Morphology and Olfactory Recognition of Leg Sensilla in Honeybee Workers of Apis cerana cerana
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Scanning Electron Microscope (SEM) Observations of Legs
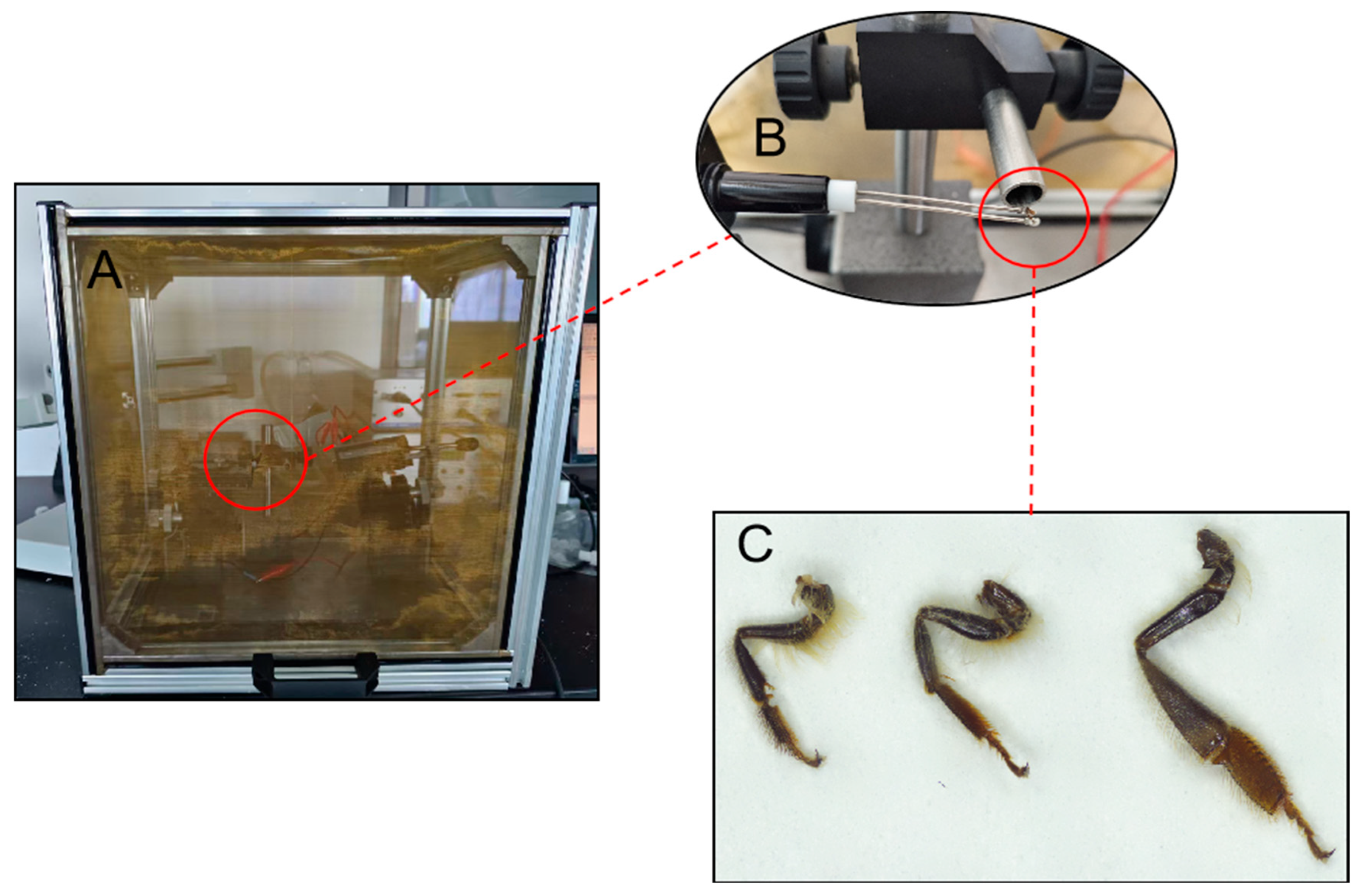
2.3. Electrophysiological Reactions to Testing Compounds
2.4. Preference Selection for Testing Compounds
2.5. Statistical Analysis
3. Results
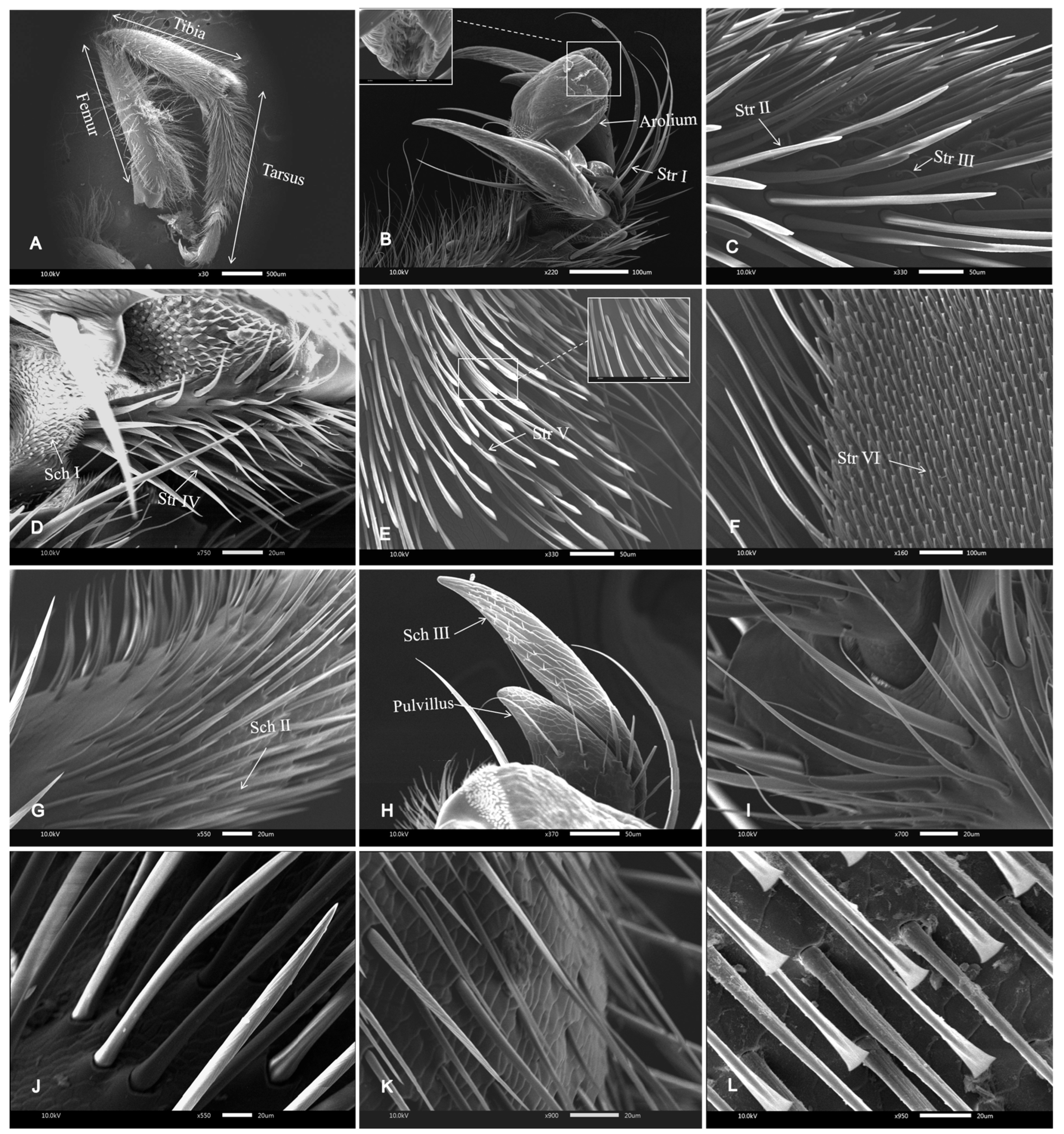
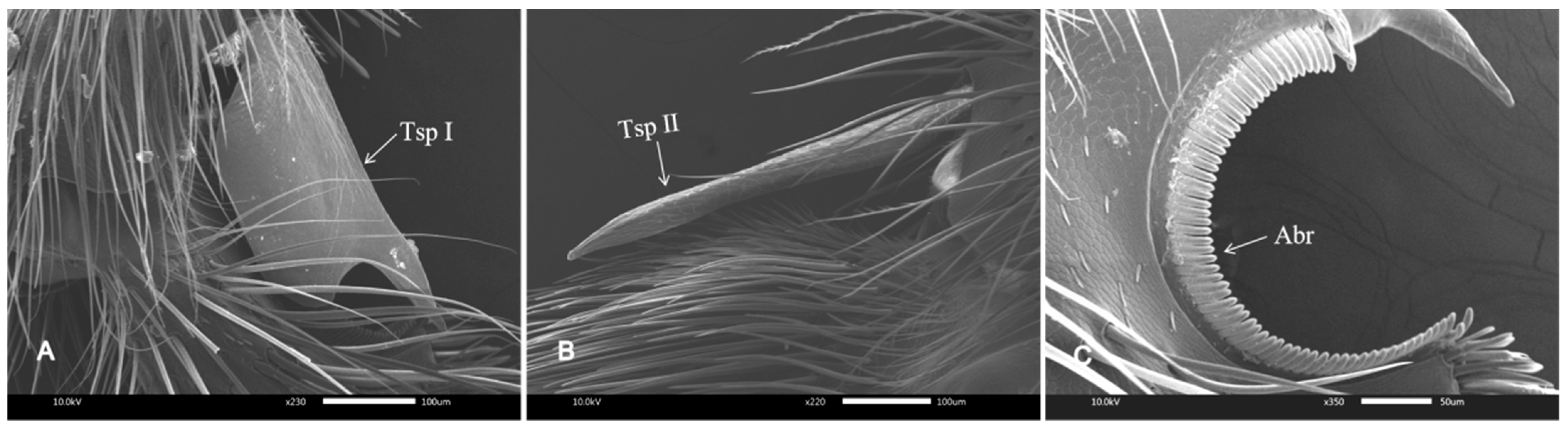
3.1. SEM of the Legs
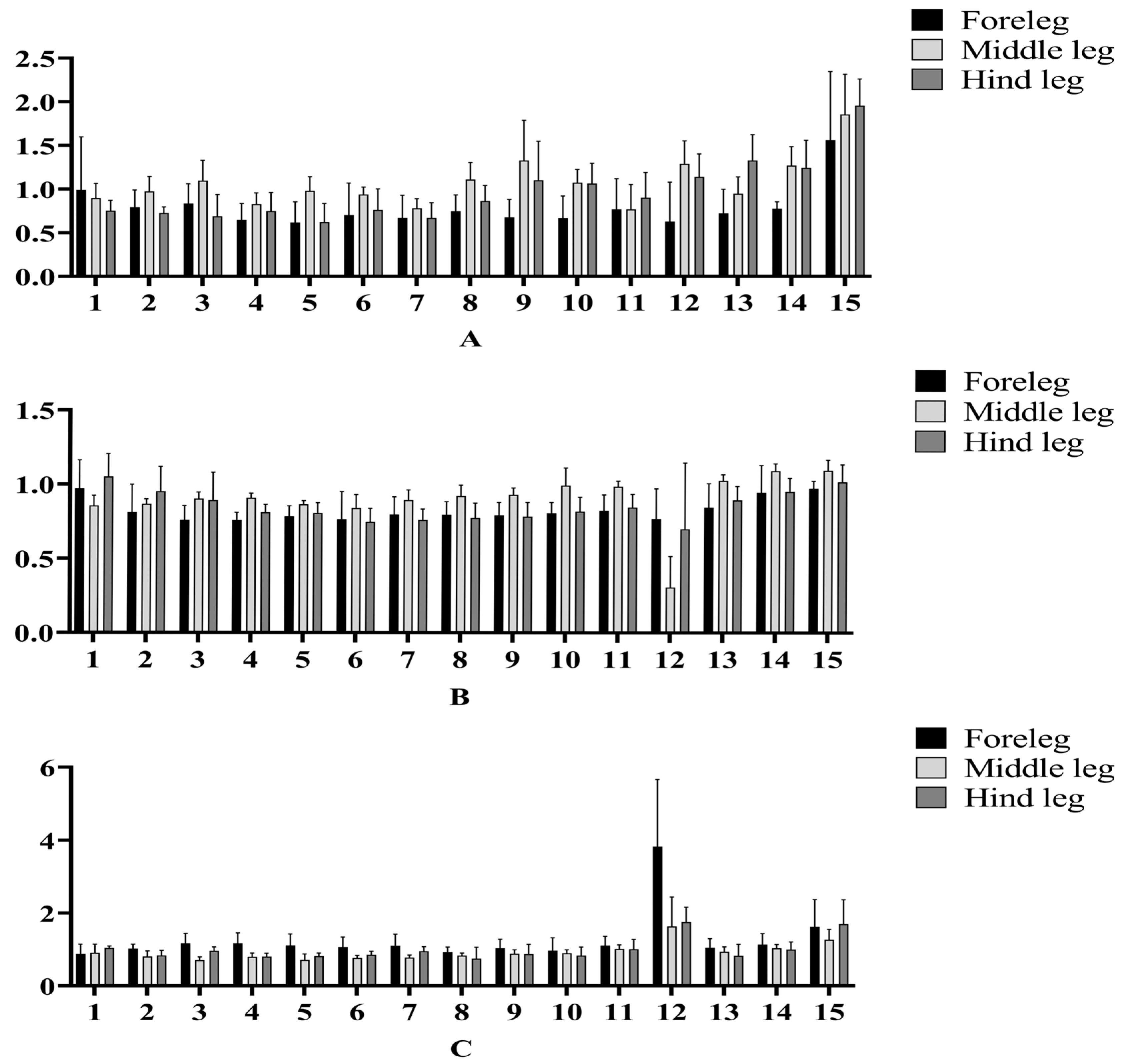
3.2. ELG Responses of A. c. cerana
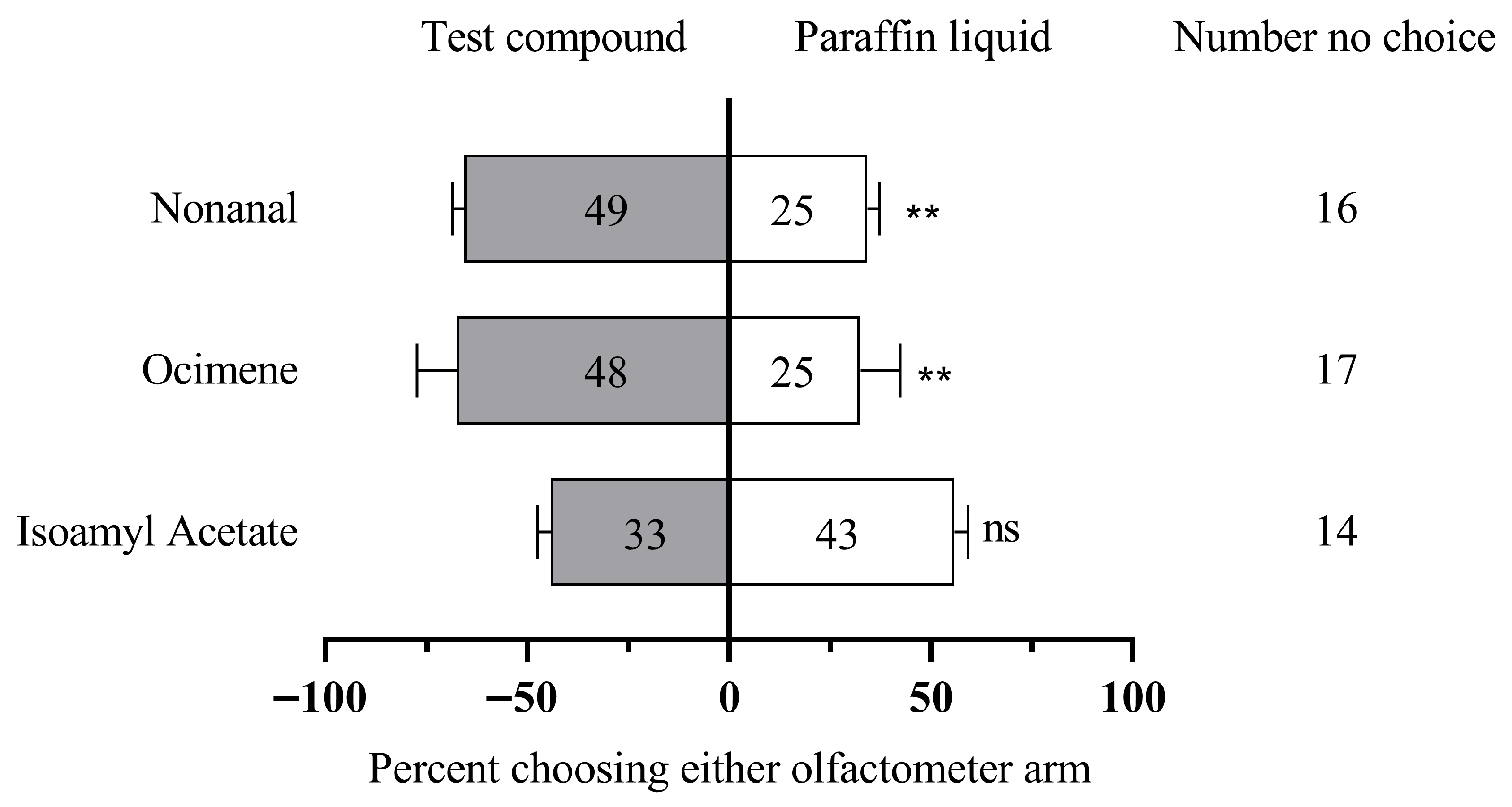
3.3. Y-Tube Behavior of A. c. cerana
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dethier, V.J. Chemical insect attractants and repellents. Q. Rev. Biol. 1947, 65, 575. [Google Scholar] [CrossRef]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in insect olfaction: To smell or not to smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef]
- Schneider, D. Insect olfaction: Deciphering system for chemical messages. Science 1969, 163, 1031–1037. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, S.; Zhang, Y.; Guo, Y.; Wang, G. Candidate olfaction genes identified within the Helicoverpa armigera antennal transcriptome. PLoS ONE 2012, 7, e48260. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liberles, S.D. Aversion and attraction through olfaction. Curr. Biol. 2015, 25, R120–R129. [Google Scholar] [CrossRef]
- Sanchez, M.G.D.B. Taste perception in honey bees. Chem. Senses 2011, 36, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.F.; Carlson, J.R. Insect olfaction from model systems to disease control. Proc. Natl. Acad. Sci. USA 2011, 108, 12987–12995. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Q.; Wang, Q.; Dong, K.; Xiao, Y.; Zhang, Y. Identification and characterization of odorant binding proteins in the forelegs of Adelphocoris lineolatus (goeze). Front. Physiol. 2017, 8, 735. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, J.; Gu, S.; Xiao, H.; Guo, Y.; Liu, Z.; Zhang, Y. Chemosensillum immunolocalization and ligand specificity of chemosensory proteins in the alfalfa plant bug Adelphocoris lineolatus (goeze). Sci. Rep. 2015, 5, 8073. [Google Scholar] [CrossRef]
- Stewart, S.; Koh, T.W.; Ghosh, A.C.; Carlson, J.R. Candidate ionotropic taste receptors in the drosophila larva. Proc. Natl. Acad. Sci. USA 2015, 112, 4195–4201. [Google Scholar] [CrossRef]
- Scott, K.; Brady, R.J.; Cravchik, A.; Morozov, P.; Rzhetsky, A.; Zuker, C.; Axel, R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 2001, 104, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, M.; Hotier, L.; Giurfa, M.; de Brito Sanchez, M.G. Aversive gustatory learning and perception in honey bees. Sci. Rep. 2018, 8, 1343. [Google Scholar] [CrossRef]
- Xu, W. How do moth and butterfly taste?—Molecular basis of gustatory receptors in Lepidoptera. Insect Sci. 2020, 27, 1148–1157. [Google Scholar] [CrossRef]
- Hou, W.; Miao, C.L.; Dong, S.L. The role of foreleg tarsus and antennae in the oviposition preference stimulated by glucosinolates in Plutella xylostella. J. Nanjing Agric. Univ. 2022, 45, 72–77. [Google Scholar]
- Zhang, Y.; Loon, J.J.; Wang, C. Tarsal taste neuron activity and proboscis extension reflex in response to sugars and amino acids in Helicoverpa armigera (hubner). J. Exp. Biol. 2010, 213, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, L.; Ge, F.; Wang, C. Tarsal taste neurons of Helicoverpa assulta (guenee) respond to sugars and amino acids, suggesting a role in feeding and oviposition. J. Insect Physiol. 2011, 57, 1332–1340. [Google Scholar] [CrossRef]
- Nowinska, A.; Franielczyk-Pietyra, B.; Polhemus, D.A. The leg sensilla of insects from different habitats—Comparison of strictly aquatic and riparian bugs (corixidae, ochteridae, gelastocoridae: Nepomorpha: Insecta: Heteroptera). Insects 2023, 14, 441. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef]
- Potts, S.; Imperatriz-Fonseca, V.; Ngo, H.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Betti, M.I.; Wahl, L.M.; Zamir, M. Age structure is critical to the population dynamics and survival of honeybee colonies. R. Soc. Open Sci. 2016, 3, 160444. [Google Scholar] [CrossRef]
- Lindauer, M.; Watkin, B. Division of labour in the honeybee colony. Bee World 1953, 34, 63–73. [Google Scholar] [CrossRef]
- Smith, D.R.; Villafuerte, L.; Otis, G.; Palmer, M.R. Biogeography of Apis cerana F. and A. nigrocincta Smith: Insights from mtDNA studies. Apidologie 2000, 31, 265–279. [Google Scholar] [CrossRef]
- Yancan, L.; Tianle, C.; Yunhan, F.; Delong, L.; Guizhi, W. Population genomics and morphological features underlying the adaptive evolution of the eastern honey bee (Apis cerana). BMC Genom. 2019, 20, 869. [Google Scholar] [CrossRef]
- Xu, Z.M.; Wei, X.P.; Lin, L.; Zhou, W.C. Observations and comparisons with different ecotypes of Apis cerana cerana. J. Bee 2018, 38, 7–11. [Google Scholar]
- Zhu, X.; Zhou, S.; Xu, X.; Wang, J.; Yu, Y.; Yang, K.C.; Luo, Q.; Xu, Y.; Wang, S.; Zhou, B. Morphological differentiation in Asian honey bee (Apis cerana) populations in the basin and highlands of southwestern China. Apic. Res. 2017, 56, 203–209. [Google Scholar] [CrossRef]
- Zhou, S.J.; Zhu, X.J.; Xu, X.J.; Gao, J.L.; Zhou, B.F. Multivariate morphometric analysis of local and introduced populations of Apis cerana (Hymenoptera: Apidae) on Hainan Island, China. Apic. Res. 2018, 57, 374–381. [Google Scholar] [CrossRef]
- de Brito Sanchez, M.G.; Lorenzo, E.; Su, S.; Liu, F.; Zhan, Y.; Giurfa, M. The tarsal taste of honey bees: Behavioral and electrophysiological analyses. Front. Behav. Neurosci. 2014, 8, 25. [Google Scholar] [CrossRef]
- Jia, X.Q.; Zhou, F.; Pan, J.; Zhao, Y.X.; Zhang, W.; Wang, K.L.; Shu, J.P. Morphological structure and distribution of hairiness on the hindleg of five species of pollinators for Camllia oleifera. Chin. J. Ecol. 2025, 44, 1793–1801. [Google Scholar]
- Du, Y.L.; Xu, K.; Ma, W.H.; Su, W.T.; Tai, M.M.; Zhao, H.T.; Jiang, Y.S.; Li, X.C. Contact chemosensory genes identified in leg transcriptome of Apis cerana cerana (Hymenoptera: Apidae). J. Econ. Entomol. 2019, 5, 5. [Google Scholar] [CrossRef]
- Ramirez, W.C.G.; Thomas, N.K.T.; Muktar, I.J.; Riabinina, O.; Ramirez, G. The neuroecology of olfaction in bees. Curr. Opin. Insect Sci. 2023, 56, 101018. [Google Scholar] [CrossRef]
- Trhlin, M.; Rajchard, J. Chemical communication in the honeybee (Apis mellifera L.): A review. Vet. Med. 2018, 56, 265–273. [Google Scholar] [CrossRef]
- Steiner, K.E. The evolution of beetle pollination in a South African orchid. Am. J. Bot. 1998, 5, 1180–1193. [Google Scholar] [CrossRef]
- Shearer, D.A.; Boch, R. 2-heptanone in the mandibular gland secretion of the honey-bee. Nature 1965, 206, 530. [Google Scholar] [CrossRef]
- Revadi, S.; Vitagliano, S.; Rossi Stacconi, M.V.; Ramasamy, S.; Mansourian, S.; Carlin, S.; Vrhovsek, U.; Becher, P.G.; Mazzoni, V.; Rota-Stabelli, O.; et al. Olfactory responses of Drosophila suzukii females to host plant volatiles. Physiol. Entomol. 2015, 40, 54–64. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Snyder, L.; Meador, C.; Watkins-DeJong, E.; Obernesser, B.T.; Brown, N.; Carroll, M.J. Diet and pheromones interact to shape honey bee (Apis mellifera) worker physiology. J. Insect Physiol. 2022, 143, 104442. [Google Scholar] [CrossRef]
- Maisonnasse, A.; Lenoir, J.C.; Beslay, D.; Crauser, D.; Le Conte, Y. E-β-ocimene, a volatile brood pheromone involved in social regulation in the honey bee colony (Apis mellifera). PLoS ONE 2010, 5, e13531. [Google Scholar] [CrossRef]
- Conte, Y.L.; Arnold, G.; Trouiller, J.; Masson, C.; Ourisson, G. Attraction of the parasitic mite varroa to the drone larvae of honey bees by simple aliphatic esters. Science 1989, 245, 638–639. [Google Scholar] [CrossRef]
- Yan, W.Y.; Le-Conte, Y.; Beslay, D.; Zeng, Z.J. Identification of brood pheromone in chinese honeybee [Apis cerana cerana (Hymenoptera: Apidae)]. Sci. Agric. Sin. 2009, 42, 2250–2254. [Google Scholar]
- Conte, Y.L.; Arnold, G.; Trouiller, J.; Masson, C.; Chappe, B. Identification of a brood pheromone in honeybees. Naturwissenschaften 1990, 77, 334–336. [Google Scholar] [CrossRef]
- Dötterl, S.; Vereecken, N.J. The chemical ecology and evolution of bee–flower interactions: A review and perspectives. Can. J. Zool. 2010, 88, 668–697. [Google Scholar] [CrossRef]
- Liang, D.S.; Wen, H.S.; Zhou, Y.X.; Wang, T.H.; Jia, G.Q.; Cui, Z.Y.; Li, A. Simultaneous qualitative and quantitative analyses of volatile components in chinese honey of six botanical origins using headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Sci. Food Agric. 2023, 103, 7631–7642. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, B.B.; Zhang, X.F.; Wang, B.X.; Wu, W.Q.; Song, H.L.; Zheng, Y.H. Identification and difference analysis of volatile odorants of pear and oilseed rape flowers. Chin. Agric. Sci. Bull. 2021, 37, 71–76. [Google Scholar]
- Wang, H.J.; Hu, C.; Guo, T.; Yang, L.X.; Zhang, J.S.; Hu, Z.Z. Analysis of Volatile Components in Robinia psendoacacia L. Flower and Application in Cigarettes. Agric. Prod. Process. 2023, 64–69. [Google Scholar] [CrossRef]
- Zhang, J.C.; Liu, J.J.; Gao, F.; Chen, M.; Jiang, Y.S.; Zhao, H.T.; Ma, W.H. Electrophysiological and behavioral responses of Apis mellifera and Bombus terrestris to melon flower volatiles. Insects 2022, 13, 973. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 91, 103–122. [Google Scholar] [CrossRef]
- Lu, A.P. The Scanning Electron-microscopic Observations on the Legs of Encarsia sp. Sun Yatsen Univ. Forum 1995, 2, 8–10+150. [Google Scholar]
- Zhao, H.Y.; Peng, Z.; Du, Y.L.; Jiang, Y.S. SEM observation on antennal sensilla of Apis cerana cerana. Apic. China 2019, 70, 68–72. [Google Scholar]
- Liu, L.; Zhang, Y.; Yan, S.C.; Yang, B.; Wang, G.R. Ultrastructural and descriptive study on the adult body surface of Heortia vitessoides (Lepidoptera: Crambidae). Insects 2023, 14, 687. [Google Scholar] [CrossRef]
- Jander, R. Grooming and pollen manipulation in bees (apoidea): The nature and evolution of movements involving the foreleg. Physiol. Entomol. 1976, 1, 179–194. [Google Scholar] [CrossRef]
- Yang, C.H. Basic knowledge of morphological anatomy of Apis cerana cerana (I)—Organization and external morphology of honeybees. J. Bee 1997, 10, 16–17. [Google Scholar]
- Michener, C.D.; Winston, M.L.; Jander, R. Pollen manipulation and related activities and structures in bees of the family apidae. Univ. Kans. Sci. Bull. 1978, 51, 575–601. [Google Scholar] [CrossRef]
- Xu, Y.D.; Liang, N.J.N.; Ma, L.B. Observation of the micro-nano structure of the pollen basket of Apis cerana cerana. Shaanxi Agric. Sci. 2016, 62, 31–32. [Google Scholar]
- Olszewski, K.; Dziechciarz, P.; Trytek, M.; Borsuk, G. A scientific note on the strategy of wax collection as rare behavior of Apis mellifera. Apidologie 2022, 53, 40. [Google Scholar] [CrossRef]
- Strauß, J. Neuronal innervation of the subgenual organ complex and the tibial campaniform sensilla in the stick insect midleg. Insects 2020, 11, 40. [Google Scholar] [CrossRef]
- Lee, M.; Kanturski, M.; Santammavong, C.; Lee, S. The first report of the aphid genus Macromyzus (Hemiptera: Aphididae) from laos, with a description of a new species and its taxonomic position. Insects 2024, 15, 1015. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, X.; Ullah, H.; Tang, Y.; Zhu, D.; Xu, H.; Wen, Q.; Tian, X.; Tan, J. Comparative SEM study of sensilla and tyloid structures in the antennae of vespinae (Hymenoptera: Vespidae). Insects 2024, 15, 448. [Google Scholar] [CrossRef]
- McIver, S.B. Structure of cuticular mechanoreceptors of arthropods. Annu. Rev. Entomol. 1975, 20, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fan, R.D.; Wu, M.Y.; Dong, Y.N.; Shi, S.S. Ultrastructure of the sensilla on the antennae of Colposcelis signata. J. Environ. Entomol. 2020, 42, 227–236. [Google Scholar]
- Faucheux, M.J. Biodiversity and Unity of Sensory Organs in Lepidopteran Insects; Société des Sciences Naturelles de l’Ouest de la France: Nantes, France, 1999; Volume 296. [Google Scholar]
- Wu, W.; Lei, J.N.; Mao, Q.; Tian, Y.Z.; Shan, H.W.; Chen, J.P. Insights into brochosome distribution, synthesis, and novel rapid-release mechanism in Maiestas dorsalis (Hemiptera: Cicadellidae). Insects 2023, 14, 734. [Google Scholar] [CrossRef] [PubMed]
- Diallo, S.; Kašparová, K.; Šulc, J.; Johny, J.; Křivánek, J.; Nebesářová, J.; Sillam-Dussès, D.; Kyjaková, P.; Vondrášek, J.; Machara, A.; et al. Identification of the trail-following pheromone receptor in termites. Elife 2025, 13, RP101814. [Google Scholar] [CrossRef]
- Ishiwari, H.; Suzuki, T.; Maeda, T. Essential compounds in herbivore-induced plant volatiles that attract the predatory mite Neoseiulus womersleyi. J. Chem. Ecol. 2007, 33, 1670–1681. [Google Scholar] [CrossRef]
- Hern, A.; Dorn, S. A female-specific attractant for the codling moth, Cydia pomonella, from apple fruit volatiles. Sci. Nat. 2004, 91, 77–80. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Shen, J.; Zhao, H.; Ma, W.; Jiang, Y. Differences in EAG response and behavioral choices between honey bee and bumble bee to tomato flower volatiles. Insects 2022, 13, 987. [Google Scholar] [CrossRef]
- Fan, N.N.; Huang, J.Y.; Liu, S.; Zhang, K.; Zhang, X.H.; Zheng, H.X. Electrophysiological and olfactory behavioral responses of adult Callosobruchus chinensis (Coleoptera: Chrysomelidae) to the volatiles from Vigna angularis and V. radiata seeds. Acta Entomol. Sin. 2024, 67, 58–67. [Google Scholar]
- Twidle, A.M.; Barker, D.; Seal, A.G.; Fedrizzi, B.; Suckling, D.M. Identification of floral volatiles and pollinator responses in kiwifruit cultivars, actinidia chinensis var. chinensis. J. Chem. Ecol. Off. J. Int. Soc. Chem. Ecol. 2018, 44, 406–415. [Google Scholar] [CrossRef]
- Sun, X.L.; Wang, G.C.; Gao, Y.; Chen, Z.M. Screening and field evaluation of synthetic volatile blends attractive to adults of the tea weevil, Myllocerinus aurolineatus. Chemoecology 2012, 22, 229–237. [Google Scholar] [CrossRef]
- Liu, Y.B.; Zeng, Z.J.; Barron, A.B.; Ma, Y.; He, Y.Z.; Liu, J.F.; Li, Z.; Yan, W.Y.; He, X.J. The involvement of a floral scent in plant-honeybee interaction. Naturwissenschaften 2022, 109, 30. [Google Scholar] [CrossRef]
- Traynor, K.S.; Conte, Y.L.; Page, R.E. Age matters: Pheromone profiles of larvae differentially influence foraging behaviour in the honeybee, Apis mellifera. Anim. Behav. 2015, 99, 1–8. [Google Scholar] [CrossRef]
- He, X.J.; Zhang, X.C.; Jiang, W.J.; Barron, A.B.; Zhang, J.H.; Zeng, Z.J. Starving honey bee (Apis mellifera) larvae signal pheromonally to worker bees. Sci. Rep. 2016, 6, 22359. [Google Scholar] [CrossRef] [PubMed]
| NO. | Chemicals | Purity% | Compound Source |
|---|---|---|---|
| 1 | Isoamyl Acetate | 98% | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| 2 | Methyl Palmitate | 97% | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| 3 | Methyl Oleate | 99% | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| 4 | Methyl Linoleate | 95% | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| 5 | Methyl Linolenate | 98% | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| 6 | Methyl Stearate | 95% | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| 7 | Ethyl Palmitate | 99% | Macklin Biochemical Technology, Shanghai, China |
| 8 | Ethyl Oleate | 95% | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| 9 | Ethyl Linoleate | 97% | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| 10 | Ethyl Stearate | 99% | Aladdin Biochemical Technology, Shanghai, China |
| 11 | Linalool | 98% | Aladdin Biochemical Technology, Shanghai, China |
| 12 | Ocimene | 90% | Aladdin Biochemical Technology, Shanghai, China |
| 13 | Ethyl Hexanoate | 99% | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| 14 | Farnesene (mixture of isomers) | 98% | Aladdin Biochemical Technology, Shanghai, China |
| 15 | Nonanal | 96% | Aladdin Biochemical Technology, Shanghai, China |
| Chemicals | 1 Day Old | 10 Days Old | 25 Days Old | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Forelegs | Middle Legs | Hind Legs | Forelegs | Middle Legs | Hind Legs | Forelegs | Middle Legs | Hind Legs | |
| Isoamyl Acetate | 0.99 ± 0.30 Ab | 0.90 ± 0.08 Acd | 0.75 ± 0.06 Adefg | 0.97 ± 0.10 Aa | 0.86 ± 0.03 Ade | 1.05 ± 0.08 Aa | 0.88 ± 0.14 Ab | 0.91 ± 0.12 Abc | 1.04 ± 0.03 Ab |
| Methyl Palmitate | 0.79 ± 0.10 ABb | 0.97 ± 0.09 Abcd | 0.73 ± 0.04 Bdefg | 0.81 ± 0.09 ABa | 0.87 ± 0.02 ABcde | 0.95 ± 0.08 ABabc | 1.02 ± 0.06 Ab | 0.81 ± 0.08 ABc | 0.84 ± 0.07 ABb |
| Methyl Oleate | 0.83 ± 0.11 BCb | 1.10 ± 0.12 ABbcd | 0.69 ± 0.13 Cefg | 0.76 ± 0.05 Ca | 0.90 ± 0.02 ABCbcde | 0.89 ± 0.09 ABCabc | 1.17 ± 0.13 Ab | 0.71 ± 0.05 Cc | 0.96 ± 0.06 ABCb |
| Methyl Linoleate | 0.65 ± 0.10 Cb | 0.83 ± 0.06 BCd | 0.75 ± 0.11 BCdefg | 0.97 ± 0.03 BCa | 0.91 ± 0.02 Bbcde | 0.81 ± 0.03 BCabc | 1.17 ± 0.14 Ab | 0.80 ± 0.05 BCc | 0.80 ± 0.05 BCb |
| Methyl Linolenate | 0.62 ± 0.12 Cb | 0.98 ± 0.08 ACbcd | 0.62 ± 0.11 Cd | 0.78 ± 0.04 BCa | 0.87 ± 0.01 ABCcde | 0.81 ± 0.03 BCabc | 1.11 ± 0.16 Ab | 0.71 ± 0.08 BCc | 0.82 ± 0.04 BCb |
| Methyl Stearate | 0.70 ± 0.18 Bb | 0.94 ± 0.04 ABbcd | 0.76 ± 0.12 ABdefg | 0.77 ± 0.09 ABa | 0.84 ± 0.05 ABe | 0.75 ± 0.05 ABbc | 1.07 ± 0.14 Ab | 0.77 ± 0.04 ABc | 0.85 ± 0.05 ABb |
| Ethyl Palmitate | 0.67 ± 0.13 Cb | 0.78 ± 0.06 BCd | 0.67 ± 0.09 Cfg | 0.80 ± 0.06 BCa | 0.89 ± 0.03 ABCbcde | 0.76 ± 0.04 BCbc | 1.10 ± 0.16 Ab | 0.78 ± 0.03 BCc | 0.95 ± 0.06 ABb |
| Ethyl Oleate | 0.67 ± 0.09 Cb | 0.78 ± 0.10 BCd | 0.67 ± 0.09 Cfg | 0.80 ± 0.04 BCa | 0.89 ± 0.04 ABCbcde | 0.77 ± 0.05 BCbc | 1.10 ± 0.07 Ab | 0.78 ± 0.03 BCc | 0.95 ± 0.16 ABb |
| Ethyl Linoleate | 0.68 ± 0.10 Bb | 1.33 ± 0.23 Ab | 1.10 ± 0.22 ABbcde | 0.79 ± 0.04 Ba | 0.93 ± 0.02 ABbcde | 0.78 ± 0.05 Bbc | 1.03 ± 0.13 ABb | 0.88 ± 0.05 Bbc | 0.87 ± 0.14 Bb |
| Ethyl Stearate | 0.67 ± 0.13 Bb | 1.07 ± 0.08 Abcd | 1.06 ± 0.12 Abcdef | 0.81 ± 0.04 ABa | 0.99 ± 0.06 ABabc | 0.82 ± 0.05 ABabc | 0.97 ± 0.18 ABb | 0.90 ± 0.05 ABbc | 0.83 ± 0.12 ABb |
| Linalool | 0.77 ± 0.18 Ab | 0.77 ± 0.14 Ad | 0.90 ± 0.15 Acdefg | 0.82 ± 0.05 Aa | 0.99 ± 0.02 Aabcd | 0.85 ± 0.04 Aabc | 1.11 ± 0.13 Ab | 1.01 ± 0.06 Abc | 1.01 ± 0.13 Ab |
| Ocimene | 0.63 ± 0.23 BCb | 1.29 ± 0.13 BCbc | 1.14 ± 0.13 BCbcd | 0.77 ± 0.10 BCa | 0.31 ± 0.10 Cf | 0.70 ± 0.22 BCc | 3.83 ± 0.92 Aa | 1.63 ± 0.41 Ba | 1.75 ± 0.20 Ba |
| Ethyl Hexanoate | 0.72 ± 0.14 Bb | 0.95 ± 0.10 Bbcd | 1.33 ± 0.15 Ab | 0.85 ± 0.08 Ba | 1.03 ± 0.02 ABab | 0.89 ± 0.05 Babc | 1.05 ± 0.13 ABb | 0.94 ± 0.07 Bbc | 0.83 ± 0.16 Bb |
| Farnesene (mixture of isomers) | 0.78 ± 0.04 Bb | 1.27 ± 0.11 Abc | 1.24 ± 0.16 Abc | 0.95 ± 0.09 ABa | 1.09 ± 0.02 ABa | 0.95 ± 0.05 ABabc | 1.13 ± 0.15 Ab | 1.03 ± 0.05 ABbc | 1.00 ± 0.10 ABb |
| Nonanal | 1.56 ± 0.39 ABCa | 1.86 ± 0.23 ABa | 1.96 ± 0.15 Aa | 0.97 ± 0.03 Ca | 1.09 ± 0.04 BCa | 1.02 ± 0.06 Cab | 1.62 ± 0.38 ABCb | 1.27 ± 0.14 ABCb | 1.70 ± 0.34 ABCa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Sun, L.; Wang, P.; Xie, J.; Guo, Y. Morphology and Olfactory Recognition of Leg Sensilla in Honeybee Workers of Apis cerana cerana. Insects 2025, 16, 961. https://doi.org/10.3390/insects16090961
Zhang H, Sun L, Wang P, Xie J, Guo Y. Morphology and Olfactory Recognition of Leg Sensilla in Honeybee Workers of Apis cerana cerana. Insects. 2025; 16(9):961. https://doi.org/10.3390/insects16090961
Chicago/Turabian StyleZhang, Huiman, Lele Sun, Peng Wang, Jiaoxin Xie, and Yuan Guo. 2025. "Morphology and Olfactory Recognition of Leg Sensilla in Honeybee Workers of Apis cerana cerana" Insects 16, no. 9: 961. https://doi.org/10.3390/insects16090961
APA StyleZhang, H., Sun, L., Wang, P., Xie, J., & Guo, Y. (2025). Morphology and Olfactory Recognition of Leg Sensilla in Honeybee Workers of Apis cerana cerana. Insects, 16(9), 961. https://doi.org/10.3390/insects16090961





