Evaluating the Effectiveness of Insecticides on Spotted Lanternfly Lycorma delicatula (Hemiptera: Fulgoridae) in Kiwifruit
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insecticides
2.2. Laboratory Ovicidal Bioassays
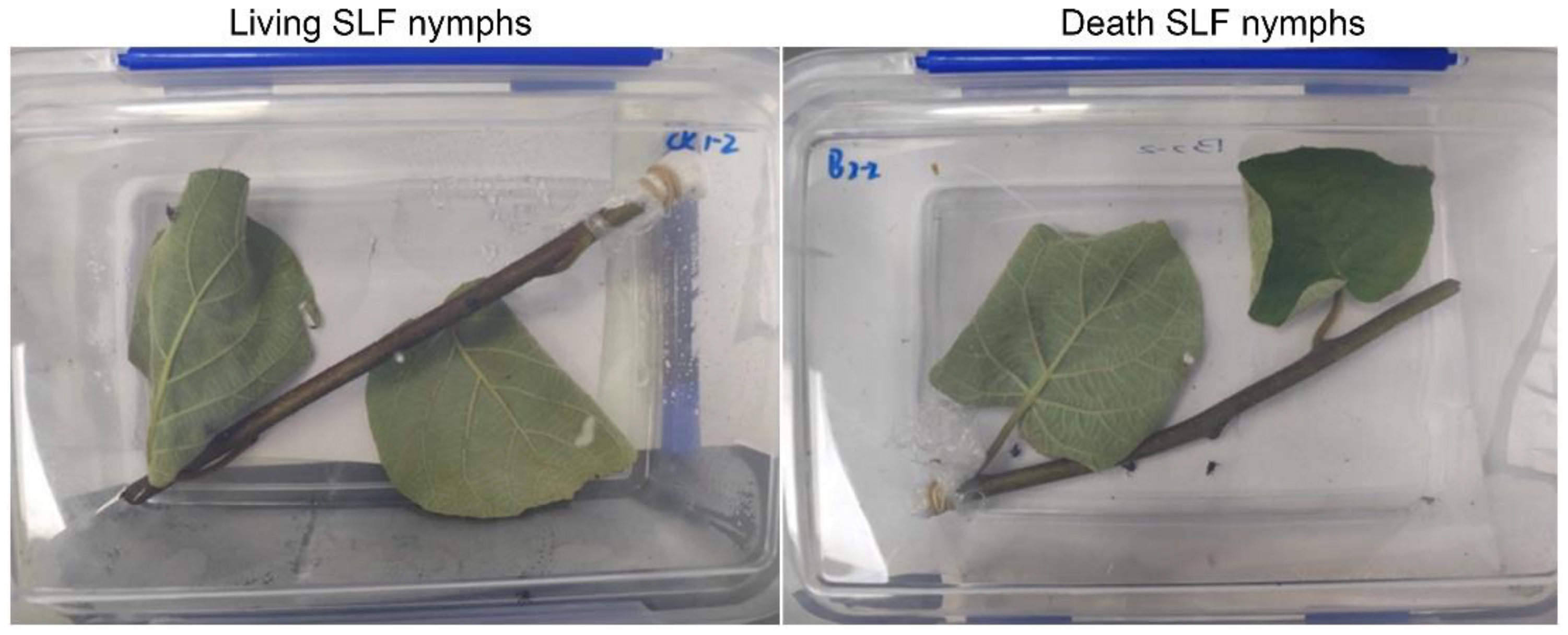
2.3. Laboratory Nymph Bioassays
2.4. Nymph Field Bioassays
2.5. Data Analysis
3. Results
3.1. Efficacy of Laboratory Ovicidal Bioassays
3.2. Efficacy of Laboratory Nymph Bioassays
3.3. Efficacy of Nymph Field Bioassays
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Kim, H.; Kim, M.; Kwon, D.H.; Park, S.; Lee, Y.; Huang, J.; Kai, S.; Lee, H.; Hong, K.; Jang, Y.; et al. Molecular comparison of Lycorma delicatula (Hemiptera: Fulgoridae) isolates in Korea, China, and Japan. J. Asia Pac. Entomol. 2013, 16, 503–506. [Google Scholar] [CrossRef]
- Barringer, L.E.; Donovall, L.R.; Spichiger, S.E.; Lynch, D.; Henry, D. The first new world record of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae). Entomol. News 2015, 125, 20–23. [Google Scholar] [CrossRef]
- Wakie, T.T.; Neven, L.G.; Yee, W.L.; Lu, Z. The establishment risk of Lycorma delicatula (Hemiptera: Fulgoridae) in the United States and globally. J. Econ. Entomol. 2020, 113, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Skrip, M.M.; Seliger, B.J.; Jones, S.; Wakie, T.; Takeuchi, Y.; Petras, V.; Petrasova, A.; Meentemeyer, R.K. Spotted lanternfly predicted to establish in California by 2033 without preventative management. Commun. Biol. 2022, 5, 558. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.M.; Leach, H. Biology and management of the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae), in the United States. Annu. Rev. Entomol. 2023, 68, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Dietrich, C.H.; Dai, W. Structure and Sensilla of the Mouthparts of the Spotted Lanternfly Lycorma delicatula (Hemiptera: Fulgoromorpha: Fulgoridae), a Polyphagous Invasive Planthopper. PLoS ONE 2016, 11, e0156640. [Google Scholar] [CrossRef]
- Kim, J.G.; Lee, E.H.; Seo, Y.M.; Kim, N.Y. Cyclic behavior of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae) on host plants. J. Insect Behav. 2011, 24, 423–435. [Google Scholar] [CrossRef]
- Lee, J.E.; Moon, S.R.; Ahn, H.G.; Cho, S.R.; Yang, J.O.; Yoon, C.; Kim, G.H. Feeding behavior of Lycorma delicatula (Hemiptera: Fulgoridae) and response on feeding stimulants of some plants. Korean J. Appl. Entomol. 2009, 48, 467–477. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Wickham, J.D.; Warden, M.L. Factors guiding the orientation of nymphal spotted lanternfly, Lycorma delicatula. Insects 2023, 14, 279. [Google Scholar] [CrossRef]
- Dara, S.K.; Barringer, L.; Arthurs, S.P. Lycorma delicatula (Hemiptera: Fulgoridae): A new invasive pest in the United States. J. Integr. Pest Manag. 2015, 6, 20. [Google Scholar] [CrossRef]
- Baker, T.C.; Smyers, E.; Urban, J.; Meng, Z.; Damadaram, K.P.; Myrick, A.J.; Cooperband, M.; Domingue, M. Progression of seasonal activities of adults of the spotted lanternfly, Lycorma delicatula, during the 2017 season of mass flight dispersal behavior in eastern Pennsylvania. J. Asia-Pac. Entomol. 2019, 22, 705–713. [Google Scholar] [CrossRef]
- Urban, J.M. Perspective: Shedding light on spotted lanternfly impacts in the USA. Pest Manag. Sci. 2020, 76, 10–17. [Google Scholar] [CrossRef]
- Wu, B.G. Preliminary investigation and control of the pests on kiwi fruit. Sichuan Agric. Sci. Technol. 2012, 7, 45. [Google Scholar]
- Pei, Z.H.; Wang, Y.A. Comprehensive prevention and control of major pests of kiwifruit occurred in the south slope of the Funiu Mountain. Deciduous Fruits 2001, 33, 53–54. [Google Scholar]
- NZKGI 2018. Simon Cook’s Nuffield Thoughts’ Part 7. Available online: https://www.nzkgi.org.nz/simon-cooks-nuffield-thoughts-part-7/ (accessed on 7 January 2019).
- Strömbom, D.; Pandey, S. Modeling the life cycle of the spotted lanternfly (Lycorma delicatula) with management implications. Math. Biosci. 2021, 340, 108670. [Google Scholar] [CrossRef]
- Shin, Y.H.; Moon, S.R.; Yoon, C.; Ahn, K.S.; Kim, G.H. Insecticidal activity of 26 insecticides against eggs and nymphs of Lycorma delicatula (Hemiptera: Fulgoridae). Korean J. Pestic. Sci. 2010, 14, 157–163. [Google Scholar]
- Park, J.D.; Kim, M.Y.; Lee, S.G.; Shin, S.C.; Kim, J.H.; Park, I.K. Biological characteristics of Lycorma delicatula and the control effects of some insecticides. Korean J. Appl. Entomol. 2009, 48, 53–57. [Google Scholar]
- Mauchline, N.; McKenna, C. BS1847: Spotted Lanternfly, Lycorma delicatula (White 1845) Review: Biology, Ecology and Pest Management with Reference to Kiwifruit; The New Zealand Institute for Plant and Food Research Limited: Auckland, New Zealand, 2019; pp. 1–49. [Google Scholar]
- Ellis, M.A.; Madden, L.V.; Wilson, L.L.; Ferree, D.C. Evaluation of organic and conventional fungicide programs for control of apple scab in Ohio. Res. Circ. Ohio Agric. Res. Dev. Cent. 1994, 298, 63–67. [Google Scholar]
- Gaskin, R.E.; Logan, D.P.; May, W.; Rowe, C.A.; Steele, K.D.; Connolly, P.G. Control of passion vine hopper and cicada eggs on kiwifruit canes with bifenthrin and a new super penetrant adjuvant. Nz. Plant Protect. 2012, 65, 100–105. [Google Scholar] [CrossRef]
- Li, D.; Jiang, K.; Wang, X.; Liu, D. Insecticide activity under changing environmental conditions: A meta-analysis. J. Pest Sci. 2024, 97, 1711–1723. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, Y.L.; Leskey, T.C. A review of biology and management of Lycorma delicatula (Hemiptera: Fulgoridae), an emerging global invasive species. J. Asia-Pac. Entomol. 2019, 22, 589–596. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar]
- Leach, H.; Biddinger, D.J.; Krawczyk, G.; Smyers, E.; Urban, J.M. Evaluation of insecticides for control of the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae), a new pest of fruit in the Northeastern US. Crop Prot. 2019, 124, 104833. [Google Scholar] [CrossRef]
- Peng, L.F.; Gibson, G.A.P.; Tang, L.U.; Xiang, J.W. Review of the species of Anastatus (Hymenoptera: Eupelmidae) known from China, with description of two new species with brachypterous females. Zootaxa 2020, 4767, 351–401. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Q.; Choi, W.Y.; Cao, L.M.; Wang, X.Y.; Hou, Z.R. A new species of Anastatus (Hymenoptera: Eulpelmidae) from China, parasitizing eggs of Lycorma delicatula (Homoptera: Fulgoridae). Zool. Syst. 2015, 40, 290–302. [Google Scholar]
- Li, T.H.; Wang, X.; Desneux, N.; Wang, S.; Zang, L.S. Egg coverings in insects: Ecological adaptation to abiotic and biotic selective pressures. Biol. Rev. 2025, 100, 99–112. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jang, M.J.; Kim, J.Y.; Kim, J.R. The effect of winter temperature on the survival of lantern fly, Lycorma delicatula (Hemiptera: Fulgoridae) eggs. Korean J. Appl. Entomol. 2014, 53, 311–315. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, D.I.; Ko, S.J.; Kang, B.R.; Park, J.D.; Kim, S.G.; Choi, K.J. Environmentally-friendly control methods and forecasting the hatching time Lycorma delicatula (Hemiptera: Fulgoridae) in Jeonnam Province. Korean J. Appl. Entomol. 2012, 51, 371–376. [Google Scholar] [CrossRef]
- Nixon, L.J.; Leskey, T.C. Evaluation of insecticide residues against spotted lanternfly (Hemiptera: Fulgoridae). J. Econ. Entomol. 2024, 117, 1582–1587. [Google Scholar] [CrossRef]
- Koehler, P.G.; Strong, C.A.; Patterson, R.S.; Valles, S.M. Differential susceptibility of German cockroach (Dictyoptera: Blattellidae) sexes and nymphal age classes to insecticides. J. Econ. Entomol. 1993, 86, 785–792. [Google Scholar] [CrossRef]
- Wang, C.J.; Liu, Z.Q. Foliar uptake of pesticides—Present status and future challenge. Pestic. Biochem. Phys. 2007, 87, 1–8. [Google Scholar] [CrossRef]
- Giorio, C.; Safer, A.; Sánchez-Bayo, F.; Tapparo, A.; Lentola, A.; Girolami, V.; van Lexmond, M.B.; Bonmatin, J.M. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 1: New molecules, metabolism, fate, and transport. Environ. Sci. Pollut. R. 2021, 28, 11716–11748. [Google Scholar] [CrossRef]
- Mukherjee, I.; Singh, R.; Govil, J.N. Risk assessment of a synthetic pyrethroid, bifenthrin on pulses. Bull. Environ. Contam. Tox. 2010, 84, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Wolfin, M.S.; Binyameen, M.; Wang, Y.; Urban, J.M.; Roberts, D.C.; Baker, T.C. Flight dispersal capabilities of female spotted lanternflies (Lycorma delicatula) related to size and mating status. J. Insect Behav. 2019, 32, 188–200. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.Y.; Cai, X.Q.; Liu, H.T. Occurrence and Control of Lycorma delicatula on Quercus mongolica in Hebei Plain. Mod. Agric. Sci. Technol. 2024, 7, 78–81. [Google Scholar]
- Li, J.J.; Yao, C.C.; Liu, Z.D. Occurrence Pattern and Control Techniques of the Spotted Lanternfly (Lycorma delicatula) in Kiwifruit Orchards on the Northern Foothills of the Qinling Mountains. Northwest Hortic. 2023, 10, 34–35. [Google Scholar]
- Wang, Z.L. Occurrence Pattern and Control of the Spotted Lanternfly. Contemp. Hortic. 2020, 43, 111–112. [Google Scholar]
- Choi, M.Y.; Yang, Z.Q.; Wang, X.Y.; Tang, Y.L.; Hou, Z.; Kim, J.H.; Byeon, Y.W. Parasitism rate of egg parasitoid Anastatus orientalis (Hymenoptera: Eupelmidae) on Lycorma delicatula (Hemiptera: Fulgoridae) in China. Korean J. Appl. Entomol. 2014, 53, 135–139. [Google Scholar] [CrossRef]
- Seo, M.; Kim, J.H.; Seo, B.Y.; Park, C.; Choi, B.R.; Kim, K.H.; Cho, J.R. Mass-rearing techniques of Anastatus orientalis (Hymenoptera: Eupelmidae), as the egg-parasitoid of Lycorma delicatula (Hemiptera: Fulgoridae): An using method of Antheraea pernyi (Lepidoptera: Saturniidae) and L. delicatula eggs in laboratory. Korean J. Appl. Entomol. 2018, 57, 243–251. [Google Scholar]

| Pesticide Active Ingredient | Technical Grade in CN | Formulation | Toxicity | Company |
|---|---|---|---|---|
| Bifenthrin | 25 g/L | Emulsifiable concentrate | II* | Shandong Shibang Agrochemical Ltd. (Heze, China) |
| Lime sulphur | 29% | Aqueous solution | III* | Sichuan Yibin Chuanan Gaoke pesticide Ltd. (Yibin, China) |
| Engulf (Polyether-modified trisiloxane) | 99% | Aqueous solution | III* | Shandong Lulong Biotechnology Ltd. (Weifang, China) |
| Thiacloprid | 25% | Water dispersible granule | II* | Qingdao Odyssey Biotechnology Ltd. (Qingdao, China) |
| Pyrethrins | 1.5% | Emulsion in water | II* | Chengdu New Sun Crop Science Ltd. (Chengdu, China) |
| Mineral oil | 97% | Emulsifiable concentrate | III* | Shanghai Hulian Bio-Pharmaceutical (Xiayi) Ltd. (Shanghai, China) |
| Types Insecticides/ Accessory Ingredient | Bifenthrin | Lime Sulphur |
|---|---|---|
| Recommend active ingredient rate per cm2 in NZ | 0.0006 mg | 0.042 mg |
| The dosage of CN product (Label solution) (/100 mL water) | 76.8 µL (80 µL) | 463.2 µL (500 µL) |
| Label rate | 19.2 µg/mL (20 µg/mL) | 1.3 mg/mL (1.4 mg/mL) |
| Treatment (Active Ingredient and Concentration) | Rate | Adjusted Mortality (%) |
|---|---|---|
| Bifenthrin at label | 19.2 µg/mL | 34.6 ± 8.9 de |
| Bifenthrin at 10× label | 192 µg/mL | 31.5 ± 7.9 de |
| Bifenthrin at 100× label | 1.9 mg/mL | 71.8 ± 8.5 a |
| Bifenthrin + Engulf at label | 19.2 µg/mL | 36.7 ± 8.2 cd |
| Bifenthrin + Engulf at 10× label | 192 µg/mL | 40.3 ± 8.5 bc |
| Bifenthrin + Engulf at 100× label | 1.9 mg/mL | 61.3 ± 8.5 ab |
| Engulf label | 990 µg/mL | 16.1 ± 10.4 def |
| Lime sulphur at label | 1.3 mg/mL | 13.3 ± 7.9 ef |
| Lime sulphur at 10× label | 13.4 mg/mL | 17.4 ± 8.9 def |
| Lime sulphur at 100× label | 134.3 mg/mL | 59.1 ± 9.3 ab |
| Treatment (Active Ingredient) | Adjusted Mortality (%) | |
|---|---|---|
| 24 h | 48 h | |
| Thiacloprid | 92.1 ± 3.1 a | 100.0 ± 0.0 a |
| Bifenthrin | 93.7 ± 3.7 a | 100.0 ± 0.0 a |
| Pyrethrins + Mineral oil | 69.8 ± 5.9 b | 83.3 ± 5.6 b |
| Treatment (Active Ingredient) | Adjusted Mortality (%) | ||
|---|---|---|---|
| Residual period | 0 day | 7 day | 14 day |
| Bifenthrin | 100.0 ± 0.0 a | 52.4 ±7.7 a | 13.3 ± 4.3 b |
| Thiacloprid | 98.9 ± 1.1 a | 72.8 ± 5.9 a | 46.7 ± 7.2 a |
| Pyrethrins + Mineral oil | 41.5 ± 12.5 b | 19.8 ± 6.8 b | 12.2 ± 3.9 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.-J.; Bai, Y.-N.; Luo, Z.-Y.; Burns, R.; Pal, C.; Zhang, F.; Shi, S.-S.; Bi, R.; Zhang, J.-P. Evaluating the Effectiveness of Insecticides on Spotted Lanternfly Lycorma delicatula (Hemiptera: Fulgoridae) in Kiwifruit. Insects 2025, 16, 954. https://doi.org/10.3390/insects16090954
Song Z-J, Bai Y-N, Luo Z-Y, Burns R, Pal C, Zhang F, Shi S-S, Bi R, Zhang J-P. Evaluating the Effectiveness of Insecticides on Spotted Lanternfly Lycorma delicatula (Hemiptera: Fulgoridae) in Kiwifruit. Insects. 2025; 16(9):954. https://doi.org/10.3390/insects16090954
Chicago/Turabian StyleSong, Zi-Jian, Yi-Na Bai, Zheng-Yu Luo, Rebecca Burns, Chandan Pal, Feng Zhang, Shu-Sen Shi, Rui Bi, and Jin-Ping Zhang. 2025. "Evaluating the Effectiveness of Insecticides on Spotted Lanternfly Lycorma delicatula (Hemiptera: Fulgoridae) in Kiwifruit" Insects 16, no. 9: 954. https://doi.org/10.3390/insects16090954
APA StyleSong, Z.-J., Bai, Y.-N., Luo, Z.-Y., Burns, R., Pal, C., Zhang, F., Shi, S.-S., Bi, R., & Zhang, J.-P. (2025). Evaluating the Effectiveness of Insecticides on Spotted Lanternfly Lycorma delicatula (Hemiptera: Fulgoridae) in Kiwifruit. Insects, 16(9), 954. https://doi.org/10.3390/insects16090954








