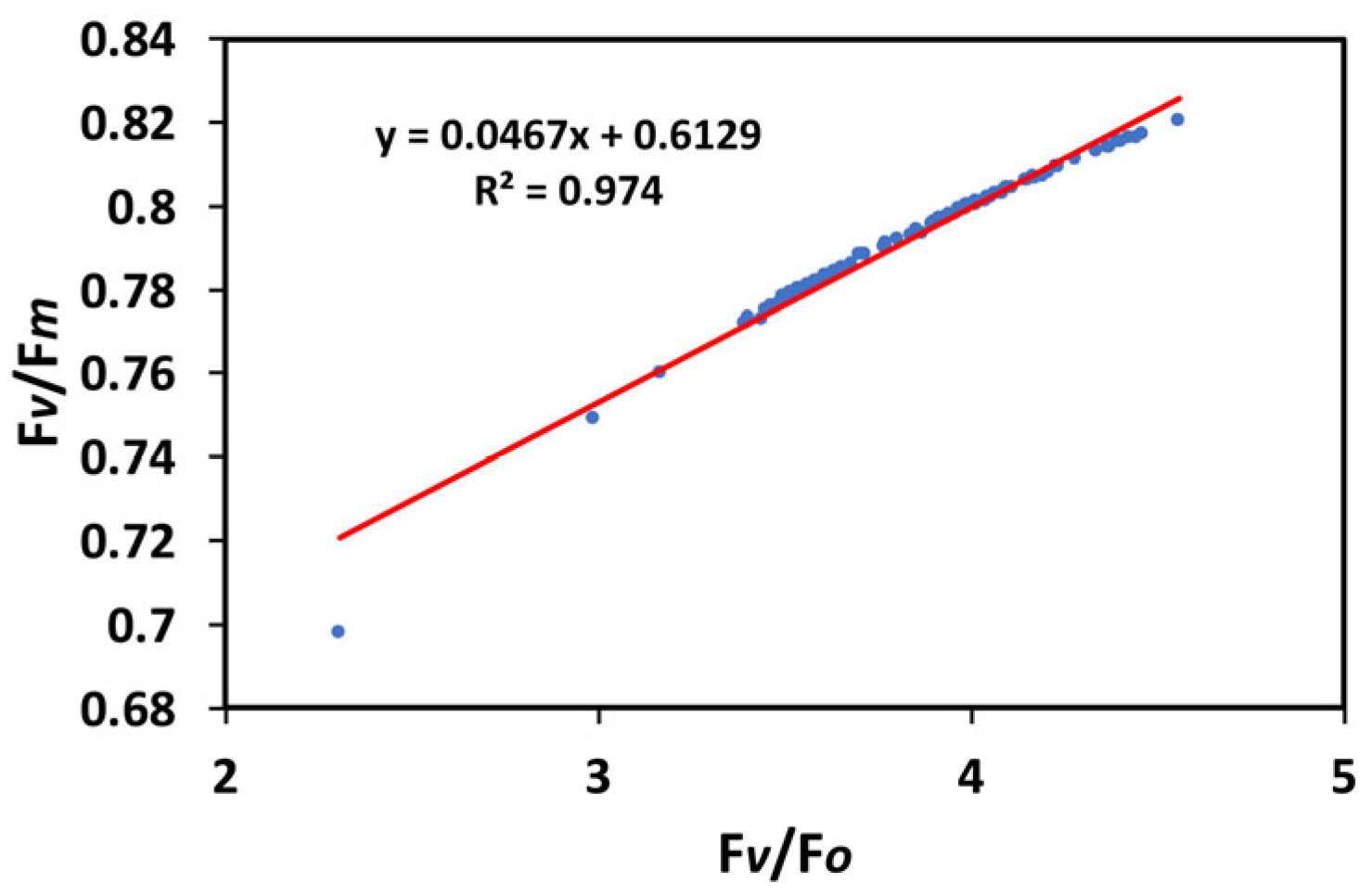
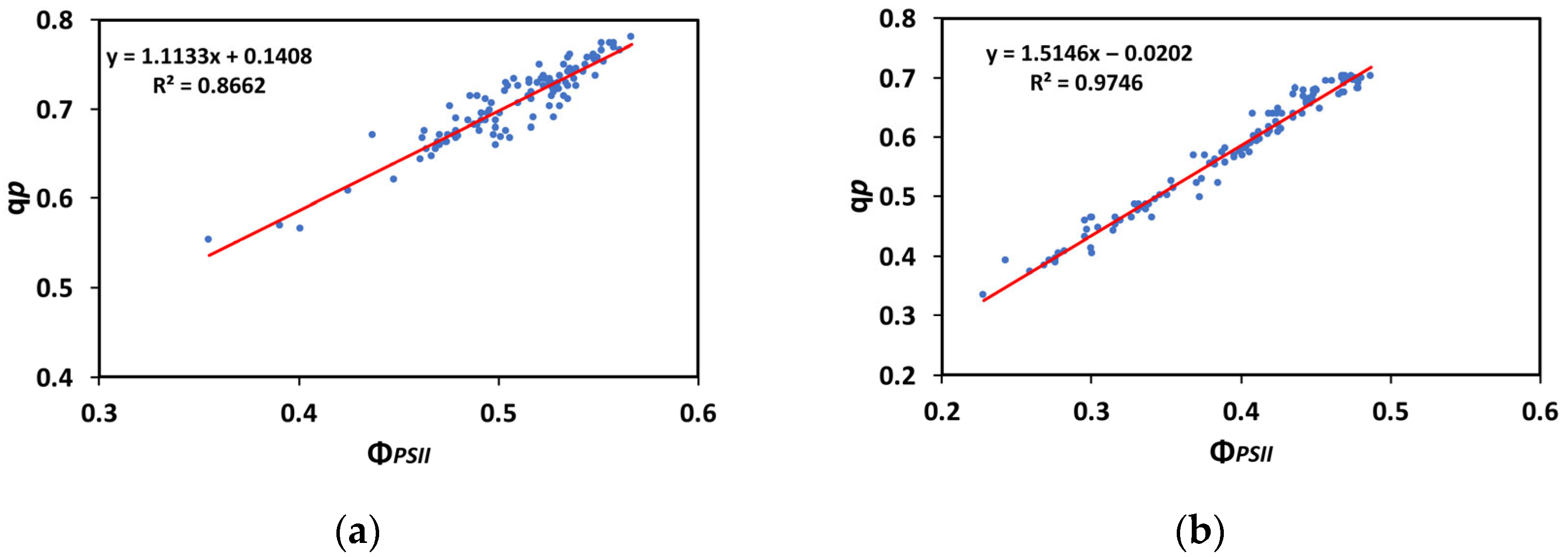
3.7. Correlation of the Efficiency of the Oxygen-Evolving Complex with the Maximum Efficiency of PSII
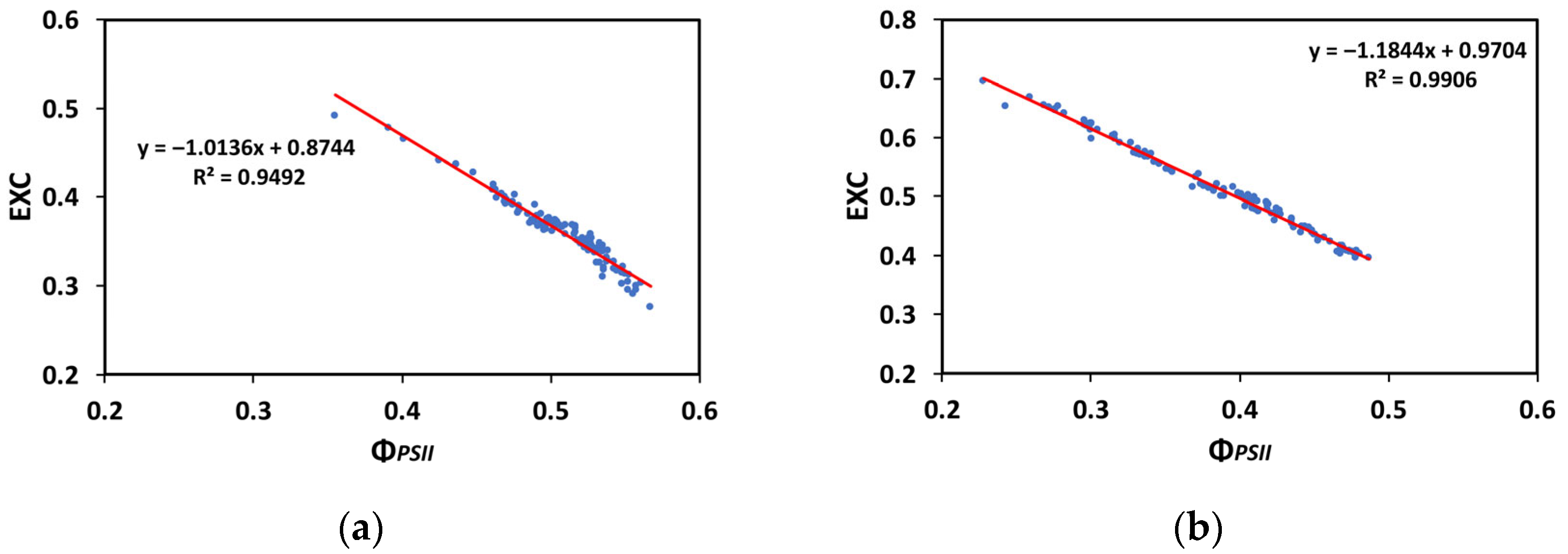
3.8. Correlation of the Excess Excitation Energy with the Effective Quantum Yield of PSII
3.9. Correlation of the Open PSII Reaction Centers with the Effective Quantum Yield of PSII
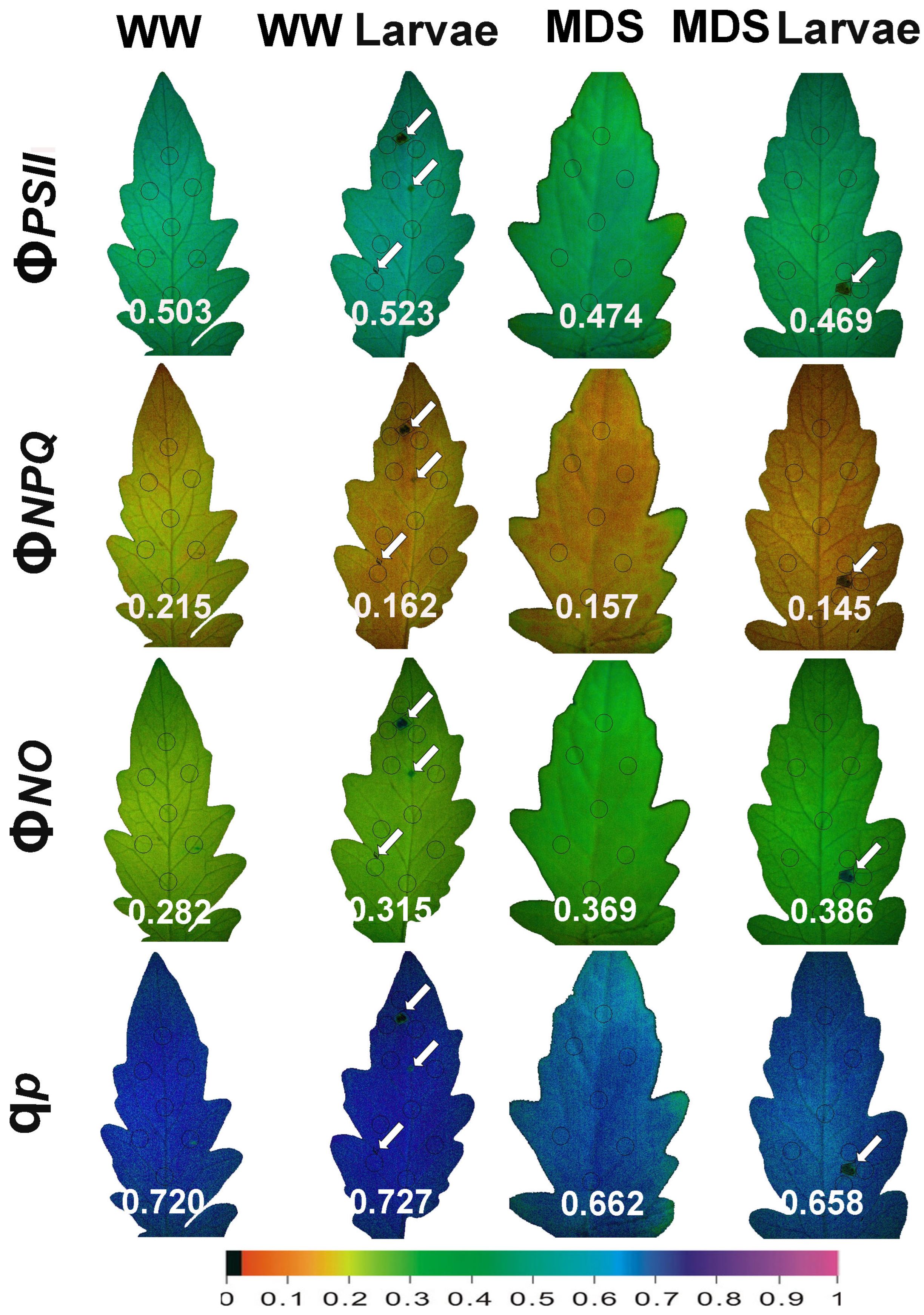
3.10. The Spatiotemporal Heterogeneity of PSII Function Before and After Herbivore Feeding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1-qL | The fraction of closed PSII reaction centres based on the “lake” model |
| 1O2 | Singlet oxygen |
| 3chl | Triplet state of chlorophyll |
| Fm′ | Maximum chlorophyll a fluorescence in the light-adapted leaf |
| Fo | Minimum chlorophyll a fluorescence in the dark-adapted leaf |
| Fo′ | Minimum chlorophyll a fluorescence in the light-adapted leaf |
| Fs | Steady-state photosynthesis |
| Fv′/Fm′ | Efficiency of the open PSII reaction centers |
| Fv/Fm | Maximum efficiency of PSII photochemistry |
| Fv/Fo | Efficiency of the oxygen-evolving complex on the donor side of PSII |
| GLI | Growth light intensity |
| H2O2 | Hydrogen peroxide |
| HIPVs | Herbivore-induced plant volatiles |
| HLI | High light intensity |
| L3 | Third-instar larvae |
| LHCII | Light-harvesting complexes of PSII |
| MDS | Mildly drought-stressed |
| NPQ | Non-photochemical quenching (dissipation of excitation energy as heat) |
| O2•− | Superoxide anion radical |
| OEC | Oxygen-evolving complex |
| PPFD | Photosynthetic photon flux density |
| PSII | Photosystem II |
| QA | Quinone A |
| qp | Photochemical quenching (fraction of open PSII reaction centers representing also the redox state of quinone A) |
| RCs | Reaction centers |
| ROS | Reactive oxygen species |
| SDs | Standard deviations |
| SPs | Saturating pulses |
| VOCs | Volatile organic compounds |
| WW | Well-watered |
| ΦNO | Quantum yield of non-regulated energy loss in PSII |
| ΦNPQ | Quantum yield of regulated non-photochemical energy loss in PSII |
| ΦPSII | Effective quantum yield of PSII photochemistry |
References
- Hu, C.; Thomas, H.R.; Wei, C.; Wu, S.; Zhu, C.; Zhou, Y.; Foyer, C.H.; Yu, J. Herbivory-triggered JA signaling suppresses photosynthesis by inducing photoinhibition in tomato. Plant Commun. 2025, 6, 101237. [Google Scholar] [CrossRef]
- Cao, H.; Ding, R.; Du, T.; Kang, S.; Tong, L.; Chen, J.; Gao, J. A meta-analysis highlights the cross-resistance of plants to drought and salt stresses from physiological, biochemical, and growth levels. Physiol. Plant. 2024, 176, e14282. [Google Scholar] [CrossRef] [PubMed]
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate change alters plant–herbivore interactions. New Phytol. 2021, 229, 1894–1910. [Google Scholar] [CrossRef]
- Gautam, M.; Kariyat, R. Drought and herbivory drive physiological and phytohormonal changes in soybean (Glycine max Merril): Insights from a meta-analysis. Plant Cell Environ. 2025, in press. [CrossRef] [PubMed]
- Adejumo, M.A.; Olowolaju, E.D.; Okunlola, G.O.; Akinyemi, D.S. Symptom expression and severity indices of Solanum lycopersicum L. and Solanum melongena L. at different growth stages under water deficit. Discov. Plants 2025, 2, 171. [Google Scholar] [CrossRef]
- Sperdouli, I.; Mellidou, I.; Moustakas, M. Harnessing chlorophyll fluorescence for phenotyping analysis of wild and cultivated tomato for high photochemical efficiency under water deficit for climate change resilience. Climate 2021, 9, 154. [Google Scholar] [CrossRef]
- Moustaka, J.; Sperdouli, I.; Moustakas, M. Light energy use efficiency in photosystem ΙΙ of tomato is related to leaf age and light intensity. Crops 2024, 4, 623–635. [Google Scholar] [CrossRef]
- Moustaka, J.; Sperdouli, I.; Panteris, E.; Adamakis, I.D.S.; Moustakas, M. Aspirin foliar spray-induced changes in light energy use efficiency, chloroplast ultrastructure, and ROS generation in tomato. Int. J. Mol. Sci. 2025, 26, 1368. [Google Scholar] [CrossRef]
- Nilson, S.E.; Assmann, S.M. The control of transpiration. Insights from Arabidopsis. Plant Physiol. 2007, 143, 19–27. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. A better energy allocation of absorbed light in photosystem II and less photooxidative damage contribute to acclimation of Arabidopsis thaliana young leaves to water deficit. J. Plant Physiol. 2014, 171, 587–593. [Google Scholar] [CrossRef]
- Zhao, T.; Dai, A. The magnitude and causes of global drought changes in the twenty-first century under a low–severe emissions scenario. J. Clim. 2015, 28, 4490–4512. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-stage detection of biotic and abiotic stress on plants by chlorophyll fluorescence imaging analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, W.; Du, T.; Kang, S.; Davies, W.J. Responses of water accumulation and solute metabolism in tomato fruit to water scarcity and implications for main fruit quality variables. J. Exp. Bot. 2020, 71, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early drought stress warning in plants: Color pictures of photosystem II photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agr. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Muktadir, M.A.; Adhikari, K.N.; Ahmad, N.; Merchant, A. Chemical composition and reproductive functionality of contrasting faba bean genotypes in response to water deficit. Physiol. Plant. 2021, 172, 540–551. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Ann. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Culliney, T. Crop losses to arthropods. In Integrated Pest Management; Pimentel, D., Peshin, R., Eds.; Springer: Dordrecht, Germany, 2014; pp. 201–225. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The evolution of fungicide resistance. Adv. Appl. Microbiol. 2015, 90, 29–92. [Google Scholar]
- Sparks, T.C.; Storer, N.; Porter, A.; Slater, R.; Nauen, R. Insecticide resistance management and industry: The origins and evolution of the Insecticide Resistance Action Committee (IRAC) and the mode of action classification scheme. Pest. Manag. Sci. 2021, 77, 2609–2619. [Google Scholar] [CrossRef]
- Gutbrodt, B.; Dorn, S.; Mody, K. Drought stress affects constitutive but not induced herbivore resistance in apple plants. Arthropod-Plant Interact. 2012, 6, 171–179. [Google Scholar] [CrossRef]
- Shivaramu, S.; Jayanthi, P.D.K.; Kempraj, V.; Anjinappa, R.; Nandagopal, B.; Chakravarty, A.K. What signals do herbivore-induced plant volatiles provide conspecific herbivores? Arthropod-Plant Interact. 2017, 11, 815–823. [Google Scholar] [CrossRef]
- Damodaram, K.J.P.; Gadad, H.S.; Parepally, S.K.; Vaddi, S.; Hunashikatti, R.H.; Bhat, R.M. Low moisture stress influences plant volatile emissions affecting herbivore interactions in tomato, Solanum lycopersicum. Ecol. Entomol. 2021, 46, 637–650. [Google Scholar] [CrossRef]
- Lin, P.A.; Paudel, S.; Bin Zainuddin, N.; Tan, C.W.; Helms, A.; Ali, J.G.; Felton, G.W. Low water availability enhances volatile-mediated direct defences but disturbs indirect defences against herbivores. J. Ecol. 2022, 110, 2759–2771. [Google Scholar] [CrossRef]
- Salerno, G.; Frati, F.; Marino, G.; Ederli, L.; Pasqualini, S.; Loreto, F.; Colazza, S.; Centritto, M. Effects of water stress on emission of volatile organic compounds by Vicia faba, and consequences for attraction of the egg parasitoid Trissolcus basalis. J. Pest Sci. 2017, 90, 635–647. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Munawar, A.; Zhu, J.; Zhong, J.; Zhang, Y.; Guo, H.; Zhu, Z.; Baldwin, I.T.; Zhou, W. Manipulating stomatal aperture by silencing StSLAC1 affects potato plant–herbivore–parasitoid tritrophic interactions under drought stress. New Phytol. 2025, 245, 2133–2149. [Google Scholar] [CrossRef] [PubMed]
- Weldegergis, B.T.; Zhu, F.; Poelman, E.H.; Dicke, M. Drought stress affects plant metabolites and herbivore preference but not host location by its parasitoids. Oecologia 2015, 177, 701–713. [Google Scholar] [CrossRef]
- Claeys, H.; Dirk, I. The agony of choice: How plants balance growth and survival under water-limiting conditions. Plant Physiol. 2013, 162, 1768–1779. [Google Scholar] [CrossRef]
- Copolovici, L.; Astrid, K.; Triinu, R.; Niinemets, Ü. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ. Exp. Bot. 2014, 100, 55–63. [Google Scholar] [CrossRef]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Rahman, S.; Rostás, M.; Vosteen, I. Drought aggravates plant stress by favouring aphids and weakening indirect defense in a sugar beet tritrophic system. J. Pest Sci. 2025, 98, 549–564. [Google Scholar] [CrossRef]
- Zhao, C.; Han, W.H.; Xiong, Y.D.; Ji, S.X.; Du, H.; Chi, Y.J.; Chen, N.; Wu, H.; Liu, S.S.; Wang, X.W. Drought suppresses plant salicylic acid defence against herbivorous insects by down-regulating the expression of ICS1 via NAC transcription factor. Plant Stress 2025, in press. [CrossRef]
- Tattini, M.; Velikova, V.; Vickers, C.; Brunetti, C.; Di Ferdinando, M.; Trivellini, A.; Fineschi, S.; Agati, G.; Ferrini, F.; Loreto, F. Isoprene production in transgenic tobacco alters isoprenoid, non-structural carbohydrate and phenylpropanoid metabolism, and protects photosynthesis from drought stress. Plant Cell Environ. 2014, 37, 1950–1964. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.M.L.; Nascimento, L.S.Q.; Souza, V.C.; Matos, E.M.; Fortini, E.A.; Grazul, R.M.; Santos, M.O.; Soltis, D.E.; Soltis, P.S.; Otono, W.C.; et al. Water stress modulates terpene biosynthesis and morphophysiology at different ploidal levels in Lippia alba (Mill.) N. E. Brown (Verbenaceae). Protoplasma 2024, 261, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef]
- Moustaka, J.; Meyling, N.V.; Hauser, T.P. Induction of a compensatory photosynthetic response mechanism in tomato leaves upon short time feeding by the chewing insect Spodoptera exigua. Insects 2021, 12, 562. [Google Scholar] [CrossRef]
- Thomson, V.P.; Cunningham, S.A.; Ball, M.C.; Nicotra, A.B. Compensation for herbivory by Cucumis sativus through increased photosynthetic capacity and efficiency. Oecologia 2003, 134, 167–175. [Google Scholar] [CrossRef]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 29, 527–557. [Google Scholar] [CrossRef]
- Peterson, R.K.D.; Higley, L.G. Biotic Stress and Yield Loss, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9780849311451. [Google Scholar]
- Delaney, K.J. Injured and uninjured leaf photosynthetic responses after mechanical injury on Nerium oleander leaves, and Danaus plexippus herbivory on Asclepias curassavica leaves. Plant Ecol. 2008, 199, 187–200. [Google Scholar] [CrossRef]
- Welter, S.C. Arthropod impact on plant gas exchange. In Insect-Plant Interactions; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 135–164. ISBN 13: 978-0-429-29091-6. [Google Scholar]
- Delaney, K.J.; Higley, L.G. An insect countermeasure impacts plant physiology: Midrib vein cutting, defoliation and leaf photosynthesis. Plant Cell Environ. 2006, 29, 1245–1258. [Google Scholar] [CrossRef]
- Retuerto, R.; Fernández-Lema, B.; Obeso, J.R. Changes in photochemical efficiency in response to herbivory and experimental defoliation in the dioecious tree Ilex aquifolium. Int. J. Plant Sci. 2006, 167, 279–289. [Google Scholar] [CrossRef]
- Zangerl, A.R.; Hamilton, J.G.; Miller, T.J.; Crofts, A.R.; Oxborough, K.; Berenbaum, M.R.; DeLucia, E.H. Impact of folivory on photosynthesis is greater than the sum of its holes. Proc. Natl. Acad. Sci. USA 2002, 99, 1088–1091. [Google Scholar] [CrossRef]
- Lenk, S.; Chaerle, L.; Pfündel, E.E.; Langsdorf, G.; Hagenbeek, D.; Lichtenthaler, H.K.; Van Der Straeten, D.; Buschmann, C. Multispectral fluorescence and reflectance imaging at the leaf level and its possible applications. J. Exp. Bot. 2007, 58, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Saglam, A.; Chaerle, L.; Van Der Straeten, D.; Valcke, R. Promising monitoring techniques for plant science: Thermal and chlorophyll fluorescence imaging. In Photosynthesis, Productivity, and Environmental Stress, 1st ed.; Ahmad, P., Ahanger, M.A., Alyemeni, M.N., Alam, P., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 241–266. [Google Scholar]
- Sperdouli, I.; Andreadis, S.; Moustaka, J.; Panteris, E.; Tsaballa, A.; Moustakas, M. Changes in light energy utilization in photosystem II and reactive oxygen species generation in potato leaves by the pinworm Tuta absoluta. Molecules 2021, 26, 2984. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Andreadis, S.S.; Adamakis, I.S.; Moustaka, J.; Koutsogeorgiou, E.I.; Moustakas, M. Reactive oxygen species initiate defence responses of potato photosystem II to sap-sucking insect feeding. Insects 2022, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, S.A.; Scholes, J.D. Chlorophyll fluorescence imaging of plant-pathogen interactions. Protoplasma 2010, 247, 163–175. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Guidi, L.; Calatayud, A. Non-invasive tools to estimate stress-induced changes in photosynthetic performance in plants inhabiting Mediterranean areas. Environ. Exp. Bot. 2014, 103, 42–52. [Google Scholar] [CrossRef]
- Moustakas, M.; Calatayud, A.; Guidi, L. Chlorophyll fluorescence imaging analysis in biotic and abiotic stress. Front. Plant Sci. 2021, 12, 658500. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping plant responses to biotic stress by chlorophyll fluorescence imaging. Front. Plant Sci. 2019, 10, 1135. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Wolosiuk, R.A.; Malkin, R. Photosynthesis. In Biochemistry & Molecular Biology of Plants, 2nd ed.; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 508–566. [Google Scholar]
- Lu, Y.; Yao, J. Chloroplasts at the crossroad of photosynthesis, pathogen infection and plant defence. Int. J. Mol. Sci. 2018, 19, 3900. [Google Scholar] [CrossRef]
- Barber, J. Photosynthetic energy conversion: Natural and artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic approach. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Imaizumi, K.; Ifuku, K. Photosystem II: Commonality and diversity with emphasis on the extrinsic subunits. Plant Cell Physiol. 2025, in press. [CrossRef] [PubMed]
- Zlobin, I.E.; Ivanov, Y.V.; Kartashov, A.V.; Sarvin, B.A.; Stavrianidi, A.N.; Kreslavski, V.D.; Kuznetsov, V.V. Impact of weak water deficit on growth, photosynthetic primary processes and storage processes in pine and spruce seedlings. Photosynth. Res. 2019, 139, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Ouzounidou, G.; Sperdouli, I.; Moustakas, M. Photosystem II is more sensitive than photosystem I to Al3+ induced phytotoxicity. Materials 2018, 11, 1772. [Google Scholar] [CrossRef]
- Pereyra, P.C.; Sanchez, N.E. Effect of two solanaceous plants on developmental and population parameters of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2006, 35, 671–676. [Google Scholar] [CrossRef]
- Ghoneim, K. Predatory insects and arachnids as potential biological control agents against the invasive tomato leafminer, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae), in perspective and prospective. J. Entomol. Zool. Stud. 2014, 2, 52–71. [Google Scholar]
- Heidari, N.; Sedaratian-Jahromi, A.; Ghane-Jahromi, M.; Zalucki, M.P. How bottom-up effects of different tomato cultivars affect population responses of Tuta absoluta (Lep.: Gelechiidae): A case study on host plant resistance. Arthropod-Plant Interact. 2020, 14, 181–192. [Google Scholar] [CrossRef]
- Fonseca Cardoso, E.; Lopes, A.R.; Dotto, M.; Pirola, K.; Moreno Giarola, C. Phenological growth stages of Gaúcho tomato based on the BBCH scale. Com. Sci. 2021, 12, e3490. [Google Scholar]
- Moustakas, M.; Panteris, E.; Moustaka, J.; Aydın, T.; Bayçu, G.; Sperdouli, I. Modulation of photosystem II function in celery via foliar-applied salicylic acid during gradual water deficit stress. Int. J. Mol. Sci. 2024, 25, 6721. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Moustakas, M. Spatio-temporal heterogeneity in Arabidopsis thaliana leaves under drought stress. Plant Biol. 2012, 14, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Panteris, E.; Adamakis, I.D.S.; Tanou, G.; Giannakoula, A.; Eleftheriou, E.P.; Moustakas, M. High anthocyanin accumulation in poinsettia leaves is accompanied by thylakoid membrane unstacking, acting as a photoprotective mechanism, to prevent ROS formation. Environ. Exp. Bot. 2018, 154, 44–55. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—Calculation of qP and Fv’/Fm’ without measuring Fo’. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Balestrini, R.; Chitarra, W.; Ghirardo, A.; Nardini, A.; Nerva, L. A stressful life: How plants cope with multiple biotic and abiotic adverse factors. Plant Stress 2022, 5, 100095. [Google Scholar] [CrossRef]
- Moustakas, M. Molecular mechanisms of plant abiotic stress tolerance. Int. J. Mol. Sci. 2025, 26, 2731. [Google Scholar] [CrossRef]
- Coolen, S.; Proietti, S.; Hickman, R.; Davila Olivas, N.H.; Huang, P.P.; Van Verk, M.C.; Van Pelt, J.A.; Wittenberg, A.H.; De Vos, M.; Prins, M.; et al. Transcriptome dynamics of Arabidopsis during sequential biotic and abiotic stresses. Plant J. 2016, 86, 249–267. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Powers, J.; Cochard, H.; Choat, B. Hanging by a thread? Forests and drought. Science 2020, 368, 261–266. [Google Scholar] [CrossRef]
- Calabrese, E.J. Evidence that hormesis represents an ‘‘overcompensation’’ response to a disruption in homeostasis. Ecotoxicol. Environ. Saf. 1999, 42, 135–137. [Google Scholar] [CrossRef]
- Sperdouli, I.; Ouzounidou, G.; Moustakas, M. Hormesis responses of photosystem II in Arabidopsis thaliana under water deficit stress. Int. J. Mol. Sci. 2023, 24, 9573. [Google Scholar] [CrossRef]
- Sperdouli, I.; Giannousi, K.; Moustaka, J.; Antonoglou, O.; Dendrinou-Samara, C.; Moustakas, M. Responses of tomato photosystem II photochemistry to pegylated zincdoped ferrite nanoparticles. Nanomaterials 2025, 15, 288. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ Int. 2019, 131, 105044. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M.; Harayama, H. On the non-monotonic, hormetic photoprotective response of plants to stress. Dose-Response 2019, 17, 1559325819838420. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Calabrese, E.J. Hormesis: The dose response for the 21st century: The future has arrived. Toxicology 2019, 425, 152249. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: Highly generalizable and beyond laboratory. Trends Plant Sci. 2020, 25, 1076–1086. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef]
- Małkowski, E.; Sitko, K.; Szopiński, M.; Gieroń, Ż.; Pogrzeba, M.; Kalaji, H.M.; Zieleźnik-Rusinowska, P. Hormesis in plants: The role of oxidative stress, auxins and photosynthesis in corn treated with Cd or Pb. Int. J. Mol. Sci. 2020, 21, 2099. [Google Scholar] [CrossRef]
- Agathokleous, E. The rise and fall of photosynthesis: Hormetic dose response in plants. J. For. Res. 2021, 32, 889–898. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Accumulator plants and hormesis. Environ. Pollut. 2021, 274, 116526. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Jiao, Q.; Agathokleous, E.; Liu, H.; Li, G.; Zhang, J.; Fahad, S.; Jiang, Y. Hormetic effects of zinc on growth and antioxidant defense system of wheat plants. Sci. Total Environ. 2022, 807, 150992. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Sonne, C.; Benelli, G.; Calabrese, E.J.; Guedes, R.N.C. Low-dose chemical stimulation and pest resistance threaten global crop production. Sci. Total Environ. 2023, 878, 162989. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Şaş, B.; İşgören, S.; Moustaka, J.; Morales, F. Mechanistic approach on melatonin-induced hormesis of photosystem II function in the medicinal plant Mentha spicata. Plants 2023, 12, 4025. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A compelling platform for sophisticated plant science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Environmental hormesis of non-specific and specific adaptive mechanisms in plants. Sci. Total Environ. 2022, 804, 150059. [Google Scholar] [CrossRef]
- Kato, M.C.; Hikosaka, K.; Hirotsu, N.; Makino, A.; Hirose, T. The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in photosystem II. Plant Cell Physiol. 2003, 44, 318–325. [Google Scholar] [CrossRef]
- Hakala, M.; Tuominen, I.; Keränen, M.; Tyystjärvi, T.; Tyystjärvi, E. Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II. Biochim. Biophys. Acta-Bioenerg. 2005, 1706, 68–80. [Google Scholar] [CrossRef]
- Ohnishi, N.; Allakhverdiev, S.I.; Takahashi, S.; Higashi, S.; Watanabe, M.; Nishiyama, Y.; Murata, N. Two-step mechanism of photodamage to photosystem II: Step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 2005, 44, 8494–8499. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of Photosystem II and photodamage of the oxygen evolving manganese cluster. Coord. Chem. Rev. 2008, 252, 361–376. [Google Scholar] [CrossRef]
- Oguchi, R.; Terashima, I.; Chow, W.S. The involvement of dual mechanisms of photoinactivation of photosystem II in Capsicum annuum L. plants. Plant Cell Physiol. 2009, 50, 1815–1825. [Google Scholar] [CrossRef]
- Campbell, D.A.; Tyystjärvi, E. Parameterization of photosystem II photoinactivation and repair. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Zavafer, A.; Koinuma, W.; Chow, W.S.; Cheah, M.H.; Mino, H. Mechanism of photodamage of the oxygen evolving Mn cluster of photosystem II by excessive light energy. Sci. Rep 2017, 7, 7604. [Google Scholar] [CrossRef]
- Oguchi, R.; Terashima, I.; Kou, J.; Chow, W.S. Operation of dual mechanisms that both lead to photoinactivation of photosystem II in leaves by visible light. Physiol. Plant. 2011, 142, 47–55. [Google Scholar] [CrossRef]
- Govindachary, S.; Bukhov, N.G.; Joly, D.; Carpentier, R. Photosystem II inhibition by moderate light under low temperature in intact leaves of chilling-sensitive and -tolerant plants. Physiol. Plant. 2004, 121, 322–333. [Google Scholar] [CrossRef]
- Pellegrini, E.; Carucci, M.G.; Campanella, A.; Lorenzini, G.; Nali, C. Ozone stress in Melissa officinalis plants assessed by photosynthetic function. Environ. Exp. Bot. 2011, 73, 94–101. [Google Scholar] [CrossRef]
- Siddiqui, H.; Ahmed, K.B.M.; Hayat, S. Comparative effect of 28-homobrassinolide and 24-epibrassinolide on the performance of different components influencing the photosynthetic machinery in Brassica juncea L. Plant Physiol. Biochem. 2018, 129, 198–212. [Google Scholar] [CrossRef]
- Mosadegh, H.; Trivellini, A.; Lucchesini, M.; Ferrante, A.; Maggini, R.; Vernieri, P.; Mensuali Sodi, A. UV-B physiological changes under conditions of distress and eustress in sweet basil. Plants 2019, 8, 396. [Google Scholar] [CrossRef]
- Gohari, G.; Farhadi, H.; Panahirad, S.; Zareei, E.; Labib, P.; Jafari, H.; Mahdavinia, G.; Hassanpouraghdam, M.B.; Ioannou, A.; Kulak, M.; et al. Mitigation of salinity impact in spearmint plants through the application of engineered chitosan-melatonin nanoparticles. Int. J. Biol. Macromol. 2023, 224, 893–907. [Google Scholar] [CrossRef]
- Kalisz, A.; Kornaś, A.; Skoczowski, A.; Oliwa, J.; Jurkow, R.; Gil, J.; Sękara, A.; Sałata, A.; Caruso, G. Leaf chlorophyll fluorescence and reflectance of oakleaf lettuce exposed to metal and metal (oid) oxide nanoparticles. BMC Plant Biol. 2023, 23, 329. [Google Scholar] [CrossRef]
- Zia, A.; Farrag, E.S.; Mahmoud, S.Y. Dieback of royal poinciana (Delonix regia) trees induced by Alternaria tenuissima and its impact on photochemical efficiency of photosystem II. Physiol. Mol. Plant Pathol. 2024, 133, 102357. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Nagy, V.; Puthur, J.T.; Kovács, L.; Garab, G. The physiological role of ascorbate as photosystem II electron donor: Protection against photoinactivation in heat-stressed leaves. Plant Physiol. 2011, 156, 382–392. [Google Scholar] [CrossRef]
- Széles, E.; Kuntam, S.; Vidal-Meireles, A.; Nagy, V.; Nagy, K.; Ábrahám, Á.; Kovács, L.; Tóth, S.Z. Single-cell microfluidics in combination with chlorophyll a fluorescence measurements to assess the lifetime of the Chlamydomonas PSBO protein. Photosynthetica 2023, 61, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Callahan, F.E.; Becker, D.W.; Cheniae, G.M. Studies on the photo-inactivation of the water-oxidizing enzyme. II. Characterization of weak light photoinhibition of PSII and its light-induced recovery. Plant Physiol. 1986, 82, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.X.; Kazimir, J.; Cheniae, G.M. Photoinhibition of hydroxylamine-extracted photosystem II membranes: Studies of the mechanism. Biochemistry 1992, 31, 11072–11083. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Park, Y.I.; Chow, W.S. Unifying model for the photoinactivation of photosystem II in vivo: A hypothesis. Photosynth. Res. 1998, 56, 1–13. [Google Scholar] [CrossRef]
- Sarvikas, P.; Hakala, M.; Pätsikkä, E.; Tyystjärvi, T.; Tyystjärvi, E. Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 391–400. [Google Scholar] [CrossRef]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Govindjee; Zhu, X.G. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2019, 139, 107–121. [Google Scholar] [CrossRef]
- Aldea, M.; Hamilton, J.G.; Resti, J.P.; Zangerl, A.R.; Berenbaum, M.R.; Frank, T.D.; DeLucia, E.H. Comparison of photosynthetic damage from arthropod herbivory and pathogen infection in understory hardwood saplings. Oecologia 2006, 149, 221–232. [Google Scholar] [CrossRef]
- Tang, J.Y.; Zielinski, R.E.; Zangerl, A.R.; Crofts, A.R.; Berenbaum, M.R.; DeLucia, E.H. The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana. J. Exp. Bot. 2006, 57, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Saito, H.; Yamamuro, K. Compensatory photosynthesis as a response to partial debudding in Ezo spruce, Picea jezoensis, seedlings. Ecol. Res. 2004, 19, 225–231. [Google Scholar] [CrossRef]
- Turnbull, T.L.; Adams, M.A.; Warren, C.R. Increased photosynthesis following partial defoliation of field-grown Eucalyptus globulus seedlings is not caused by increased leaf nitrogen. Tree Physiol. 2007, 27, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Ruban, A.V. Light harvesting control in plants. FEBS Lett. 2018, 592, 3030–3039. [Google Scholar] [CrossRef]
- Sun, H.; Shi, Q.; Zhang, S.-B.; Huang, W. Coordination of cyclic electron flow and water–water cycle facilitates photoprotection under fluctuating light and temperature stress in the epiphytic orchid Dendrobium officinale. Plants 2021, 10, 606. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustaka, J.; Ouzounidou, G.; Moustakas, M. Leaf age-dependent photosystem II photochemistry and oxidative stress responses to drought stress in Arabidopsis thaliana are modulated by flavonoid accumulation. Molecules 2021, 26, 4157. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.D.S. Reactive oxygen species in chloroplasts and chloroplast antioxidants under abiotic stress. Front. Plant Sci. 2023, 14, 1208247. [Google Scholar] [CrossRef]
- Ruban, A.V.; Murchie, E.H. Assessing the photoprotective effectiveness of non-photochemical chlorophyll fluorescence quenching: A new approach. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 977–982. [Google Scholar] [CrossRef]
- Foo, C.C.; Burgess, A.J.; Retkute, R.; Tree-Intong, P.; Ruban, A.V.; Murchie, E.H. Photoprotective energy dissipation is greater in the lower, not the upper, regions of a rice canopy: A 3D analysis. J. Exp. Bot. 2020, 71, 7382–7392. [Google Scholar] [CrossRef]
- Zavafer, A.; Iermak, I.; Cheah, M.H.; Chow, W.S. Two quenchers formed during photodamage of phostosystem II and the role of one quencher in preemptive photoprotection. Sci. Rep. 2019, 9, 17275. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Hendrickson, L.; Chow, W.S. Photoinactivation of photosystem II in high light-acclimated grapevines. Funct. Plant Biol. 2001, 28, 755–764. [Google Scholar] [CrossRef]
- Derks, A.; Schaven, K.; Bruce, D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim. Biophys. Acta-Bioenerg. 2015, 1847, 468–485. [Google Scholar] [CrossRef] [PubMed]
- Zavafer, A.; Chow, W.S.; Cheah, M.H. The action spectrum of Photosystem II photoinactivation in visible light. J. Photochem. Photobiol. B 2015, 152, 247–260. [Google Scholar] [CrossRef]
- Zuo, G. Non-photochemical quenching (NPQ) in photoprotection: Insights into NPQ levels required to avoid photoinactivation and photoinhibition. New Phytol. 2025, 246, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Dietz, K.J.J.; Turkan, I.; Krieger-Liszkay, A. Redox- and reactive oxygen species dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef]
- Takagi, D.; Takumi, S.; Hashiguchi, M.; Sejima, T.; Miyake, C. Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant. Physiol. 2016, 171, 1626–1634. [Google Scholar] [CrossRef]
- Moustakas, M. Plant photochemistry, reactive oxygen species, and photoprotection. Photochem 2022, 2, 5–8. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Grandin-Courbet, A.; Morvan-Bertrand, A.; Dehail, M.; Hennequart, F.; Prud’homme, M.P. Laminaria digitata extract improved leaf meristem protection under drought and nitrogen uptake after rehydration through hormesis-based chemical priming in Lolium perenne. Plant Cell Environ. 2025, 48, 6674–6690. [Google Scholar] [CrossRef] [PubMed]
- Kasajima, I.; Ebana, K.; Yamamoto, T.; Takahara, K.; Yano, M.; Kawai-Yamada, M.; Uchimiya, H. Molecular distinction in genetic regulation of nonphotochemical quenching in rice. Proc. Natl. Acad. Sci. USA 2011, 108, 13835–13840. [Google Scholar] [CrossRef]
- Gawroński, P.; Witoń, D.; Vashutina, K.; Bederska, M.; Betliński, B.; Rusaczonek, A.; Karpiński, S. Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol. Plant 2014, 7, 1151–1166. [Google Scholar] [CrossRef]
- Vitale, L.; Vitale, E.; Costanzo, G.; De Maio, A.; Arena, C. Photo-protective mechanisms and the role of poly (ADP-ribose) polymerase activity in a facultative CAM plant exposed to long-term water deprivation. Plants 2020, 9, 1192. [Google Scholar] [CrossRef]
- McNaughton, S.J. Compensatory plant growth as a response to herbivory. Oikos 1983, 40, 329–336. [Google Scholar] [CrossRef]
- Karban, R.; Myers, J.H. Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 1989, 20, 331–348. [Google Scholar] [CrossRef]
- Carvajal Acosta, A.N.; Agrawal, A.A.; Mooney, K. Plant water-use strategies as mediators of herbivore drought response: Ecophysiology, host plant quality and functional traits. J. Ecol. 2022, 111, 687–700. [Google Scholar] [CrossRef]
- Barrett, L.G.; Heil, M. Unifying concepts and mechanisms in the specificity of plant–enemy interactions. Trends Plant Sci. 2012, 17, 282–292. [Google Scholar] [CrossRef]
- Kessler, A. Detecting the enemy or being manipulated by your attacker? Herbivore-derived elicitors of plant responses: An introduction to a Virtual Issue. New Phytol. 2025, 247, 431–435. [Google Scholar] [CrossRef]
| Parameter | Well-Watered Plants | Mildly Drought-Stressed |
|---|---|---|
| Leaf Water Content 1 | 86.19 ± 0.97 | 83.99 ± 0.08 |
| Soil Water Content 2 | 0.51 ± 0.03 | 0.22 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustaka, J.; Sperdouli, I.; Andreadis, S.S.; Stoikou, N.; Giannousi, K.; Dendrinou-Samara, C.; Moustakas, M. The Compensatory Response of Photosystem II Photochemistry to Short-Term Insect Herbivory Is Suppressed Under Water Deficit. Insects 2025, 16, 984. https://doi.org/10.3390/insects16090984
Moustaka J, Sperdouli I, Andreadis SS, Stoikou N, Giannousi K, Dendrinou-Samara C, Moustakas M. The Compensatory Response of Photosystem II Photochemistry to Short-Term Insect Herbivory Is Suppressed Under Water Deficit. Insects. 2025; 16(9):984. https://doi.org/10.3390/insects16090984
Chicago/Turabian StyleMoustaka, Julietta, Ilektra Sperdouli, Stefanos S. Andreadis, Nikoletta Stoikou, Kleoniki Giannousi, Catherine Dendrinou-Samara, and Michael Moustakas. 2025. "The Compensatory Response of Photosystem II Photochemistry to Short-Term Insect Herbivory Is Suppressed Under Water Deficit" Insects 16, no. 9: 984. https://doi.org/10.3390/insects16090984
APA StyleMoustaka, J., Sperdouli, I., Andreadis, S. S., Stoikou, N., Giannousi, K., Dendrinou-Samara, C., & Moustakas, M. (2025). The Compensatory Response of Photosystem II Photochemistry to Short-Term Insect Herbivory Is Suppressed Under Water Deficit. Insects, 16(9), 984. https://doi.org/10.3390/insects16090984









