Phonotaxis in Male Field Crickets: The Role of Flight Experience, Serotonin and Octopamine Neurotransmission
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
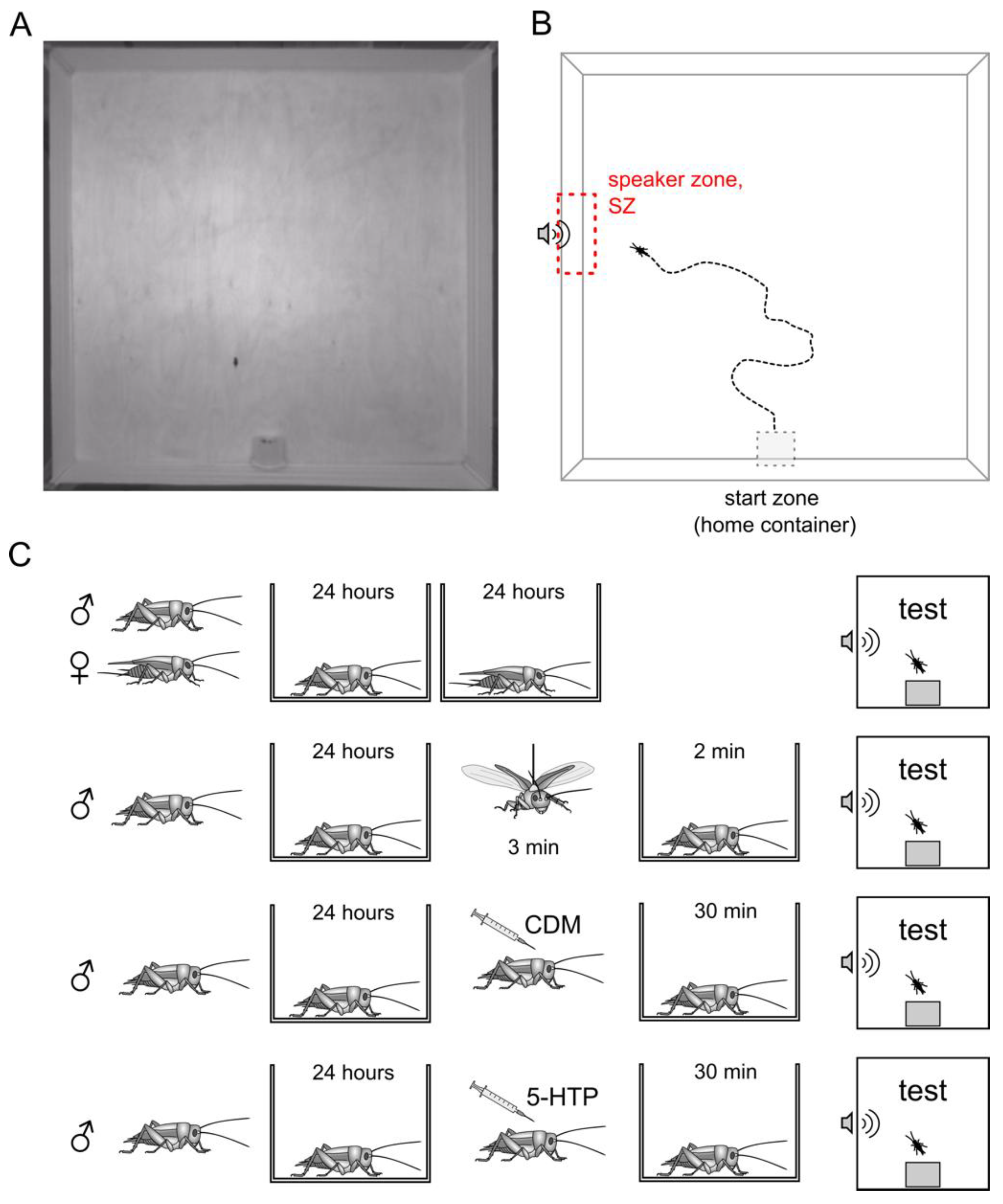
2.2. Behavioral Test Equipment
2.3. Behavioral Analysis
2.4. Tethered Flight
2.5. Pharmacological Treatments
2.6. Statistical Analysis
3. Results
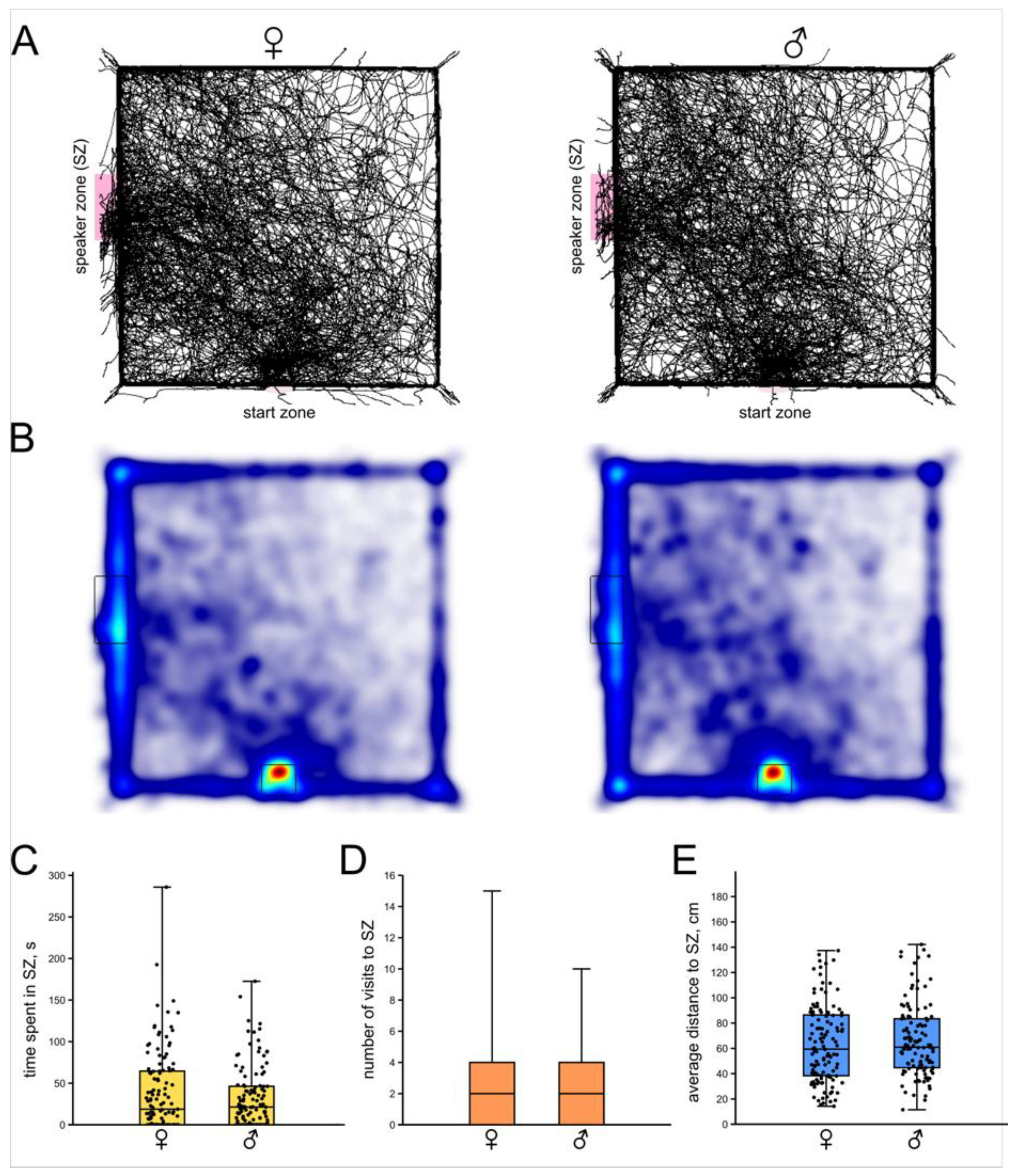
3.1. Phonotactic Behavior of Intact Female and Male Crickets
3.2. Effects of Flight Experience on the Phonotactic Behavior of Male Crickets
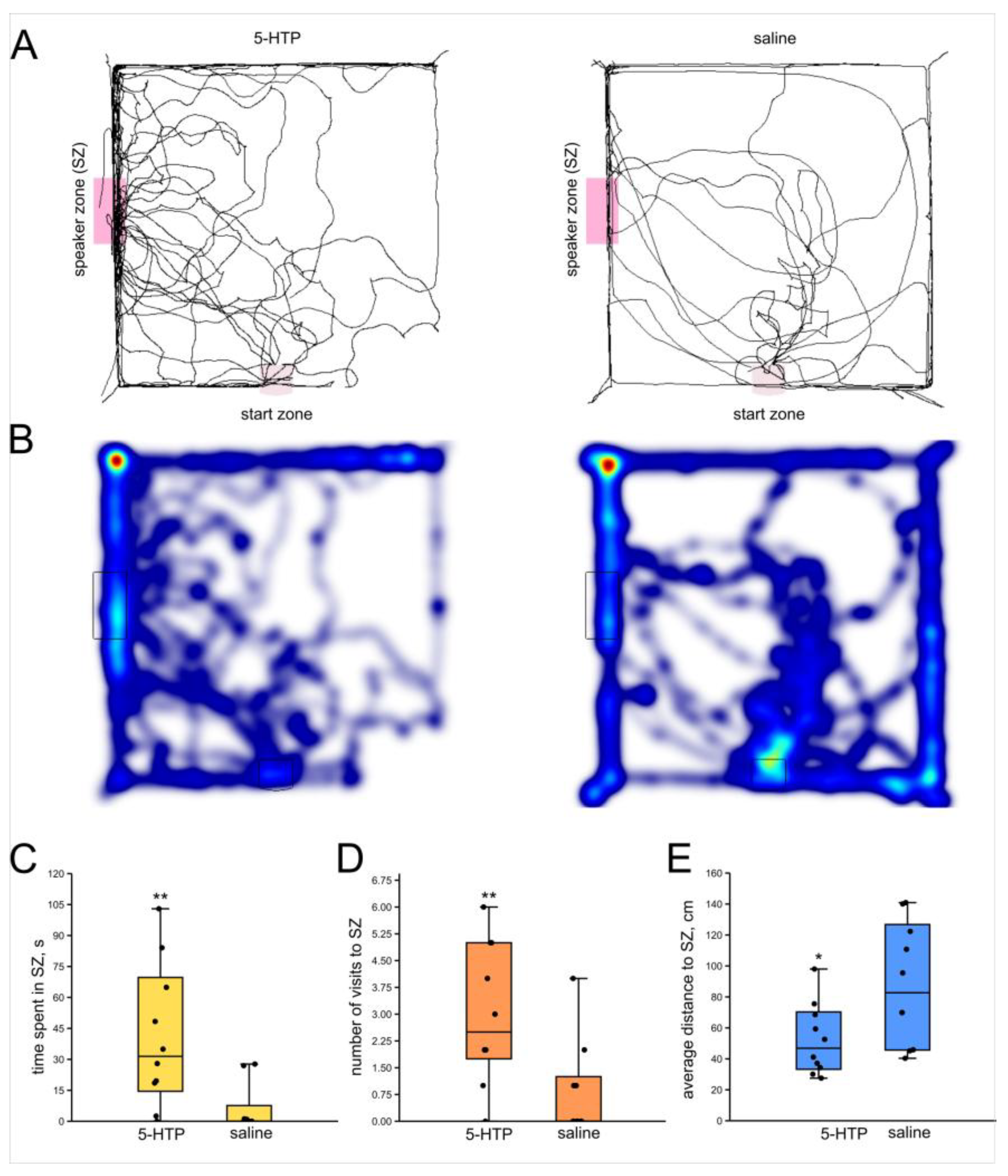
3.3. Effect of Serotonin Precursor (5-HTP, 20 mM) on Phonotactic Behavior of Male Crickets
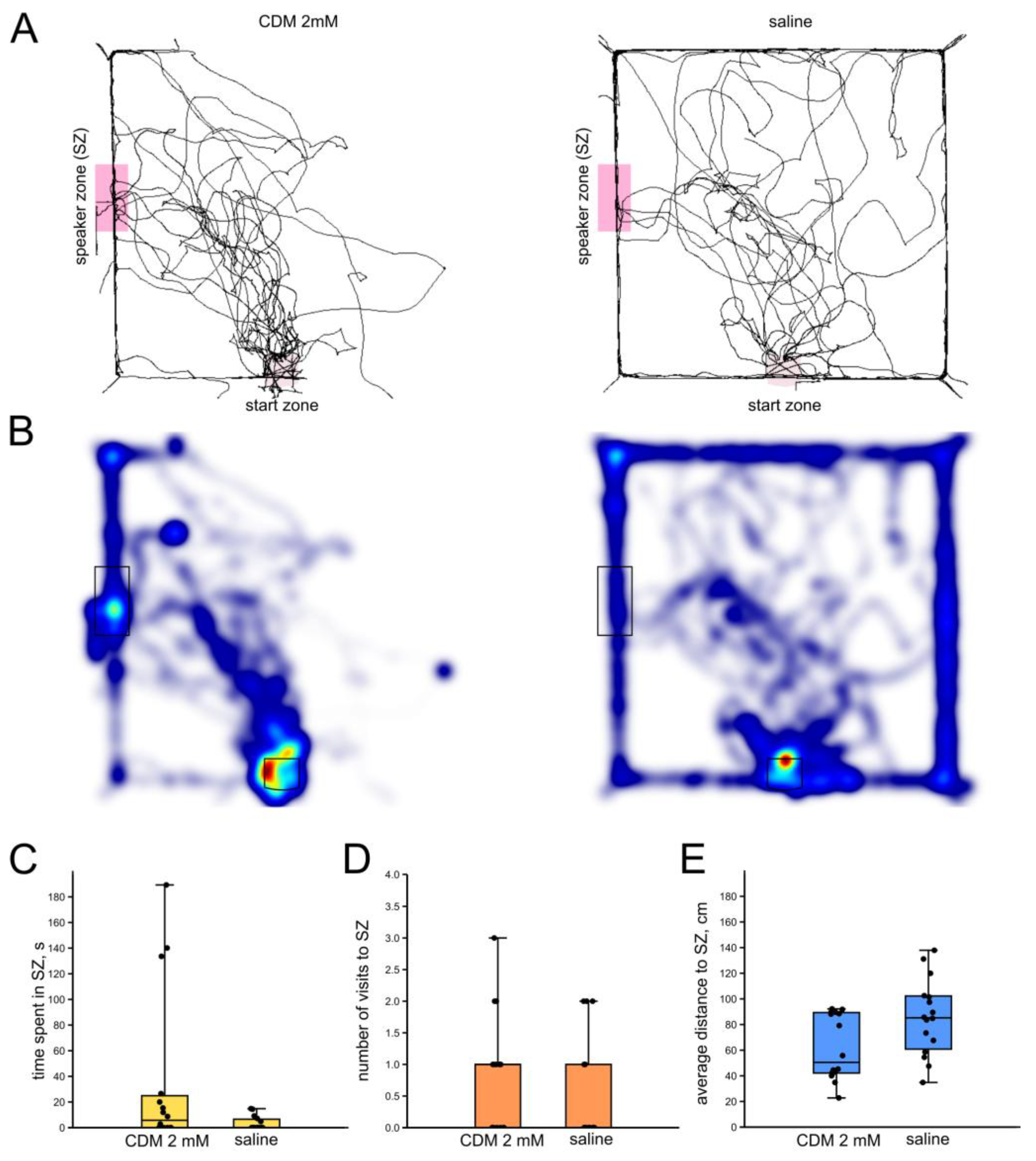
3.4. Effects of the Octopamine Receptor Agonist Chlordimeform on the Phonotaxis of Male Crickets
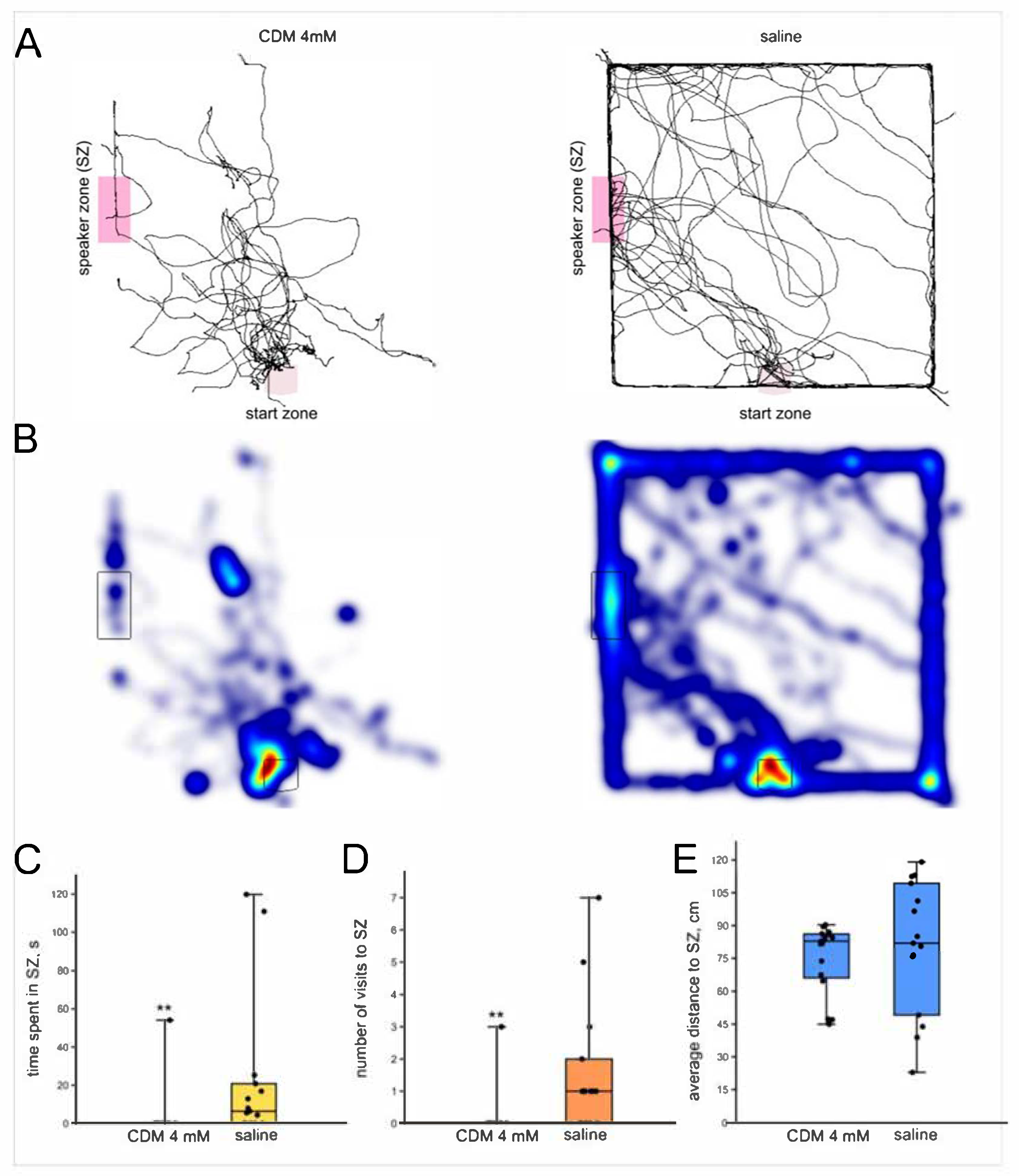
3.4.1. Chlordimeform 2 mM
3.4.2. Chlordimeform 4 mM
4. Discussion
4.1. Phonotaxis of Male Crickets
4.2. Flight and Male Phonotaxis
4.3. Serotonin, Octopamine and Phonotaxis
4.4. Monoamines and Aggregation
4.5. Conclusions
- No obvious sex differences were observed neither in intensity of phonotactic responses to calling signal, nor in the modulation of this response by previous behavior or by monoamines.
- Phonotaxis in male crickets is enhanced by flight. While factors reducing phonotaxis in male crickets (e.g., age, illness, recent copulation) are known, enhancers were previously studied only in female crickets. Here we show that the prior flight experience boosts phonotaxis in male crickets., This may promote aggregation and may compensate for flight-induced reproductive decline (e.g., the oogenesis-flight syndrome).
- Serotonergic signaling is associated with enhanced phonotaxis while octopaminergic system suppresses phonotaxis both in male and female crickets.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HTP | 5-hydroxytryptophan |
| CDM | chlordimeform |
| SZ | speaker zone |
References
- Prokopy, R.J.; Roitberg, B.D. Joining and avoidance behavior in nonsocial insects. Annu. Rev. Entomol. 2001, 46, 631–665. [Google Scholar] [CrossRef]
- Couzin-Fuchs, E.; Ayali, A. The social brain of ‘non-eusocial’ insects. Curr. Opin. Insect Sci. 2021, 48, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Robinson, D. Acoustic signalling in Orthoptera. Adv. Insect Physiol. 2021, 61, 1–99. [Google Scholar]
- Robinson, D.J.; Hall, M.J. Sound signalling in Orthoptera. Adv. Insect Physiol. 2002, 29, 151–278. [Google Scholar]
- Song, H.; Béthoux, O.; Shin, S.; Donath, A.; Letsch, H.; Liu, S.; McKenna, D.D.; Meng, G.; Misof, B.; Podsiadlowski, L.; et al. Phylogenomic analysis sheds light on the evolutionary pathways towards acoustic communication in Orthoptera. Nat. Commun. 2020, 11, 4939. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.V.; Shuvalov, V.F. Phonotactic behavior of crickets. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1977, 119, 111–126. [Google Scholar] [CrossRef]
- Schildberger, K. Behavioral and neuronal mechanisms of cricket phonotaxis. Experientia 1988, 44, 408–415. [Google Scholar] [CrossRef]
- Alexander, R.D. Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae). Behaviour 1961, 17, 130–223. [Google Scholar] [CrossRef]
- Leonard, A.S.; Hedrick, A.V. Male and female crickets use different decision rules in response to mating signals. Behav. Ecol. 2009, 20, 1175–1184. [Google Scholar] [CrossRef]
- Jang, Y. Male responses to conspecific advertisement signals in the field cricket Gryllus rubens (Orthoptera: Gryllidae). PLoS ONE 2011, 6, e16063. [Google Scholar] [CrossRef]
- McCarthy, T.M.; Keyes, J.; Cade, W.H. Phonotactic behavior of male field crickets (Gryllus texensis) in response to acoustic calls from conspecific males. J. Insect Behav. 2013, 26, 634–648. [Google Scholar] [CrossRef]
- Bent, A.; Hedwig, B. Phonotaxis of male field crickets, Gryllus bimaculatus, to conspecific calling song. Anim. Behav. 2023, 205, 173–181. [Google Scholar] [CrossRef]
- Balenger, S.L.; Zuk, M. Roaming Romeos: Male crickets evolving in silence show increased locomotor behaviours. Anim. Behav. 2015, 101, 213–219. [Google Scholar] [CrossRef]
- Cade, W.H. Field cricket spacing, and the phonotaxis of crickets and parasitoid flies to clumped and isolated cricket songs. Z. Tierpsychol. 1981, 55, 365–375. [Google Scholar] [CrossRef]
- Zuk, M.; Rotenberry, J.T.; Tinghitella, R.M. Silent night: Adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2006, 2, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Ponce, E.J. An Analysis of Phonotactic Behaviour in the Cricket Gryllus Bimaculatus. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2019. [Google Scholar]
- Cade, W.H.; Cade, E.S. Male mating success, calling and searching behaviour at high and low densities in the field cricket, Gryllus integer. Anim. Behav. 1992, 43, 49–56. [Google Scholar] [CrossRef]
- Adamo, S.A.; Hoy, R.R. Agonistic behaviour in male and female field crickets, Gryllus bimaculatus, and how behavioural context influences its expression. Anim. Behav. 1995, 49, 1491–1501. [Google Scholar] [CrossRef]
- Khazraıe, K.; Campan, M. The role of prior agonistic experience in dominance relationships in male crickets Gryllus bimaculatus (Orthoptera: Gryllidae). Behav. Process. 1999, 44, 341–348. [Google Scholar] [CrossRef]
- Bailey, N.W.; Gray, B.; Zuk, M. Acoustic experience shapes alternative mating tactics and reproductive investment in male field crickets. Curr. Biol. 2010, 20, 845–849. [Google Scholar] [CrossRef]
- Stevenson, P.A.; Rillich, J. The decision to fight or flee–insights into underlying mechanism in crickets. Front. Neurosci. 2012, 6, 118. [Google Scholar] [CrossRef]
- Stevenson, P.A.; Schildberger, K. Mechanisms of experience dependent control of aggression in crickets. Curr. Opin. Neurobiol. 2013, 23, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Hommaru, N.; Shidara, H.; Ando, N.; Ogawa, H. Internal state transition to switch behavioral strategies in cricket phonotaxis. J. Exp. Biol. 2020, 223, jeb229732. [Google Scholar] [CrossRef] [PubMed]
- Levy, K.; Barnea, A.; Tauber, E.; Ayali, A. Crickets in the spotlight: Exploring the impact of light on circadian behavior. J. Comp. Physiol. A 2024, 210, 267–279. [Google Scholar] [CrossRef]
- Shuvalov, V.F.; Rüting, T.; Popov, A.V. The influence of auditory and visual experience on the phonotactic behavior of the cricket, Gryllus bimaculatus. J. Insect Behav. 1990, 3, 289–302. [Google Scholar] [CrossRef]
- Bailey, N.W.; Zuk, M. Acoustic experience shapes female mate choice in field crickets. Proc. R. Soc. B Biol. Sci. 2008, 275, 2645–2650. [Google Scholar] [CrossRef]
- Bailey, N.W.; Zuk, M. Field crickets change mating preferences using remembered social information. Biol. Lett. 2009, 5, 449–451. [Google Scholar] [CrossRef]
- Judge, K.A.; Ting, J.J.; Gwynne, D.T. Condition dependence of female choosiness in a field cricket. J. Evol. Biol. 2014, 27, 2529–2540. [Google Scholar] [CrossRef]
- Lierheimer, V.F.; Tinghitella, R.M. Quantity and quality of available mates alters female responsiveness but not investment in the Pacific field cricket, Teleogryllus oceanicus. Behav. Ecol. Sociobiol. 2017, 71, 80. [Google Scholar] [CrossRef]
- Atwell, A.; Wagner, W.E., Jr. Female mate choice plasticity is affected by the interaction between male density and female age in a field cricket. Anim. Behav. 2014, 98, 177–183. [Google Scholar] [CrossRef]
- Atwell, A.; Wagner, W.E., Jr. Along came a spider who sat down beside her: Perceived predation risk, but not female age, affects female mate choosiness. Behav. Process. 2015, 115, 143–148. [Google Scholar] [CrossRef]
- Beckers, O.M.; Wagner, W.E., Jr. Parasitoid infestation changes female mating preferences. Anim. Behav. 2013, 85, 791–796. [Google Scholar] [CrossRef]
- Loher, W.; Weber, T.; Huber, F. The effect of mating on phonotactic behaviour in Gryllus bimaculatus (De Geer). Physiol. Entomol. 1993, 18, 57–66. [Google Scholar] [CrossRef]
- Tanner, J.C.; Garbe, L.M.; Zuk, M. When virginity matters: Age and mating status affect female responsiveness in crickets. Anim. Behav. 2019, 147, 83–90. [Google Scholar] [CrossRef]
- Mezheritskiy, M.; Vorontsov, D.; Lapshin, D.; Dyakonova, V. Previous flight facilitates partner finding in female crickets. Sci. Rep. 2020, 10, 22328. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.V.; Shuvalov, V.F.; Markovich, A.M. The spectrum of the calling signals, phonotaxis, and the auditory system in the cricket Gryllus bimaculatus. Neurosci. Behav. Physiol. 1976, 7, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.V.; Markovich, A.M. Auditory interneurones in the prothoracic ganglion of the cricket, Gryllus bimaculatus. J. Comp. Physiol. 1982, 146, 351–359. [Google Scholar] [CrossRef]
- Dyakonova, V.E.; Schürmann, F.W.; Sakharov, D.A. Effects of serotonergic and opioidergic drugs on escape behaviors and social status of male crickets. Naturwissenschaften 1999, 86, 435–437. [Google Scholar] [CrossRef]
- Stevenson, P.A.; Dyakonova, V.; Rillich, J.; Schildberger, K. Octopamine and experience-dependent modulation of aggression in crickets. J. Neurosci. 2005, 25, 1431–1441. [Google Scholar] [CrossRef]
- Hedwig, B. Pulses, patterns and paths: Neurobiology of acoustic behaviour in cricket. J. Comp. Physiol. A 2006, 192, 677–689. [Google Scholar] [CrossRef]
- Abbey-Lee, R.; Uhrig, E.J.; Garnham, L.; Lundgren, K.; Child, S.; Løvlie, H. Experimental manipulation of monoamine levels alters personality in crickets. Sci. Rep. 2018, 8, 16211. [Google Scholar] [CrossRef]
- Schöneich, S. Neuroethology of acoustic communication in field crickets-from signal generation to song recognition in an insect brain. Prog. Neurobiol. 2020, 194, 101882. [Google Scholar] [CrossRef]
- Itoh, M.T. The influence of self-generated song during aggression on brain serotonin levels in male crickets. J. Insect Sci. 2024, 24, 29. [Google Scholar] [CrossRef]
- Mizunami, M. What is learned in Pavlovian conditioning in crickets? Revisiting the SS and SR learning theories. Front. Behav. Neurosci. 2021, 15, 661225. [Google Scholar] [CrossRef]
- Matsumoto, Y. Learning and memory in the cricket Gryllus bimaculatus. Physiol. Entomol. 2022, 47, 147–161. [Google Scholar] [CrossRef]
- Libersat, F.; Pflueger, H.J. Monoamines and the orchestration of behavior. Bioscience 2004, 54, 17–25. [Google Scholar] [CrossRef]
- Anstey, M.L.; Rogers, S.M.; Ott, S.R.; Burrows, M.; Simpson, S.J. Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 2009, 323, 627–630. [Google Scholar] [CrossRef] [PubMed]
- D’yakonova, V.E. Neurotransmitter mechanisms of context-dependent behavior. Neurosci. Behav. Physiol. 2014, 44, 256–267. [Google Scholar] [CrossRef]
- Sinakevitch, I.T.; Wolff, G.H.; Pflüger, H.J.; Smith, B.H. Editorial: Biogenic Amines and Neuromodulation of Animal Behavior. Front. Syst. Neurosci. 2018, 12, 31. [Google Scholar] [CrossRef]
- Kamhi, J.F.; Arganda, S.; Moreau, C.S.; Traniello, J.F. Origins of aminergic regulation of behavior in complex insect social systems. Front. Syst. Neurosci. 2017, 11, 74. [Google Scholar] [CrossRef]
- Selcho, M.; Pauls, D. Linking physiological processes and feeding behaviors by octopamine. Curr. Opin. Insect Sci. 2019, 36, 125–130. [Google Scholar] [CrossRef]
- Ramesh, D.; Brockmann, A. Mass spectrometric quantification of arousal associated neurochemical changes in single honey bee brains and brain regions. ACS Chem. Neurosci. 2018, 10, 1950–1959. [Google Scholar] [CrossRef]
- Chatterjee, A.; Bais, D.; Brockmann, A.; Ramesh, D. Search behavior of individual foragers involves neurotransmitter systems characteristic for social scouting. Front. Insect Sci. 2021, 1, 664978. [Google Scholar] [CrossRef] [PubMed]
- Mezheritskiy, M.I.; Vorontsov, D.D.; Dyakonova, V.E.; Zakharov, I.S. Behavioral functions of octopamine in adult insects under stressful conditions. Biol. Bull. Rev. 2024, 14, 535–547. [Google Scholar] [CrossRef]
- Mezheritskiy, M.; Melnikova, V.; Dyakonova, V.; Vorontsov, D. Monoaminergic Systems in Flight-Induced Potentiation of Phonotactic Behavior in Female Crickets Gryllus bimaculatus. Insects 2024, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.; Yoon, J.; Lee, K.; Koo, R.; Chung, K.; Zdor, J.; Magno, D.; Cho, E.B.; Kim, C.; Gonzalez-Socoloske, D.; et al. Nanoinjection of neurotransmitters into the prothoracic ganglion of female cricket Acheta domesticus changes phonotactic selectivity. Physiol. Entomol. 2020, 45, 131–139. [Google Scholar] [CrossRef]
- Hofmann, H.A.; Stevenson, P.A. Flight restores fight in crickets. Nature 2000, 403, 613. [Google Scholar] [CrossRef] [PubMed]
- Dyakonova, V.E.; Krushinsky, A.L. Previous motor experience enhances courtship behavior in male cricket Gryllus bimaculatus. J. Insect Behav. 2008, 21, 172–180. [Google Scholar] [CrossRef]
- Ureshi, M.; Dainobu, M.; Sakai, M. Serotonin precursor (5-hydroxytryptophan) has a profound effect on the post-copulatory time-fixed sexually refractory stage in the male cricket, Gryllus bimaculatus DeGeer. J. Comp. Physiol. A 2002, 188, 767–779. [Google Scholar]
- Dyakonova, V.E.; Krushinsky, A.L. Serotonin precursor (5-hydroxytryptophan) causes substantial changes in the fighting behavior of male crickets, Gryllus bimaculatus. J. Comp. Physiol. A 2013, 199, 601–609. [Google Scholar] [CrossRef]
- Rillich, J.; Stevenson, P.A. Serotonin mediates depression of aggression after acute and chronic social defeat stress in a model insect. Front. Behav. Neurosci. 2018, 12, 233. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Rop, C.J. Cricket behavior: Observing insects to learn about Science & Scientific Inquiry. Am. Biol. Teach. 2008, 70, 235–240. [Google Scholar] [PubMed]
- Ebina, H.; Mizunami, M. Appetitive and aversive social learning with living and dead conspecifics in crickets. Sci. Rep. 2020, 10, 9340. [Google Scholar] [CrossRef] [PubMed]
- Hedwig, B. Trackball systems for analysing cricket phonotaxis. In The Cricket as a Model Organism: Development, Regeneration, and Behavior; Springer: Tokyo, Japan, 2017; pp. 303–312. [Google Scholar]
- Lorenz, M.W. Oogenesis-flight syndrome in crickets: Age-dependent egg production, flight performance, and biochemical composition of the flight muscles in adult female Gryllus bimaculatus. J. Insect Physiol. 2007, 8, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Guo, X.; Guo, S.; Yang, N.; Huang, Y. Trade-off between flight capability and reproduction in Acridoidea (Insecta: Orthoptera). Ecol. Evol. 2021, 11, 16849–16861. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Wu, K. Biological characteristics and energy metabolism of migrating insects. Metabolites 2023, 13, 439. [Google Scholar] [CrossRef]
- Ren, Y.S.; Zhang, B.; Zeng, Y.; Zhu, D.H. Effects of Flight on Reproductive Development in Long-Winged Female Crickets (Velarifictorus aspersus Walker; Orthoptera: Gryllidae) with Differences in Flight Behavior. Insects 2023, 14, 79. [Google Scholar] [CrossRef]
- Zera, A.J.; Rankin, M.A. Wing dimorphism in Gryllus rubens: Genetic basis of morph determination and fertility differences between morphs. Oecologia 1989, 80, 249–255. [Google Scholar] [CrossRef]
- Zera, A.J.; Denno, R.F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 1997, 42, 207–231. [Google Scholar] [CrossRef]
- Guerra, P.A.; Pollack, G.S. A life history trade-off between flight ability and reproductive behavior in male field crickets (Gryllus texensis). J. Insect Behav. 2007, 20, 377–387. [Google Scholar] [CrossRef]
- Mitra, C.; Wagner, W.E.; Zera, A.J.; Tolle, A.E. Variation in singing behaviour among morphs of the sand field cricket, Gryllus firmus. Ecol. Entomol. 2011, 36, 152–160. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, D.H. Trade-off between flight capability and reproduction in male Velarifictorus asperses crickets. Ecol. Entomol. 2012, 37, 244–251. [Google Scholar] [CrossRef]
- Guerra, P.A.; Pollack, G.S. Flight behaviour attenuates the trade-off between flight capability and reproduction in a wing polymorphic cricket. Biol. Lett. 2009, 5, 229–231. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, D.H.; Zhao, L.Q. Critical flight time for switch from flight to reproduction in the wing dimorphic cricket Velarifictorus aspersus. Evol. Biol. 2014, 41, 397–403. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, J.Y.; Zhu, D.H. Variations in calling behaviour of wing dimorphic male crickets. Ecol. Entomol. 2022, 47, 1044–1050. [Google Scholar] [CrossRef]
- Bertram, S.M. Positive relationship between signalling time and flight capability in the Texas field cricket, Gryllus texensis. Ethology 2007, 113, 875–880. [Google Scholar] [CrossRef]
- Murakami, S.; Itoh, M.T. Effects of aggression and wing removal on brain serotonin levels in male crickets, Gryllus bimaculatus. J. Insect Physiol. 2001, 47, 1309–1312. [Google Scholar] [CrossRef]
- Rillich, J.; Rillich, B.; Stevenson, P.A. Differential modulation of courtship behavior and subsequent aggression by octopamine, dopamine and serotonin in male crickets. Horm. Behav. 2019, 114, 104542. [Google Scholar] [CrossRef]
- Huber, F. Akustische Verständigung im Tierreich: Studien aus der Welt der Vögel und der Insekten; Bayerische Akademie der Wissenschaften: Munich, Germany, 1991. [Google Scholar]
- D’iakonova, V.E. Behavioral functions of serotonin and octopamine: Some paradoxes of comparative physiology. Uspekhi Fiziol. Nauk 2007, 38, 3–20. [Google Scholar]
- Roeder, T.; Degen, J.; Gewecke, M. Epinastine, a highly specific antagonist of insect neuronal octopamine receptors. Eur. J. Pharmacol. 1998, 349, 171–177. [Google Scholar] [CrossRef]
- Magno, D. The Role of Octopamine in Syllable-Period Selective Phonotaxis in Female Cricket Acheta domesticus. Bachelor’s Thesis, Andrews University, Berrien County, MI, USA, 2017. [Google Scholar]
- Rogers, S.M.; Matheson, T.; Sasaki, K.; Kendrick, K.; Simpson, S.J.; Burrows, M. Substantial changes in central nervous system neurotransmitters and neuromodulators accompany phase change in the locust. J. Exp. Biol. 2004, 207, 3603–3617. [Google Scholar] [CrossRef]
- Rogers, S.M.; Cullen, D.A.; Anstey, M.L.; Burrows, M.; Despland, E.; Dodgson, T.; Matheson, T.; Ott, S.R.; Stettin, K.; Sword, G.A.; et al. Rapid behavioural gregarization in the desert locust, Schistocerca gregaria entails synchronous changes in both activity and attraction to conspecifics. J. Insect Physiol. 2014, 65, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.M.; Ott, S.R. Differential activation of serotonergic neurons during short-and long-term gregarization of desert locusts. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142062. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qiu, R.; Li, X.; Cheng, Y.; Gao, S.; Kong, F.; Liu, L.; Zhu, Y. Social attraction in Drosophila is regulated by the mushroom body and serotonergic system. Nat. Commun. 2020, 11, 5350. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, Z.; Kang, L. Serotonin enhances solitariness in phase transition of the migratory locust. Front. Behav. Neurosci. 2013, 7, 129. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, W.; Guo, X.; Wang, X.; Kang, L. Modulation of behavioral phase changes of the migratory locust by the catecholamine metabolic pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 3882–3887. [Google Scholar] [CrossRef]
- Alessi, A.M.; O’Connor, V.; Aonuma, H.; Newland, P.L. Dopaminergic modulation of phase reversal in desert locusts. Front. Behav. Neurosci. 2014, 8, 371. [Google Scholar] [CrossRef]
- Yang, M.; Wei, Y.; Jiang, F.; Wang, Y.; Guo, X.; He, J.; Kang, L. MicroRNA-133 inhibits behavioral aggregation by controlling dopamine synthesis in locusts. PLoS Genet. 2014, 10, e1004206. [Google Scholar] [CrossRef]
- Guo, X.; Ma, Z.; Kang, L. Two dopamine receptors play different roles in phase change of the migratory locust. Front. Behav. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef]
- Morton, D.B.; Evans, P.D. Octopamine distribution in solitarious and gregarious forms of the locust, Schistocerca americana gregaria. Insect Biochem. 1983, 13, 177–183. [Google Scholar] [CrossRef]
- Verlinden, H.; Vleugels, R.; Marchal, E.; Badisco, L.; Pflüger, H.J.; Blenau, W.; Broeck, J.V. The role of octopamine in locusts and other arthropods. J. Insect Physiol. 2010, 56, 854–867. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, X.; Lei, H.; Li, T.; Hao, S.; Kang, L. Octopamine and tyramine respectively regulate attractive and repulsive behavior in locust phase changes. Sci. Rep. 2015, 5, 8036. [Google Scholar] [CrossRef]

| Agent | Concentration | Volume of Injection | Vehicle | Remarks |
|---|---|---|---|---|
| Chlordimeform (CDM, the octopamine receptors agonist) (Sigma-Aldrich, Burlington, MA, USA) | 2 mM 4 mM | 100 μL | saline | Crickets were injected 30 min prior to testing. Chlordimeform acted for at least 1.5–2 h after injection. |
| 5-hydroxytryptophan (5-HTP), immediate metabolic precursor of serotonin | 20 mM | 100 μL | saline | Crickets were injected 30 min prior to testing. 5-HTP acted for at least 1.5–2 h after injection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezheritskiy, M.; Vorontsov, D.; Dyakonova, V. Phonotaxis in Male Field Crickets: The Role of Flight Experience, Serotonin and Octopamine Neurotransmission. Insects 2025, 16, 887. https://doi.org/10.3390/insects16090887
Mezheritskiy M, Vorontsov D, Dyakonova V. Phonotaxis in Male Field Crickets: The Role of Flight Experience, Serotonin and Octopamine Neurotransmission. Insects. 2025; 16(9):887. https://doi.org/10.3390/insects16090887
Chicago/Turabian StyleMezheritskiy, Maxim, Dmitry Vorontsov, and Varvara Dyakonova. 2025. "Phonotaxis in Male Field Crickets: The Role of Flight Experience, Serotonin and Octopamine Neurotransmission" Insects 16, no. 9: 887. https://doi.org/10.3390/insects16090887
APA StyleMezheritskiy, M., Vorontsov, D., & Dyakonova, V. (2025). Phonotaxis in Male Field Crickets: The Role of Flight Experience, Serotonin and Octopamine Neurotransmission. Insects, 16(9), 887. https://doi.org/10.3390/insects16090887







