Modeling the Wildlife–Livestock Interface of Cattle Fever Ticks in the Southern United States
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species Occurrence Data
2.2. Explanatory Variables for Habitat Suitability Models
2.3. Habitat Suitability Models
2.4. Model Validation
2.5. Assessing Overlap Between Wildlife Hosts and Cattle
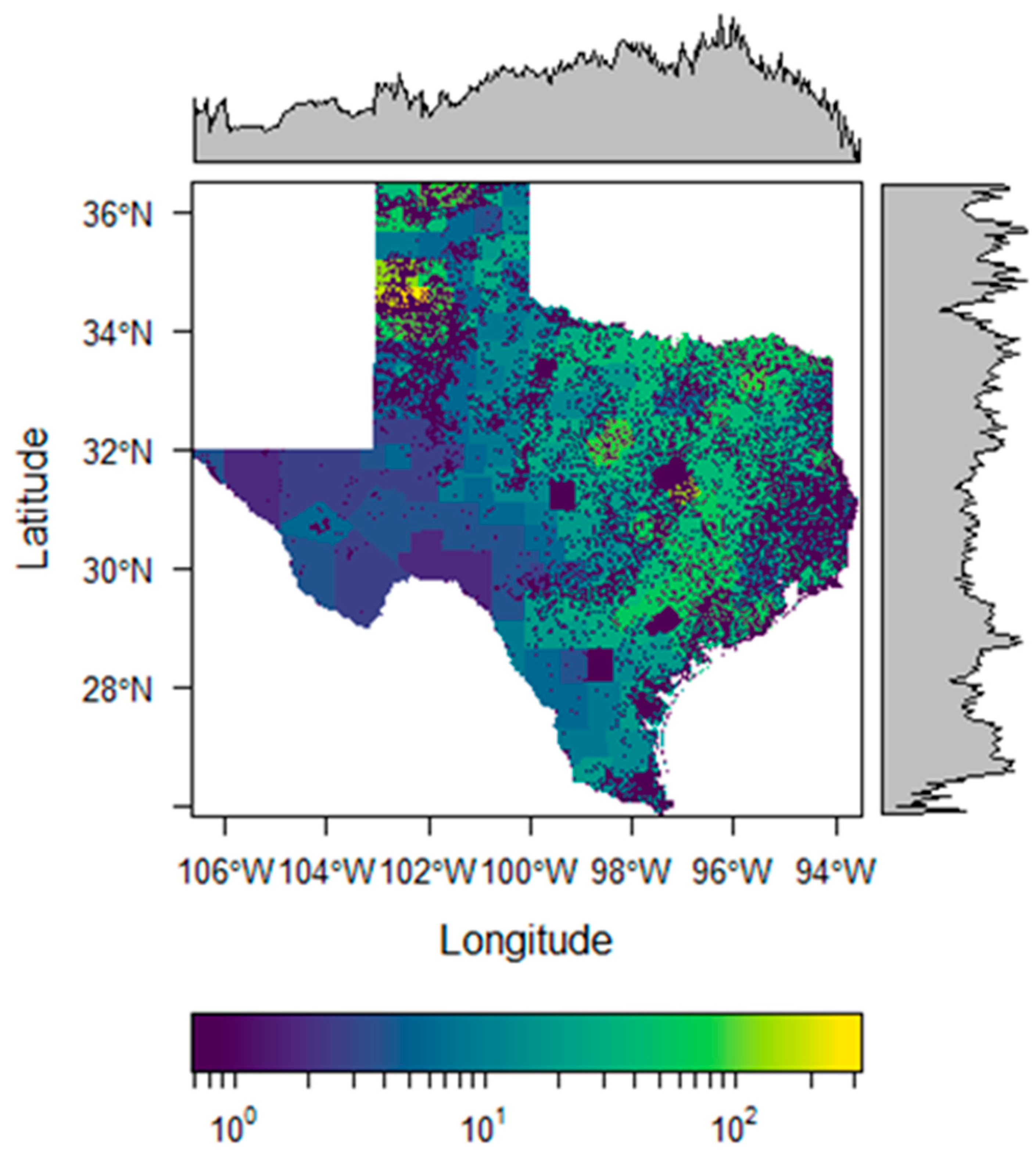
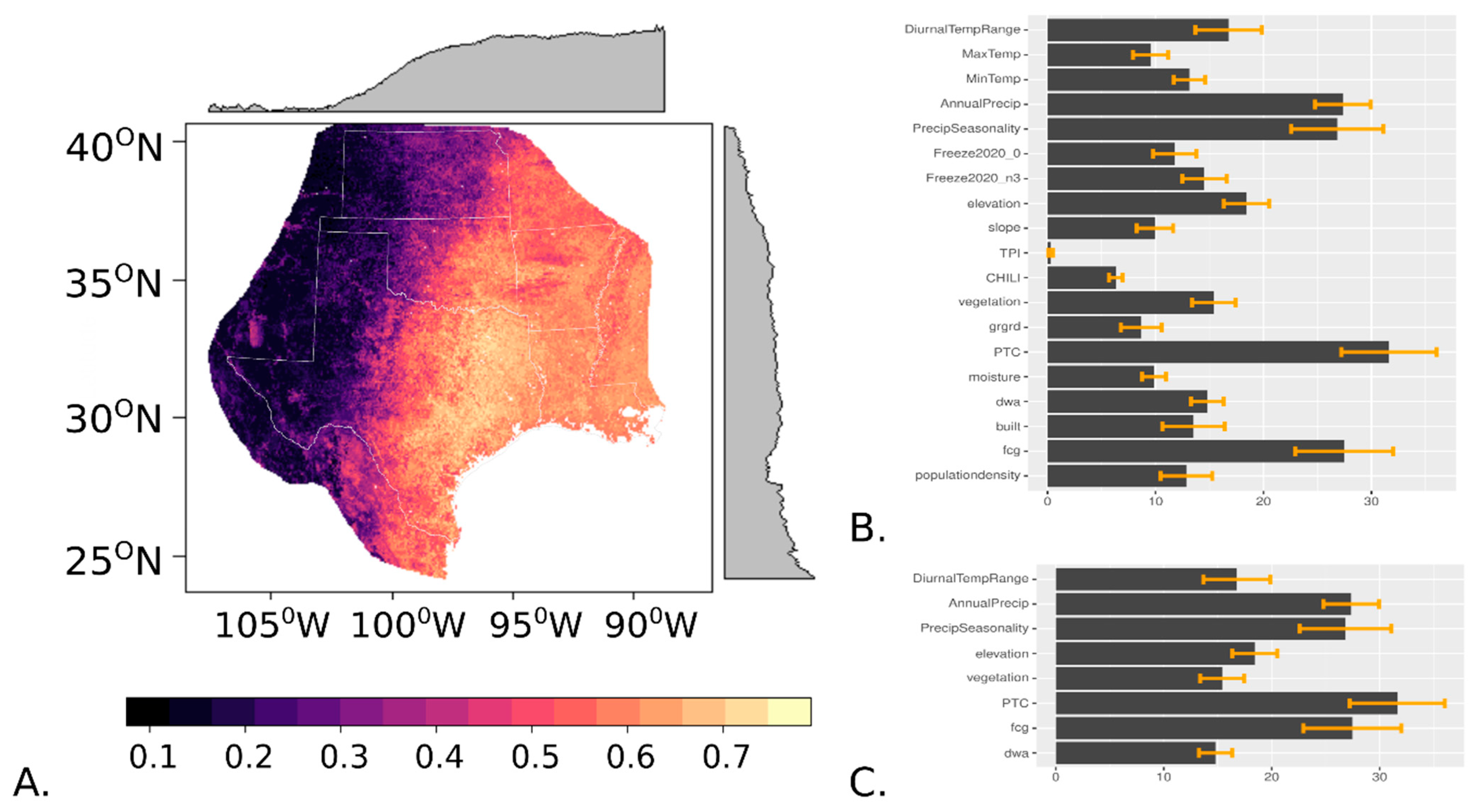
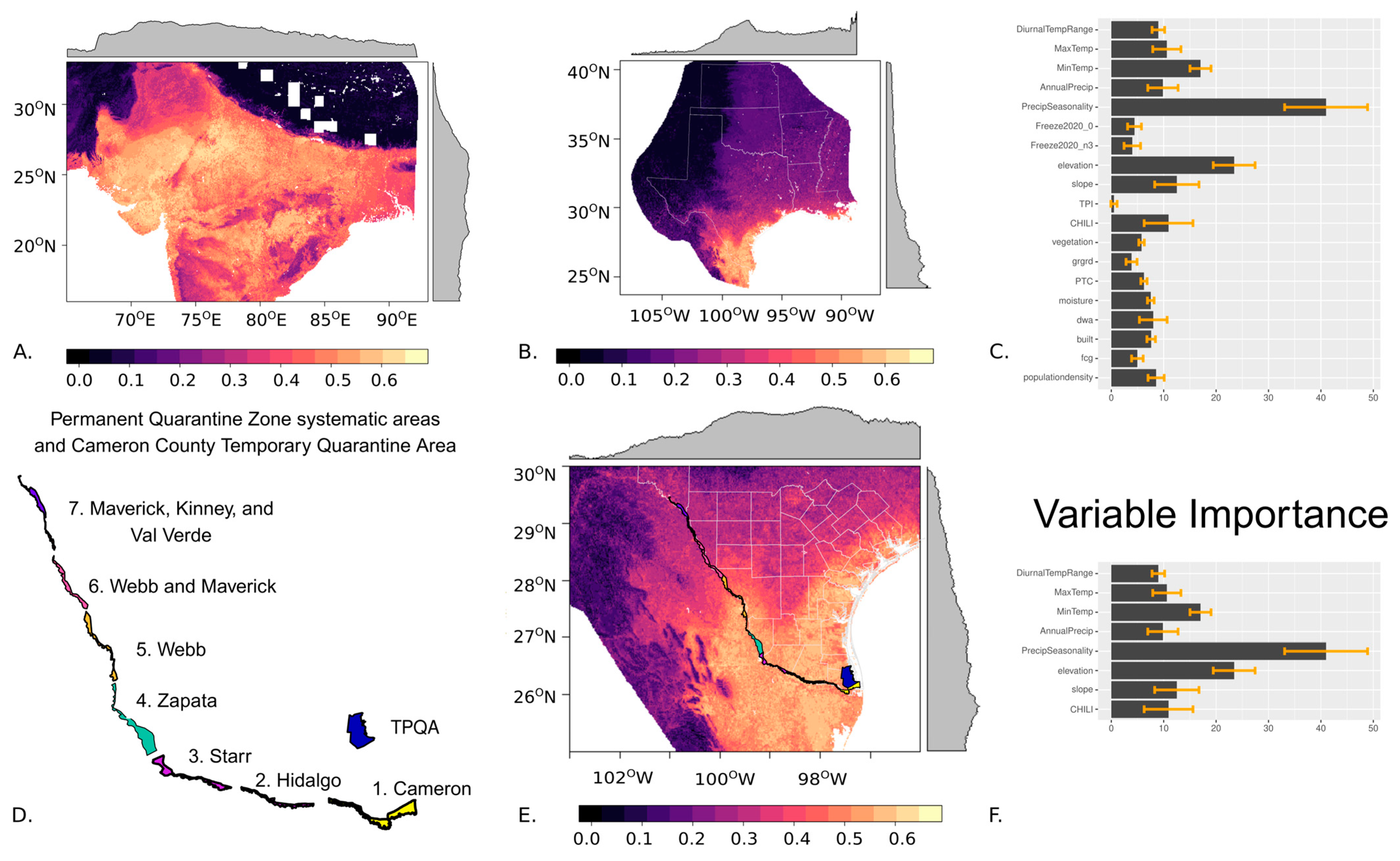
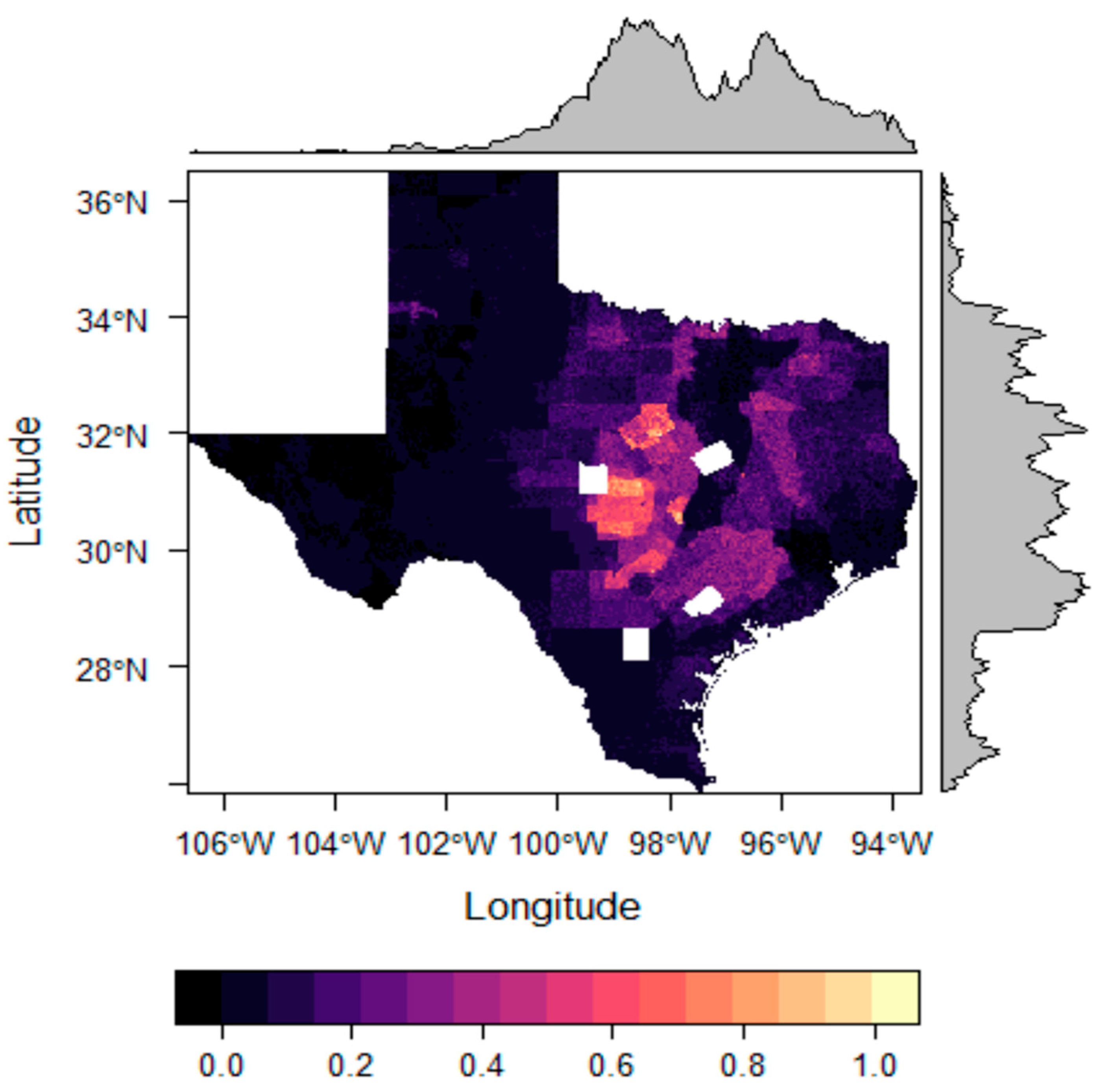
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Uilenberg, G. Babesia—A historical overview. Vet. Parasitol. 2006, 138, 3–10. [Google Scholar] [CrossRef]
- Rodriguez-Vivas, R.I.; Jonsson, N.N.; Bhushan, C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 2018, 117, 3–29. [Google Scholar] [CrossRef]
- Suarez, C.E.; Alzan, H.F.; Silva, M.G.; Rathinasamy, V.; Poole, W.A.; Cooke, B.M. Unravelling the cellular and molecular pathogenesis of bovine babesiosis: Is the sky the limit? Int. J. Parasitol. 2019, 49, 183–197. [Google Scholar] [CrossRef]
- Giles, J.R.; Peterson, A.T.; Busch, J.D.; Olafson, P.U.; Scoles, G.A.; Davey, R.B.; Pound, J.M.; Kammlah, D.M.; Lohmeyer, K.H.; Wagner, D.M. Invasive potential of cattle fever ticks in the southern United States. Parasites Vectors 2014, 7, 189. [Google Scholar] [CrossRef]
- Osbrink, W.L.A.; Thomas, D.B.; Lohmeyer, K.H.; Temeyer, K.B. Climate change and alternative hosts complicate the eradication of cattle fever ticks (Acari: Ixodidae) in the southern United States, a review. Ann. Entomol. Soc. Am. 2022, 115, 39–55. [Google Scholar] [CrossRef]
- Lohmeyer, K.H.; May, M.A.; Thomas, D.B.; Pérez de León, A.A. Implication of nilgai antelope (Artiodactyla: Bovidae) in reinfestations of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in South Texas: A review and update. J. Med. Entomol. 2018, 55, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Goolsby, J.A.; Maestas, L.P.; Garcia, R.; May, M.; Lohmeyer, K.H.; Picanso, J.; Anderson, D.; Coy, J.; Bonilla, D. Preventative methods to reduce the spread of cattle fever ticks on wildlife and protect local endangered species in South Texas. Southwest. Entomol. 2023, 48, 289–302. [Google Scholar] [CrossRef]
- Olafson, P.U.; Thomas, D.B.; May, M.A.; Buckmeier, B.G.; Duhaime, R.A. Tick vector and disease pathogen surveillance of nilgai antelope (Boselaphus tragocamelus) in southeastern Texas, USA. J. Wildl. Dis. 2018, 54, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, M.J.; Conway, W.C.; Arsuffi, T.L.; Lockwood, M.L.; Grisham, B.A. Density of axis deer in Texas: Management implications for native white-tailed deer and associated habitats. J. Fish Wildl. Manag. 2023, 14, 27–40. [Google Scholar] [CrossRef]
- Kistner, T.P.; Hayes, F.A. White-tailed deer as hosts of cattle fever-ticks. J. Wildl. Dis. 1970, 6, 437–440. [Google Scholar] [CrossRef]
- George, J.E. Wildlife as a constraint to the eradication of Boophilus spp. (Acari: Ixodidae). J. Agric. Entomol. 1990, 7, 119–125. [Google Scholar]
- Pound, J.M.; George, J.E.; Kammlah, D.M.; Lohmeyer, K.H.; Davey, R.B. Evidence for role of white-tailed deer (Artiodactyla: Cervidae) in epizootiology of cattle ticks and southern cattle ticks (Acari: Ixodidae) in reinfestations along the Texas/Mexico border in South Texas: A review and update. J. Econ. Entomol. 2010, 103, 211–218. [Google Scholar] [CrossRef]
- Davey, R.B.; Cooksey, L.M.; Despins, J.L. Survival of larvae of Boophilus annulatus, Boophilus microplus, and Boophilus hybrids (Acari: Ixodidae) in different temperature and humidity regimes in the laboratory. Vet. Parasitol. 1991, 40, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.B.; Pound, J.M.; Cooksey, L.M. Comparative reproduction and nonparasitic development of Boophilus microplus and hybridized Boophilus ticks (Acari: Ixodidae) under natural field conditions in subtropical South Texas. Exp. Appl. Acarol. 1994, 18, 185–200. [Google Scholar] [CrossRef]
- Senbill, H.; Hazarika, L.K.; Baruah, A.; Borah, D.K.; Bhattacharyya, B.; Rahman, S. Life cycle of the southern cattle tick, Rhipicephalus (Boophilus) microplus Canestrini 1888 (Acari: Ixodidae) under laboratory conditions. Syst. Appl. Acarol. 2018, 23, 1169. [Google Scholar] [CrossRef]
- Webb, S.L.; Hewitt, D.G.; Hellickson, M.W. Scale of management for mature male white-tailed deer as influenced by home range and movements. J. Wildl. Manag. 2007, 71, 1507–1512. [Google Scholar] [CrossRef]
- Hellickson, M.W.; Campbell, T.A.; Miller, K.V.; Marchinton, R.L.; DeYoung, C.A. Seasonal ranges and site fidelity of adult male white-tailed deer (Odocoileus virginianus) in southern Texas. Southwest. Nat. 2008, 53, 1–8. [Google Scholar] [CrossRef]
- Moczygemba, J.D.; Hewitt, D.G.; Campbell, T.A.; Ortega-S., J.A.; Field, J.; Hellickson, M.W. Home ranges of the nilgai antelope (Boselaphus tragocamelus) in Texas. Southwest. Nat. 2012, 57, 26–30. [Google Scholar] [CrossRef]
- Foley, A.M.; Goolsby, J.A.; Ortega-S, A., Jr.; Ortega-S., J.A.; Pérez de León, A.; Singh, N.K.; Schwartz, A.; Ellis, D.; Hewitt, D.G.; Campbell, T.A. Movement patterns of nilgai antelope in South Texas: Implications for cattle fever tick management. Prev. Vet. Med. 2017, 146, 166–172. [Google Scholar] [CrossRef]
- Sliwa, K.M.; Baumgardt, J.A.; DeYoung, R.W.; Ortega-S, J.A.; Hewitt, D.G.; Goolsby, J.A.; Lohmeyer, K.H. Movement ecology of exotic nilgai antelope: A threat to the re-emergence of cattle fever ticks in the southern USA. Ecosphere 2023, 14, e4401. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Carreón, D.; Almazán, C.; de la Fuente, J. Modeling the impact of climate and landscape on the efficacy of white-tailed deer vaccination for cattle tick control in northeastern Mexico. PLoS ONE 2014, 9, e102905. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, A.L. The first line of defense: Inventing the infrastructure to combat animal diseases. J. Econ. Hist. 2009, 69, 327. [Google Scholar] [CrossRef]
- Leal, B.; Thomas, D.B.; Dearth, R.K. Population dynamics of off-host Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) larvae in response to habitat and seasonality in South Texas. Vet. Sci. 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- USDA—National Agricultural Statistics Service. Census of Agriculture. 2023. Available online: https://www.nass.usda.gov/AgCensus/ (accessed on 1 January 2023).
- R Core Team. R: A Language and Environment for Statistical Computing. 2023. Available online: http://www.r-project.org/ (accessed on 1 January 2023).
- Owens, H.; Barve, V.; Chamberlain, S.; Ram, K.; Hart, T. Spocc: Interface to Species Occurrence Data Sources. 2023. Available online: https://docs.ropensci.org/spocc/ (accessed on 1 January 2023).
- Beck, J.; Böller, M.; Erhardt, A.; Schwanghart, W. Spatial bias in the GBIF database and its effect on modeling species’ geographic distributions. Ecol. Inform. 2014, 19, 10–15. Available online: https://doi.org/10.1016/j.ecoinf.2013.11.002 (accessed on 1 January 2023).
- Crego, R.D.; Stabach, J.A.; Connette, G. Implementation of species distribution models in Google Earth Engine. Divers. Distrib. 2022, 28, 904–916. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Brun, P.; Zimmermann, N.E.; Hari, C.; Pellissier, L.; Karger, D.N. Global climate-related predictors at kilometer resolution for the past and future. Earth Syst. Sci. Data 2022, 14, 5573–5603. [Google Scholar] [CrossRef]
- ASTER. ASTER Global Digital Elevation Model V003. ASTGTM v003; 2019. Available online: https://www.earthdata.nasa.gov/data/catalog/lpcloud-astgtm-003 (accessed on 1 January 2023). [CrossRef]
- Goolsby, J.A.; Saelao, P.; May, M.; Goldsmith, B. Nilgai (Boselaphus tragocamelus) mortality levels in South Texas after historic freeze event. Subtrop. Agric. Environ. 2021, 72, 7–11. [Google Scholar]
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodriguez, E.; Roth, L.; et al. The shuttle radar topography mission. Rev. Geophys. 2007, 45, 2005RG000183. [Google Scholar] [CrossRef]
- Theobald, D.M.; Harrison-Atlas, D.; Monahan, W.B.; Albano, C.M. Ecologically-relevant maps of landforms and physiographic diversity for climate adaptation planning. PLoS ONE 2015, 10, e0143619. [Google Scholar] [CrossRef]
- Potapov, P.; Hansen, M.C.; Pickens, A.; Hernandez-Serna, A.; Tyukavina, A.; Turubanova, S.; Zalles, V.; Li, X.; Khan, A.; Stolle, F.; et al. The global 2000–2020 land cover and land use change dataset derived from the Landsat archive: First results. Front. Remote Sens. 2022, 3, 856903. [Google Scholar] [CrossRef]
- European Commission Joint Research Centre. Copernicus Sentinel DataCopernicus Data Space Ecosystem. Available online: https://dataspace.copernicus.eu/data-collections/copernicus-sentinel-data (accessed on 1 January 2023).
- Warszawski, L.; Frieler, K.; Huber, V.; Piontek, F.; Serdeczny, O.; Schewe, J. Gridded Population of the World, Version 4 (GPWv4): Population Density; Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2017. [Google Scholar] [CrossRef]
- Ho, T.K. Random decision forests. In Proceedings of the 3rd International Conference on Document Analysis and Recognition, Montreal, QC, Canada, 14–16 August 1995; Volume 1, pp. 278–282. [Google Scholar]
- Roberts, D.R.; Bahn, V.; Ciuti, S.; Boyce, M.S.; Elith, J.; Guillera-Arroita, G.; Hauenstein, S.; Lahoz-Monfort, J.J.; Schröder, B.; Thuiller, W.; et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography 2017, 40, 913–929. [Google Scholar] [CrossRef]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. BLOCK CV: An R package for generating spatially or environmentally separated folds for k-fold cross-validation of species distribution models. Methods Ecol. Evol. 2019, 10, 225–232. [Google Scholar] [CrossRef]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Modelling species presence-only data with random forests. Ecography 2021, 44, 1731–1742. [Google Scholar] [CrossRef]
- Evans, J.S.; Murphy, M.A.; Holden, Z.A.; Cushman, S.A. Modeling species distribution and change using random forest. In Predictive Species and Habitat Modeling in Landscape Ecology; Drew, C.A., Wiersma, Y.F., Huettmann, F., Eds.; Springer: New York, NY, USA, 2011; pp. 139–159. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Sillero, N.; Arenas-Castro, S.; Enriquez-Urzelai, U.; Vale, C.G.; Sousa-Guedes, D.; Martínez-Freiría, F.; Real, R.; Barbosa, A.M. Want to model a species niche? a step-by-step guideline on correlative ecological niche modelling. Ecol. Model. 2021, 456, 109671. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Hoeting, J.A.; Jarnevich, C.S. The area under the precision-recall curve as a performance metric for rare binary events. Methods Ecol. Evol. 2019, 10, 565–577. [Google Scholar] [CrossRef]
- Cain, A. White-tailed deer harvest recommendations. Tex. Parks Wildl. 2022. [Google Scholar]
- Gray, S.S. Mule deer harvest recommendations. Tex. Parks Wildl. 2022. [Google Scholar]
- Lamigueiro, O.P.; Hijmans, R. RasterVis. 2023. Available online: https://oscarperpinan.github.io/rastervis/ (accessed on 1 January 2023).
- Sheffield, W.J.; Ables, E.D.; Fall, B.A. Geographic and ecologic distribution of nilgai antelope in Texas. J. Wildl. Manag. 1971, 35, 250–257. [Google Scholar] [CrossRef]
- Sheffield, W.J. Food habits of nilgai antelope in Texas. J. Range Manag. 1983, 36, 316. [Google Scholar] [CrossRef]
- Schmidtmann, E.T.; Schlater, J.L.; Maupin, G.O.; Mertins, J.W. Vegetational associations of host-seeking adult blacklegged ticks, Ixodes scapularis Say (Acari: Ixodidae), on dairy farms in northwestern Wisconsin. J. Dairy Sci. 1998, 81, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Mathisson, D.C.; Kross, S.M.; Palmer, M.I.; Diuk-Wasser, M.A. Effect of Vegetation on the Abundance of Tick Vectors in the Northeastern United States: A Review of the Literature. J. Med. Entomol. 2021, 58, 2030–2037. [Google Scholar] [CrossRef]
- Hanberry, B.B.; Hanberry, P. Regaining the history of deer populations and densities in the southeastern United States. Wildl. Soc. Bull. 2020, 44, 512–518. [Google Scholar] [CrossRef]
- Nuttall, P.A. Climate change impacts on ticks and tick-borne infections. Biologia 2022, 77, 1503–1512. [Google Scholar] [CrossRef]
- Bennett, G.F. Oviposition of Boophilus microplus (Canestrini) (Acarida: Ixodidae). Acarologia 1974, 16, 52–61. [Google Scholar]
- Ouhelli, H.; Pandey, V.S.; Choukri, M. The effects of temperature, humidity, photoperiod and weight of the engorged female on oviposition of Boophilus annulatus (Say, 1821). Vet. Parasitol. 1982, 11, 231–239. [Google Scholar] [CrossRef]
- Van Campen, H.; Rhyan, J. The role of wildlife in diseases of cattle. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 147–161. [Google Scholar] [CrossRef]

| Variables | Source (Resolution) |
|---|---|
| Environmental variables | |
| Diurnal temperature range Max temperature | Chelsa BioClim+ (~1 km) Chelsa BioClim+ (~1 km) |
| Min temperature | Chelsa BioClim+ (~1 km) |
| Annual precipitation | Chelsa BioClim+ (~1 km) |
| Precipitation seasonality | Chelsa BioClim+ (~1 km) |
| Average annual number of days below 0 °C based on night land surface temperature, 2016–2020 | MODIS Aqua Land Surface Temperature (daily, 1 km) |
| Average annual number of days below −3 °C based on night land surface temperature, 2016–2020 | MODIS Aqua Land Surface Temperature (daily, 1 km) |
| Topographic variables | |
| Elevation | NASA SRTM DEM (~30 m) |
| Slope | NASA SRTM DEM (~30 m) |
| Multi-scale topographic position index (mTPI) | JAXA AVE (~270 m) |
| Topographic shading/heat insolation (CHILI) | JAXA AVE (~270 m) |
| Land cover and configuration variables | |
| Average vegetation/productivity (NDVI) | Sentinel 2 (weekly, 10 m) |
| Grassland cover gradient | GLAD project (100 m) |
| Percent tree canopy cover (PTC) | MODIS (250 m) |
| Moisture (MNDWI) | Sentinel 2 (weekly, 20 m) |
| Distance to water | GLAD project (100 m) |
| Normalized difference built-up index (NDBI) | Sentinel 2 (weekly, 20 m) |
| Forest and grassland or cropland edge | GLAD project (100 m) |
| Land use variables | |
| Human population density | NASA SEDAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeiffer, V.W.; García-Carrasco, J.-M.; Crowder, D.W.; Ueti, M.W.; Poh, K.C.; Gutierrez Illán, J. Modeling the Wildlife–Livestock Interface of Cattle Fever Ticks in the Southern United States. Insects 2025, 16, 940. https://doi.org/10.3390/insects16090940
Pfeiffer VW, García-Carrasco J-M, Crowder DW, Ueti MW, Poh KC, Gutierrez Illán J. Modeling the Wildlife–Livestock Interface of Cattle Fever Ticks in the Southern United States. Insects. 2025; 16(9):940. https://doi.org/10.3390/insects16090940
Chicago/Turabian StylePfeiffer, Vera W., José-María García-Carrasco, David W. Crowder, Massaro W. Ueti, Karen C. Poh, and Javier Gutierrez Illán. 2025. "Modeling the Wildlife–Livestock Interface of Cattle Fever Ticks in the Southern United States" Insects 16, no. 9: 940. https://doi.org/10.3390/insects16090940
APA StylePfeiffer, V. W., García-Carrasco, J.-M., Crowder, D. W., Ueti, M. W., Poh, K. C., & Gutierrez Illán, J. (2025). Modeling the Wildlife–Livestock Interface of Cattle Fever Ticks in the Southern United States. Insects, 16(9), 940. https://doi.org/10.3390/insects16090940






