Syntaxin-1A Silencing by RNAi Disrupts Growth and Reproduction in the Asian Citrus Psyllid, Diaphorina citri
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects Rearing and Sample Preparation
2.2. Identification of Syx1A and Bioinformatic Analysis
2.3. RNA Isolation and cDNA Synthesis
2.4. RT-qPCR Analysis
2.5. Syx1A Expression Analysis of Different Developmental and Tissue
2.6. RNA Interference
2.7. Effect of Syx1A on the Survival Rate and Body Weight of Asian Citrus Psyllid
2.8. Effect of on Syx1A Female Fecundity
2.9. Effect of Syx1A Silencing on the Expression of Vitellogenin and Its Receptor Genes
2.10. Statistical Analysis
3. Results
3.1. Sequence Characterization and Phylogenetic Insights into Syx1A
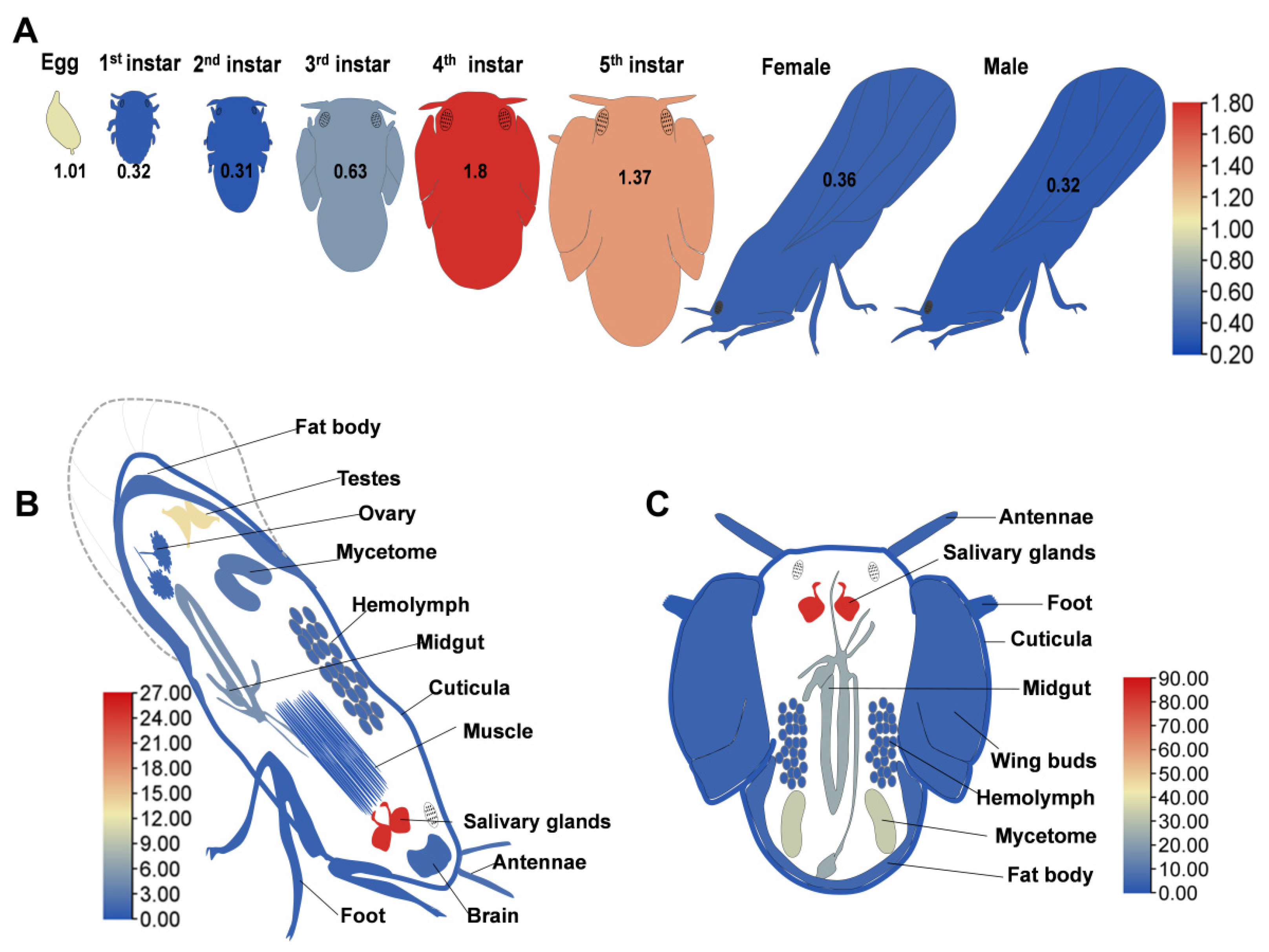
3.2. Expression Profiles of Syx1A Across Developmental Stages and Tissues
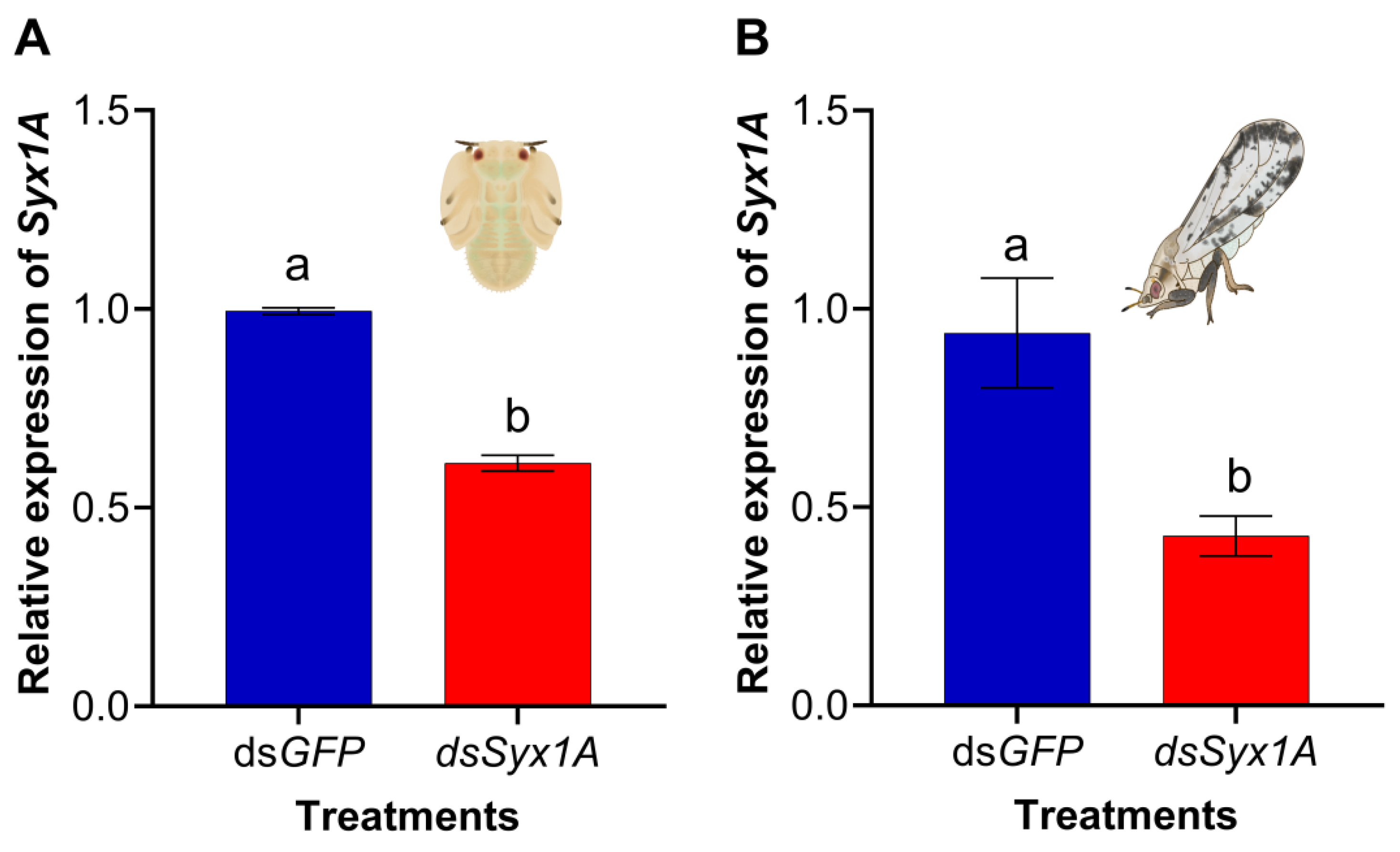
3.3. Evaluation of dsRNA-Mediated Silencing Efficiency of Exogenous Syx1A in Nymphs and Adults of Diaphorina citri
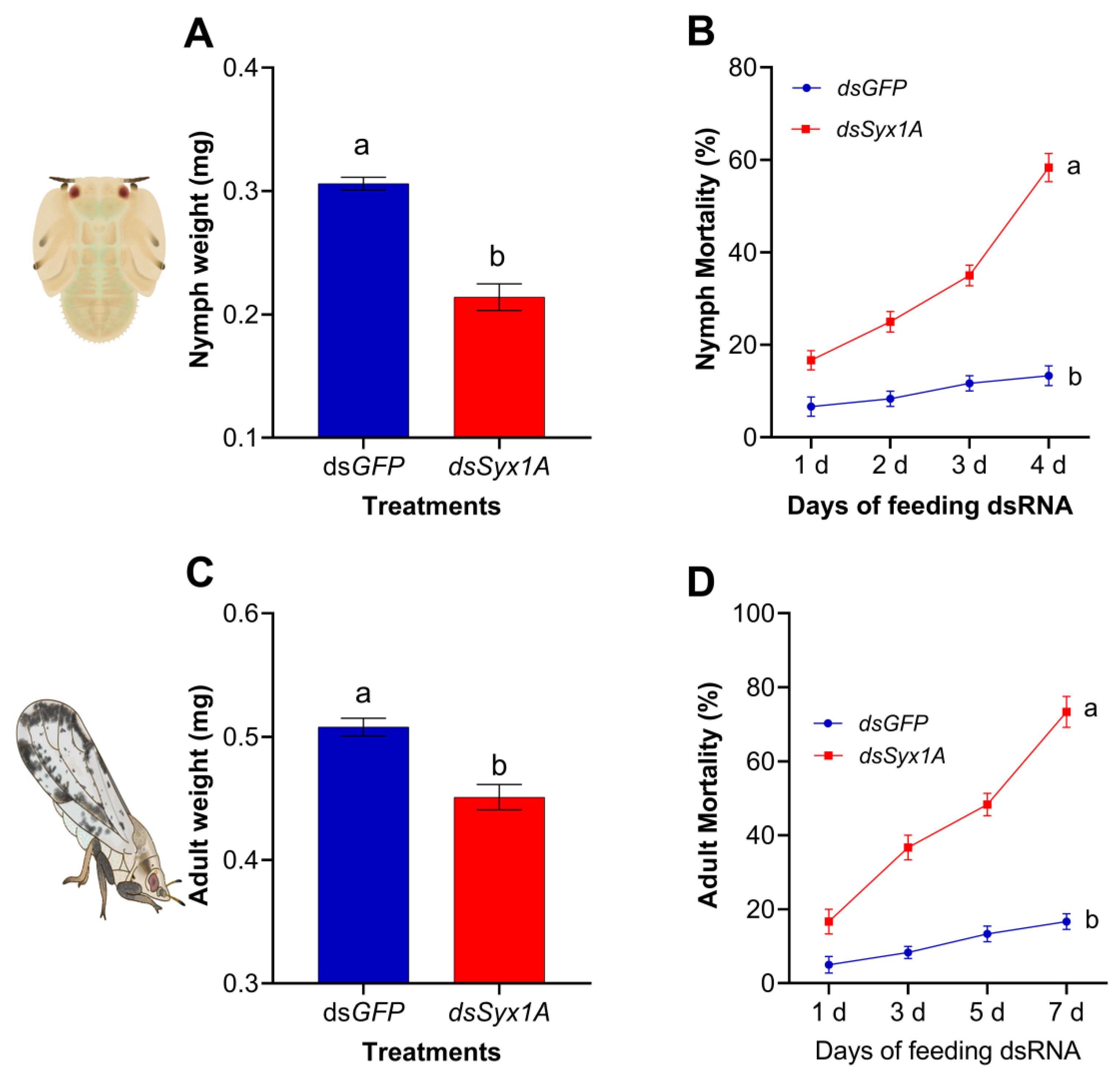
3.4. Effects of Syx1A Silencing on Body Weight and Survival of Diaphorina citri Nymphs and Adults
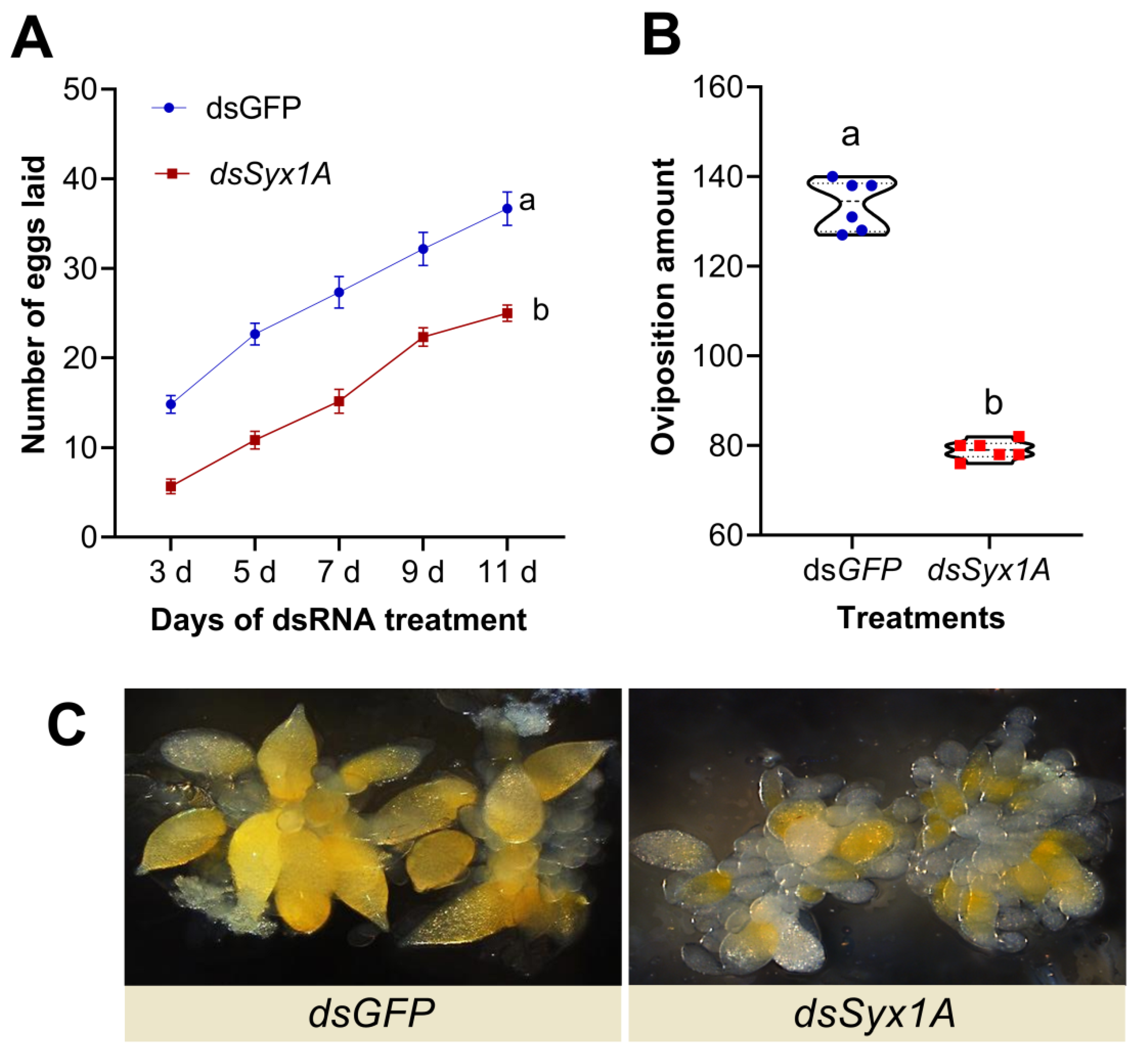
3.5. Impact of Syx1A Silencing on Oviposition and Ovarian Development in Adult Diaphorina citri
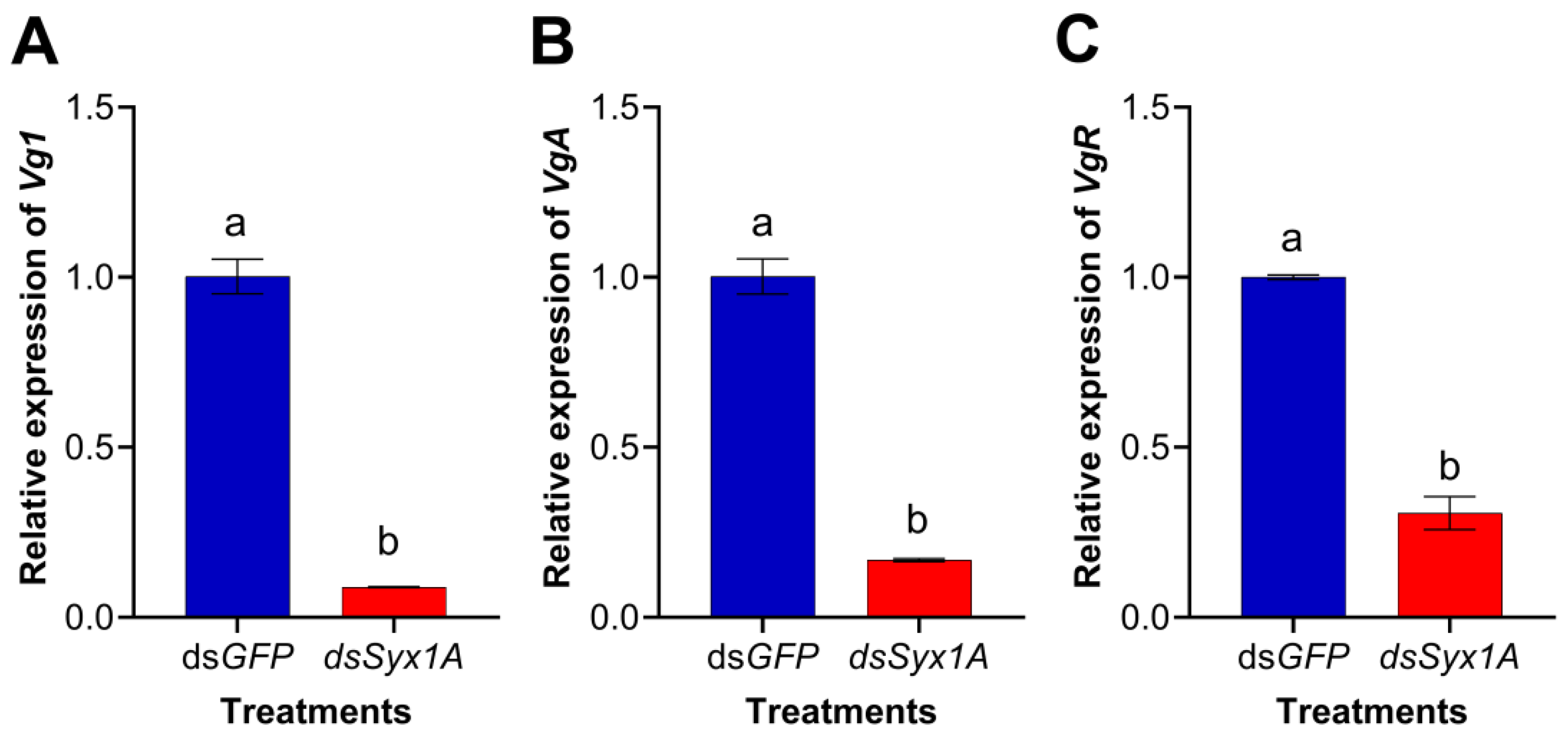
3.6. Influence of Syx1A Silencing on the Expression of Vg1, VgA, and VgR in Adult Diaphorina citri
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, N.; Trivedi, P. Citrus Huanglongbing: A Newly Relevant Disease Presents Unprecedented Challenges. Phytopathology 2013, 103, 652–665. [Google Scholar] [CrossRef]
- Bove, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Graham, J.H.; Bassanezi, R.B.; Dawson, W.O.; Dantzler, R. Management of Huanglongbing of Citrus: Lessons from São Paulo and Florida. Annu. Rev. Phytopathol. 2024, 62, 243–262. [Google Scholar] [CrossRef]
- Hall, D.G.; Richardson, M.L.; Ammar, E.D.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Li, X.; Ruan, H.; Zhou, C.; Meng, X.; Chen, W. Controlling Citrus Huanglongbing: Green Sustainable Development Route Is the Future. Front. Plant Sci. 2021, 12, 760481. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Hoddle, M.S.; Alferez, F.; Tena, A.; Wade, T.; Chakravarty, S.; Wang, N.; Stelinski, L.L.; Urbaneja, A. Huanglongbing (HLB) and its vectors: Recent research advances and future challenges. Entomol. Gen. 2025, 45, 17–35. [Google Scholar] [CrossRef]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and Management of Asian Citrus Psyllid, Vector of the Huanglongbing Pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef]
- Tiwari, S.; Pelz-Stelinski, K.; Stelinski, L.L. Effect of Candidatus Liberibacter asiaticus infection on susceptibility of Asian citrus psyllid, Diaphorina citri, to selected insecticides. Pest Manag. Sci. 2011, 67, 94–99. [Google Scholar] [CrossRef]
- Alquézar, B.; Carmona, L.; Bennici, S.; Miranda, M.P.; Bassanezi, R.B.; Peña, L. Cultural Management of Huanglongbing: Current Status and Ongoing Research. Phytopathology 2022, 112, 11–25. [Google Scholar] [CrossRef]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef]
- Chen, X.D.; Neupane, S.; Gossett, H.; Pelz-Stelinski, K.S.; Stelinski, L.L. Insecticide rotation scheme restores insecticide susceptibility in thiamethoxam-resistant field populations of Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), in Florida. Pest Manag. Sci. 2021, 77, 464–473. [Google Scholar] [CrossRef]
- Chen, X.D.; Stelinski, L.L. Resistance Management for Asian Citrus Psyllid, Diaphorina citri Kuwayama, in Florida. Insects 2017, 8, 103. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Y.; Yuan, C.; Gao, Y.; Fan, J.; Yuan, G.; Wang, J.; Dou, W. Genetic mutations and insecticide resistance in Diaphorina citri: A comparative study across Chinese citrus regions. Pest Manag. Sci. 2025, 81, 4831–4838. [Google Scholar] [CrossRef]
- Lopez, S.B.G.; Guimarães-Ribeiro, V.; Rodriguez, J.V.G.; Dorand, F.A.P.S.; Salles, T.S.; Sá-Guimarães, T.E.; Alvarenga, E.S.L.; Melo, A.C.A.; Almeida, R.V.; Moreira, M.F. RNAi-based bioinsecticide for Aedes mosquito control. Sci. Rep. 2019, 9, 4038. [Google Scholar] [CrossRef]
- Yu, N.; Christiaens, O.; Liu, J.; Niu, J.; Cappelle, K.; Caccia, S.; Huvenne, H.; Smagghe, G. Delivery of dsRNA for RNAi in insects: An overview and future directions. Insect Sci. 2013, 20, 4–14. [Google Scholar] [CrossRef]
- Kebede, M.; Fite, T. RNA interference (RNAi) applications to the management of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae): Its current trends and future prospects. Front. Mol. Biosci. 2022, 9, 944774. [Google Scholar] [CrossRef]
- Gamez, S.; Srivastav, S.; Akbari, O.S.; Lau, N.C. Diverse Defenses: A Perspective Comparing Dipteran Piwi-piRNA Pathways. Cells. 2020, 9, 2180. [Google Scholar] [CrossRef]
- Haller, S.; Widmer, F.; Siegfried, B.D.; Zhuo, X.; Romeis, J. Responses of two ladybird beetle species (Coleoptera: Coccinellidae) to dietary RNAi. Pest Manag. Sci. 2019, 75, 2652–2662. [Google Scholar] [CrossRef]
- Schvartzman, C.; Fresia, P.; Murchio, S.; Mujica, M.V.; Dalla-Rizza, M. RNAi in Piezodorus guildinii (Hemiptera: Pentatomidae): Transcriptome Assembly for the Development of Pest Control Strategies. Front. Plant Sci. 2022, 13, 804839. [Google Scholar] [CrossRef]
- Liu, S.; Jaouannet, M.; Dempsey, D.M.A.; Imani, J.; Coustau, C.; Kogel, K.-H. RNA-based technologies for insect control in plant production. Biotechnol. Adv. 2020, 39, 107463. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Lu, W.; Yin, X.; An, S. Application progress of plant-mediated RNAi in pest control. Front. Bioeng. Biotechnol. 2022, 10, 963026. [Google Scholar] [CrossRef]
- Cagliari, D.; Dias, N.P.; Galdeano, D.M.; dos Santos, E.Á.; Smagghe, G.; Zotti, M.J. Management of Pest Insects and Plant Diseases by Non-Transformative RNAi. Front. Plant Sci. 2019, 10, 1319. [Google Scholar] [CrossRef]
- Cooper, A.M.; Silver, K.; Zhang, J.; Park, Y.; Zhu, K.Y. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef]
- Cooper, A.M.W.; Yu, Z.; Biondi, M.; Song, H.; Silver, K.; Zhang, J.; Zhu, K.Y. Stability of double-stranded RNA in gut contents and hemolymph of Ostrinia nubilalis larvae. Pestic. Biochem. Physiol. 2020, 169, 104672. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Palli, S.R. Mechanisms, Applications, and Challenges of Insect RNA Interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Nunes, M.A.; España, M.U.; Namin, H.H.; Jin, P.; Bensoussan, N.; Zhurov, V.; Rahman, T.; De Clercq, R.; Hilson, P.; et al. RNAi-based reverse genetics in the chelicerate model Tetranychus urticae: A comparative analysis of five methods for gene silencing. PLoS ONE 2017, 12, e0180654. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, N.; Thiruvengadam, V.; Sushil, S.N. Nanoparticle-mediated dsRNA delivery for precision insect pest control: A comprehensive review. Mol. Biol. Rep. 2024, 51, 355. [Google Scholar] [CrossRef]
- Kolge, H.; Kadam, K.; Ghormade, V. Chitosan nanocarriers mediated dsRNA delivery in gene silencing for Helicoverpa armigera biocontrol. Pestic. Biochem. Physiol. 2023, 189, 105292. [Google Scholar] [CrossRef]
- Yan, S.; Ren, B.; Shen, J. Nanoparticle-mediated double-stranded RNA delivery system: A promising approach for sustainable pest management. Insect Sci. 2021, 28, 21–34. [Google Scholar] [CrossRef]
- Ma, Z.; Zheng, Y.; Chao, Z.; Chen, H.; Zhang, Y.; Yin, M.; Shen, J.; Yan, S. Visualization of the process of a nanocarrier-mediated gene delivery: Stabilization, endocytosis and endosomal escape of genes for intracellular spreading. J. Nanobiotechnol. 2022, 20, 124. [Google Scholar] [CrossRef]
- Lu, Z.; Xia, T.; Zhang, C.; He, Q.; Zhong, H.; Fu, S.; Yuan, X.; Liu, X.; Liu, Y.; Chen, W.; et al. Characterization of an RR-2 cuticle protein DcCP8 and its potential application based on SPc nanoparticle-wrapped dsRNA in Diaphorina citri. Pest Manag. Sci. 2024, 80, 6262–6275. [Google Scholar] [CrossRef]
- El-Shesheny, I.; Hajeri, S.; El-Hawary, I.; Gowda, S.; Killiny, N. Silencing Abnormal Wing Disc Gene of the Asian Citrus Psyllid, Diaphorina citri Disrupts Adult Wing Development and Increases Nymph Mortality. PLoS ONE 2013, 8, e65392. [Google Scholar] [CrossRef] [PubMed]
- Galdeano, D.M.; Breton, M.C.; Spotti Lopes, J.R.; Falk, B.W.; Machado, M.A. Oral delivery of double-stranded RNAs induces mortality in nymphs and adults of the Asian citrus psyllid, Diaphorina citri. PLoS ONE 2017, 12, e0171847. [Google Scholar] [CrossRef]
- Pacheco, I.d.S.; Galdeano, D.M.; Maluta, N.K.P.; Lopes, J.R.S.; Machado, M.A. Gene silencing of Diaphorina citri candidate effectors promotes changes in feeding behaviors. Sci. Rep. 2020, 10, 5992. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Z.; Yu, H. Silencing of Glycogen Synthase Kinase 3 Significantly Inhibits Chitin and Fatty Acid Metabolism in Asian Citrus Psyllid, Diaphorina citri. Int. J. Mol. Sci. 2022, 23, 9654. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Qiu, J.; Hu, Y.; Xu, P.; Deng, Y.; Tian, L.; Wei, Y.; Sang, W.; Liu, Y.; Qiu, B. Silencing of V-ATPase-E gene causes midgut apoptosis of Diaphorina citri and affects its acquisition of Huanglongbing pathogen. Insect Sci. 2023, 30, 1022–1034. [Google Scholar] [CrossRef]
- Yang, S.; Zou, Z.; Xin, T.; Cai, S.; Wang, X.; Zhang, H.; Zhong, L.; Xia, B. Knockdown of hexokinase in Diaphorina citri Kuwayama (Hemiptera: Liviidae) by RNAi inhibits chitin synthesis and leads to abnormal phenotypes. Pest Manag. Sci. 2022, 78, 4303–4313. [Google Scholar] [CrossRef]
- D’Agostino, M.; Risselada, H.J.; Lürick, A.; Ungermann, C.; Mayer, A. A tethering complex drives the terminal stage of SNARE-dependent membrane fusion. Nature 2017, 551, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.M.; Stein, A.; Behrmann, E.; Riedel, D.; Cypionka, A.; Farsi, Z.; Walla, P.J.; Raunser, S.; Jahn, R. Membrane Fusion Intermediates via Directional and Full Assembly of the SNARE Complex. Science 2012, 336, 1581–1584. [Google Scholar] [CrossRef]
- Liu, H.; Dang, R.; Zhang, W.; Hong, J.; Li, X. SNARE proteins: Core engines of membrane fusion in cancer. Biochim. Biophys. Acta 2024, 189148. [Google Scholar] [CrossRef]
- Lobingier, B.T.; Merz, A.J. Sec1/Munc18 protein Vps33 binds to SNARE domains and the quaternary SNARE complex. Mol. Biol. Cell 2012, 23, 4611–4622. [Google Scholar] [CrossRef]
- Fang, Q.A.-O.; Zhao, Y.; Herbst, A.D.; Kim, B.N.; Lindau, M. Positively charged amino acids at the SNAP-25 C terminus determine fusion rates, fusion pore properties, and energetics of tight SNARE complex zippering. J. Neurosci. 2015, 35, 3230–3239. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Ebine, K.; Nishimura, K.; Tsutsumi, N.; Ueda, T. Longin R-SNARE is retrieved from the plasma membrane by ANTH domain-containing proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 25150–25158. [Google Scholar] [CrossRef]
- Song, J.; Mizrak, A.; Lee, C.-W.; Cicconet, M.; Lai, Z.W.; Tang, W.; Lu, C.; Mohr, S.E.; Farese, R.V.; Walther, T.C. Identification of two pathways mediating protein targeting from ER to lipid droplets. Nat. Cell Biol. 2022, 24, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Surpin, M.; Zheng, H.; Morita, M.T.; Saito, C.; Avila, E.; Blakeslee, J.J.; Bandyopadhyay, A.; Kovaleva, V.; Carter, D.; Murphy, A.; et al. The VTI Family of SNARE Proteins Is Necessary for Plant Viability and Mediates Different Protein Transport Pathways. Plant Cell 2003, 15, 2885–2899. [Google Scholar] [CrossRef]
- Schulze, K.L.; Broadie, K.; Perin, M.S.; Bellen, H.J. Genetic and electrophysiological studies of drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell 1995, 80, 311–320. [Google Scholar] [CrossRef]
- Irfan, M.; Gopaul, K.R.; Miry, O.; Hökfelt, T.; Stanton, P.K.; Bark, C. SNAP-25 isoforms differentially regulate synaptic transmission and long-term synaptic plasticity at central synapses. Sci. Rep. 2019, 9, 6403. [Google Scholar] [CrossRef]
- Megighian, A.; Zordan, M.; Pantano, S.; Scorzeto, M.; Rigoni, M.; Zanini, D.; Rossetto, O.; Montecucco, C. Evidence for a radial SNARE super-complex mediating neurotransmitter release at the Drosophila neuromuscular junction. J. Cell Sci. 2013, 126, 3134–3140. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Li, Y.; Zhang, J. Targeting Syntaxin 1A via RNA interference inhibits feeding and midgut development in Locusta migratoria. Insect Sci. 2025, 32, 385–397. [Google Scholar] [CrossRef]
- Chen, Q.; He, J.; Ma, C.; Yu, D.; Kang, L. Syntaxin 1A modulates the sexual maturity rate and progeny egg size related to phase changes in locusts. Insect Biochem. Mol. Biol. 2015, 56, 1–8. [Google Scholar] [CrossRef]
- Guo, C.; Pan, H.; Zhang, L.; Ou, D.; Lu, Z.; Khan, M.M.; Qiu, B. Comprehensive Assessment of Candidate Reference Genes for Gene Expression Studies Using RT-qPCR in Tamarixia radiata, a Predominant Parasitoid of Diaphorina citri. Genes 2020, 11, 1178. [Google Scholar] [CrossRef]
- Kong, W.; Lv, X.; Ran, X.; Mukangango, M.; Eric Derrick, B.; Qiu, B.; Guo, C. Comprehensive Assessment of Reference Gene Expression within the Whitefly Dialeurodes citri Using RT-qPCR. Genes 2024, 15, 318. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, S.; Chen, S.; Chen, H.; Liu, Y.; Liu, X.; Xu, H. Vestigial mediates the effect of insulin signaling pathway on wing-morph switching in planthoppers. PLoS Genet. 2021, 17, e1009312. [Google Scholar] [CrossRef]
- Lu, L.; Feng, Q.; Wang, S.; Ghafar, M.A.; Cheng, H.; Zhou, C.; Wang, L. miR-278-3p targets ATG16L1 to modulate autophagy and suppresses CLas proliferation in Diaphorina citri. Int. J. Biol. Macromol. 2025, 308, 142441. [Google Scholar] [CrossRef]
- Sutton, R.B.; Fasshauer, D.; Jahn, R.; Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 1998, 395, 347–353. [Google Scholar] [CrossRef]
- Farkaš, R.; Beňová-Liszeková, D.; Mentelová, L.; Mahmood, S.; Ďatková, Z.; Beňo, M.; Pečeňová, L.; Raška, O.; Šmigová, J.; Chase, B.A.; et al. Vacuole dynamics in the salivary glands of Drosophila melanogaster during prepupal development. Dev. Growth Differ. 2015, 57, 74–96. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, W.; Lin, W.; Lai, T.; Chien, C. Nak regulates Dlg basal localization in Drosophila salivary gland cells. Biochem. Biophys. Res. Commun. 2009, 382, 108–113. [Google Scholar] [CrossRef]
- Kuang, Y.; Shangguan, C.; Wang, C.; Gao, L.; Yu, X. Salivary effector DcE1 suppresses plant defense and facilitates the successful feeding of Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2025, 81, 1717–1726. [Google Scholar] [CrossRef]
- Zhao, S.; Ran, X.; Huang, Y.; Sang, W.; Derrick, B.E.; Qiu, B. Transcriptomic response of citrus psyllid salivary glands to the infection of citrus Huanglongbing pathogen. Bull. Entomol. Res. 2024, 114, 210–229. [Google Scholar] [CrossRef]
- Wu, Z.; Qu, M.; Chen, M.; Lin, J. Proteomic and transcriptomic analyses of saliva and salivary glands from the Asian citrus psyllid, Diaphorina citri. J. Proteom. 2021, 238, 104136. [Google Scholar] [CrossRef]
- Zhuo, J.; Xue, J.; Lu, J.; Huang, H.; Xu, H.; Zhang, C. Effect of RNAi-mediated knockdown of NlTOR gene on fertility of male Nilaparvata lugens. J. Insect Physiol. 2017, 98, 149–159. [Google Scholar] [CrossRef]
- Zha, X.; Wang, H.; Sun, W.; Zhang, H.; Wen, J.; Huang, X.; Lu, C.; Shen, Y. Characteristics of the Peritrophic Matrix of the Silkworm, Bombyx mori and Factors Influencing Its Formation. Insects 2021, 12, 516. [Google Scholar] [CrossRef]
- Isoe, J.; Rascón, A.A.; Kunz, S.; Miesfeld, R.L. Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol. 2009, 39, 903–912. [Google Scholar] [CrossRef]
- Browning, R.; Karim, S. RNA interference-mediated depletion of N-ethylmaleimide Sensitive Fusion Protein and Synaptosomal Associated Protein of 25 kDa results in the inhibition of blood feeding of the Gulf Coast tick, Amblyomma maculatum. Insect Mol. Biol. 2013, 22, 245–257. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Palli, S.R. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2011, 41, 294–305. [Google Scholar] [CrossRef]
- Li, H.; Mo, J.; Wang, X.; Pan, B.; Xu, S.; Li, S.; Zheng, X.; Lu, W. IPS (In-Plant System) Delivery of Double-Stranded Vitellogenin and Vitellogenin receptor via Hydroponics for Pest Control in Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Int. J. Mol. Sci. 2023, 24, 9497. [Google Scholar] [CrossRef]
- Nian, X.; Luo, Y.; He, X.; Wu, S.; Li, J.; Wang, D.; Holford, P.; Beattie, G.A.C.; Cen, Y.; Zhang, S.; et al. Infection with ‘Candidatus Liberibacter asiaticus’ improves the fecundity of Diaphorina citri aiding its proliferation: A win-win strategy. Mol. Ecol. 2024, 33, e17214. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, D.; Wang, X.; Qiu, B.; Chang, C.; Guo, C. Syntaxin-1A Silencing by RNAi Disrupts Growth and Reproduction in the Asian Citrus Psyllid, Diaphorina citri. Insects 2025, 16, 901. https://doi.org/10.3390/insects16090901
Dong D, Wang X, Qiu B, Chang C, Guo C. Syntaxin-1A Silencing by RNAi Disrupts Growth and Reproduction in the Asian Citrus Psyllid, Diaphorina citri. Insects. 2025; 16(9):901. https://doi.org/10.3390/insects16090901
Chicago/Turabian StyleDong, Dingming, Xingmin Wang, Baoli Qiu, Changqing Chang, and Changfei Guo. 2025. "Syntaxin-1A Silencing by RNAi Disrupts Growth and Reproduction in the Asian Citrus Psyllid, Diaphorina citri" Insects 16, no. 9: 901. https://doi.org/10.3390/insects16090901
APA StyleDong, D., Wang, X., Qiu, B., Chang, C., & Guo, C. (2025). Syntaxin-1A Silencing by RNAi Disrupts Growth and Reproduction in the Asian Citrus Psyllid, Diaphorina citri. Insects, 16(9), 901. https://doi.org/10.3390/insects16090901





