Mesoporous Silica Nanoparticles Impair Physiology and Reproductive Fitness of Tuta absoluta Through Plant-Mediated Oxidative Stress and Enzymatic Disruption
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Mesoporous Silica Nanoparticles (MSNs)
2.2. Tomato Cultivation and MSN Application
2.3. Insect Rearing and Feeding Bioassay
2.4. Biochemical Assays
2.4.1. Plant (Leaf) Assays
2.4.2. Insect (Larval) Assays
2.5. Life Table and Reproductive Performance
2.6. Statistical and Correlation Analysis
3. Results
3.1. Characterization of Mesoporous Silica Nanoparticles (MSNs)
3.2. Physiological Effects of MSNs on Tomato Plants
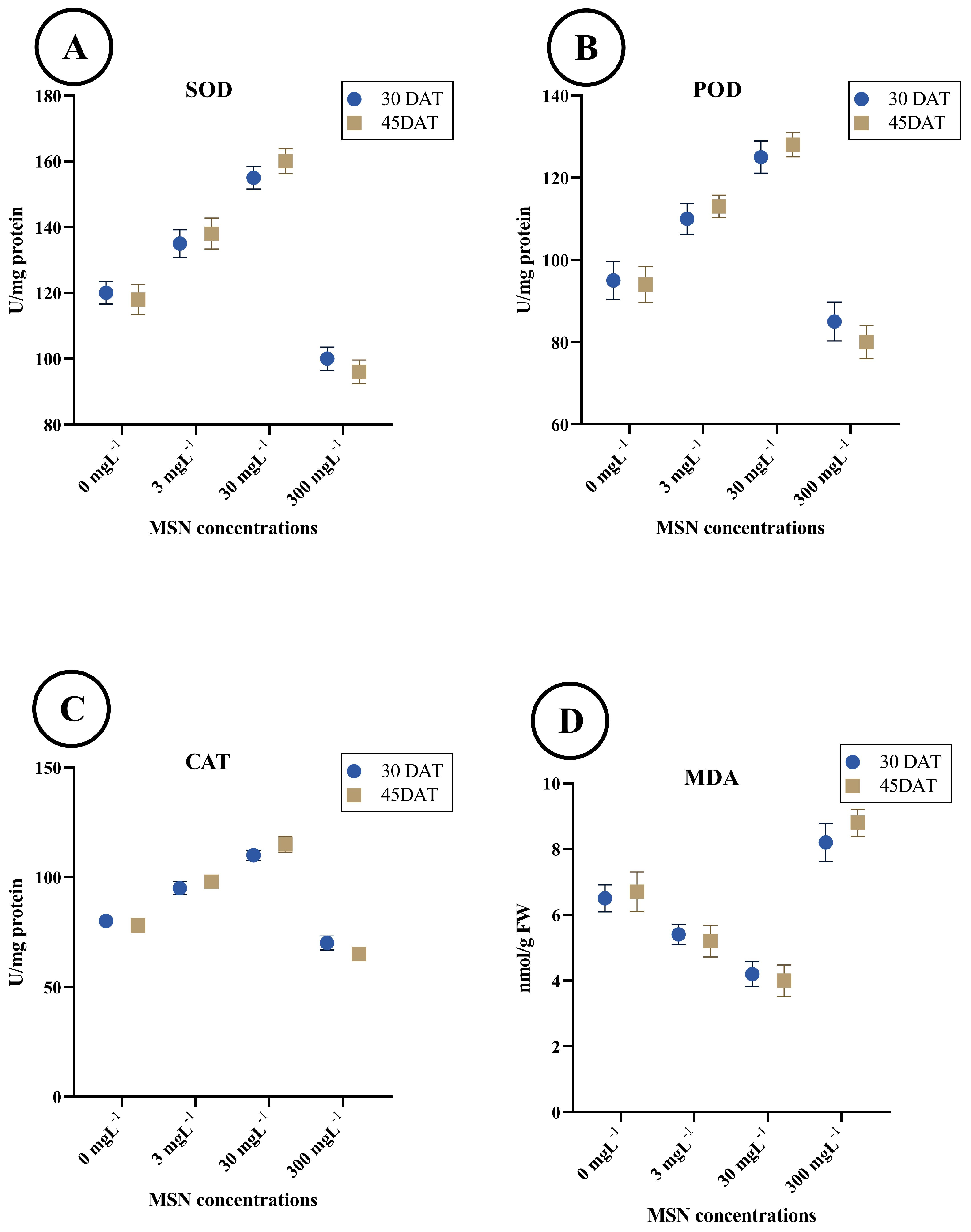
3.3. Antioxidant Enzyme Activities
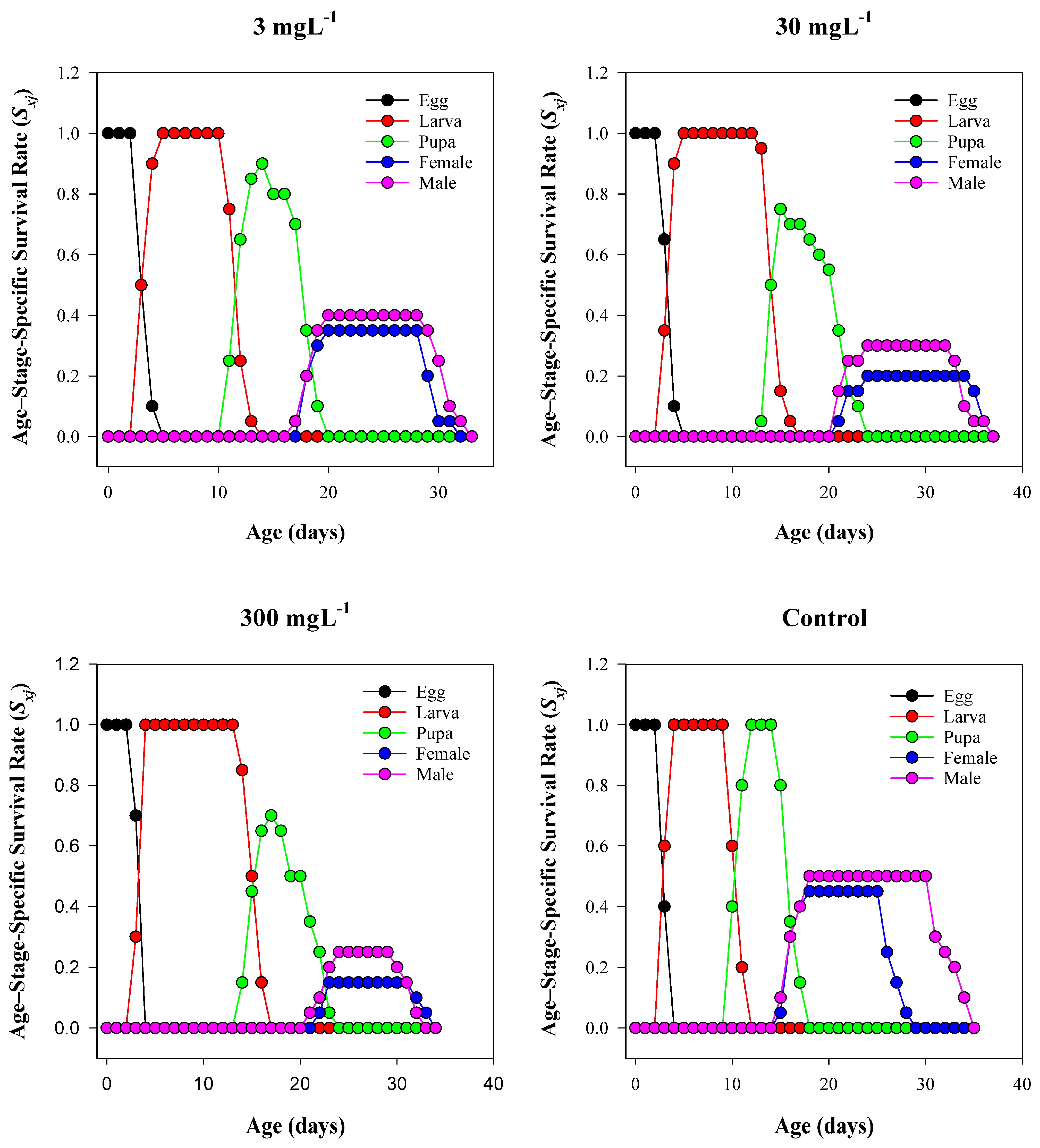
3.4. Age–Stage-Specific Survival Rate (Sₓⱼ)
3.5. Life Table Parameters of T. absoluta Under MSN Exposure
3.6. Age–Stage-Specific Developmental Time
3.7. Age–Stage-Specific Reproductive Value (Vₓⱼ) of T. absoluta
3.8. Reproductive Capacity, Pre-Oviposition Periods, and Survivorship
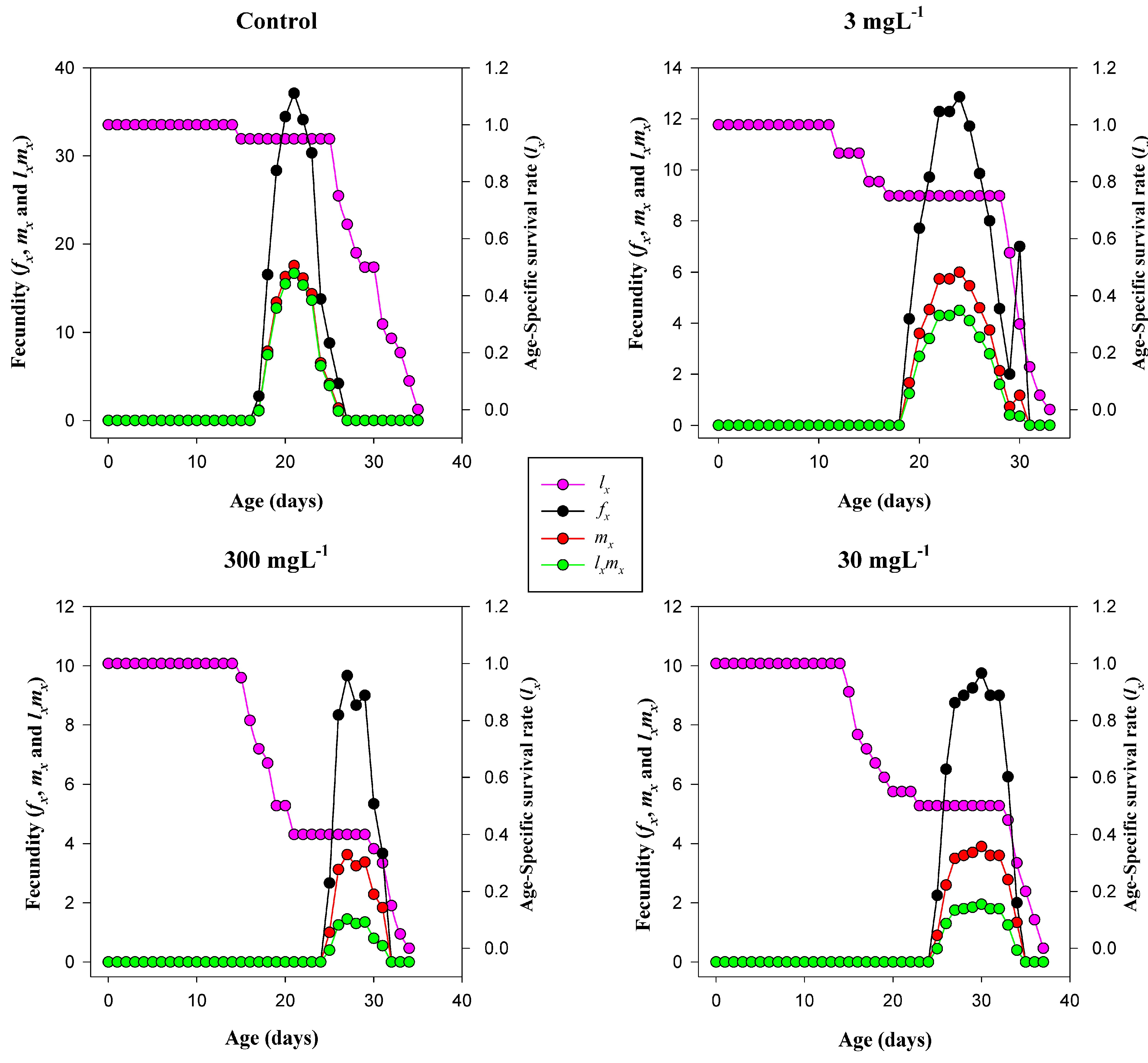
3.9. Age-Specific Survival and Fecundity Trends
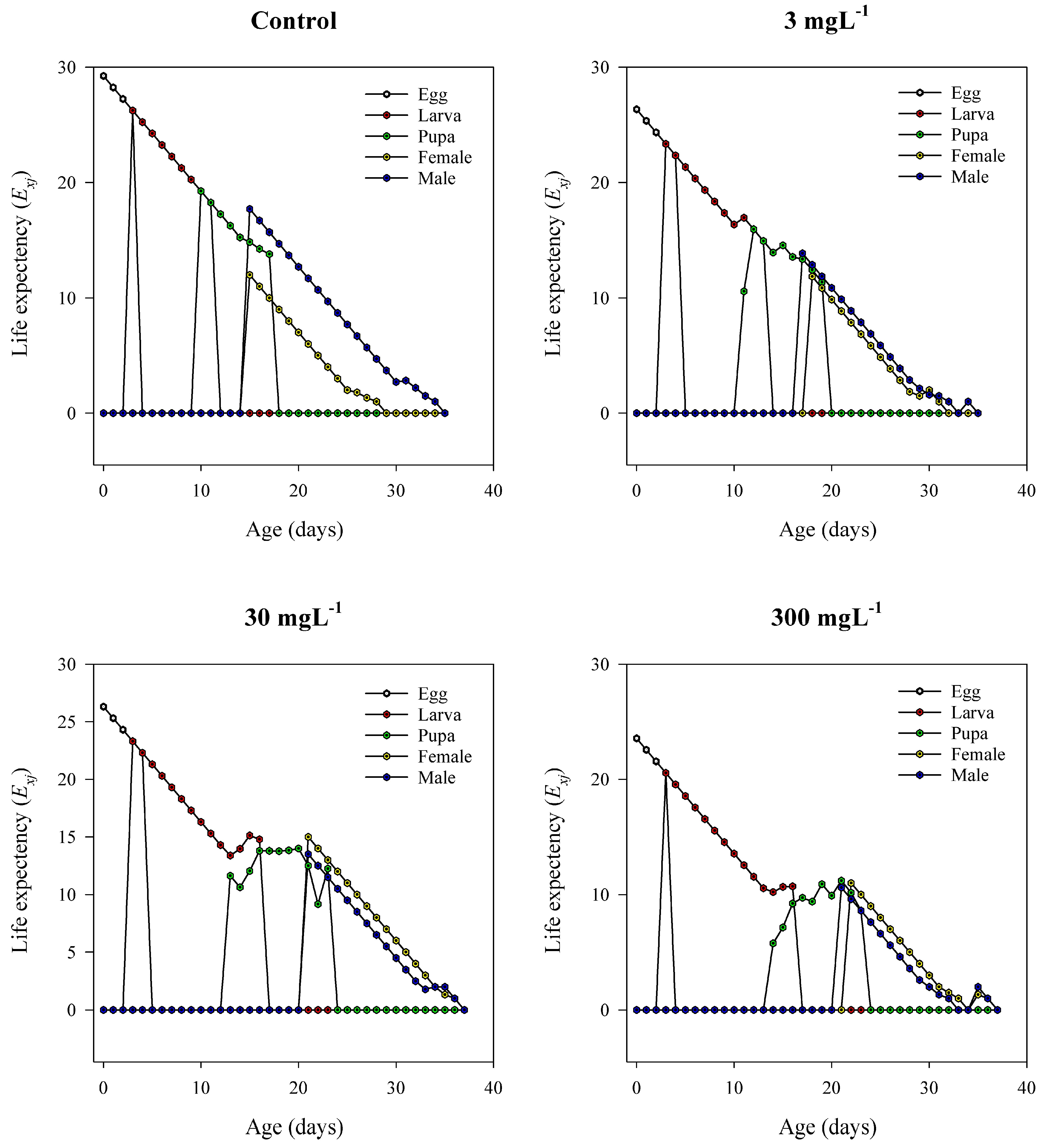
3.10. Age–Stage Life Expectancy (exj)
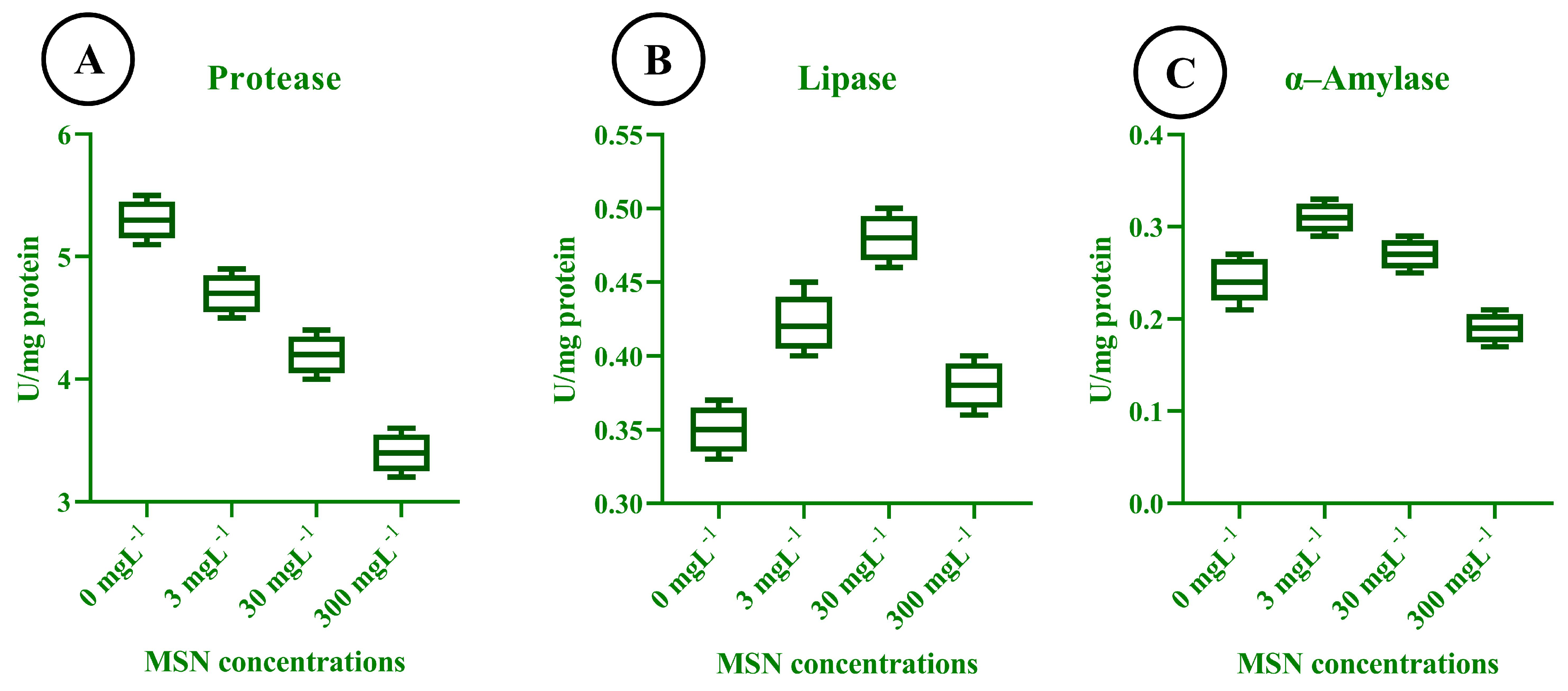
3.11. Digestive Enzyme Activity in T. absoluta
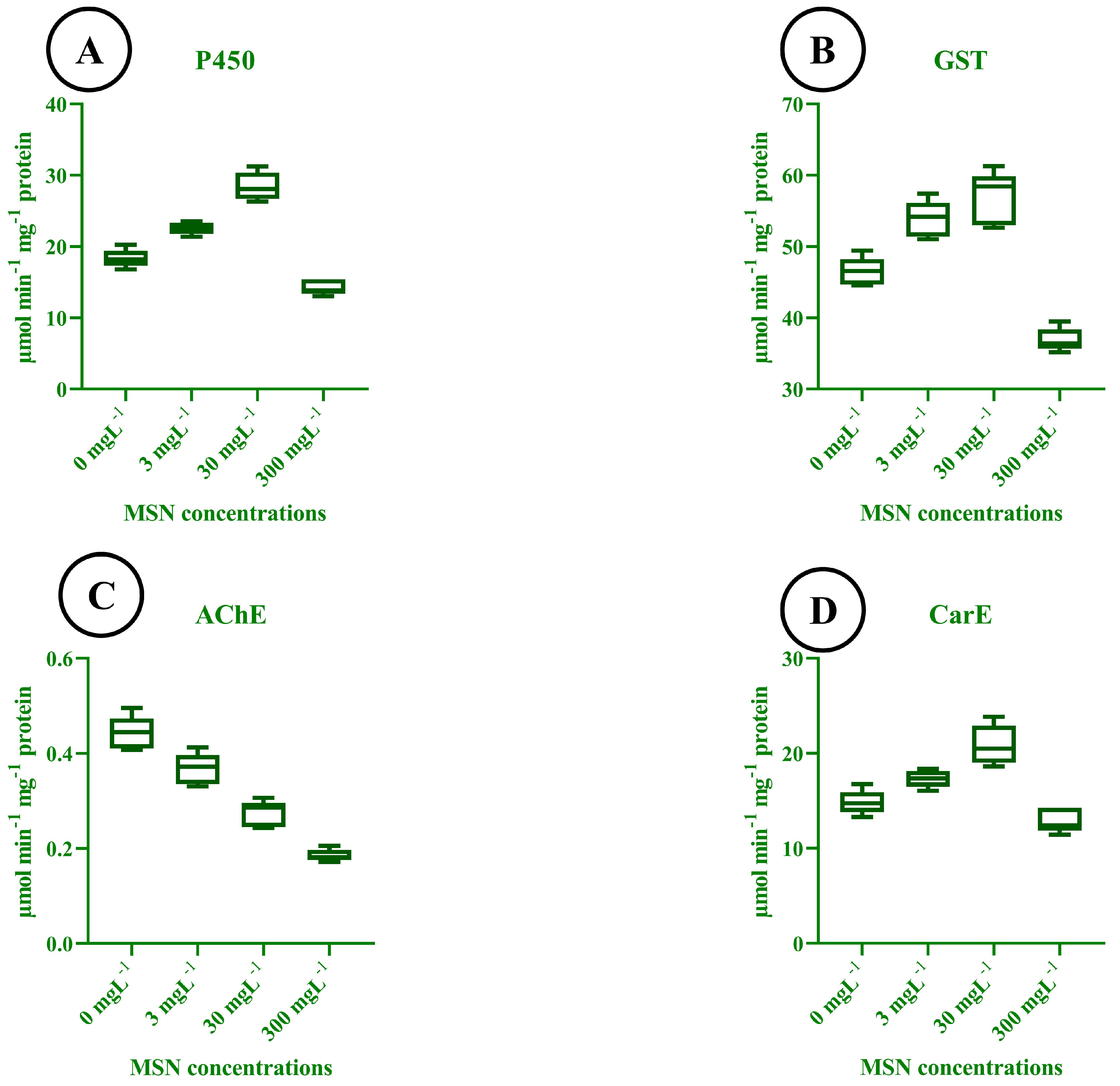
3.12. Enzymatic Response to MSN Treatments
3.13. Correlation Analysis of Plant and Insect Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSN(s) | Mesoporous Silica Nanoparticle(s) |
| T. absoluta | Tuta absoluta |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| MDA | Malondialdehyde |
| P450 | Cytochrome P450 Monooxygenases |
| AChE | Acetylcholinesterase |
| CarE | Carboxylesterase |
| GST | Glutathione S-Transferase |
| RWC | Relative Water Content |
| Pn | Photosynthetic Rate |
| gs | Stomatal Conductance |
| r | Intrinsic Rate of Increase |
| λ | Finite Rate of Increase |
| R0 | Net Reproductive Rate |
| GRR | Gross Reproductive Rate |
| T | Mean Generation Time |
| APOP | Adult Pre-Oviposition Period |
| TPOP | Total Pre-Oviposition Period |
| Sa | Preadult Survivorship |
| fx | Age-Specific Fecundity |
| lx | Age-Specific Survival Rate |
| Vₓⱼ | Age–Stage Reproductive Value |
| exj | Age–Stage Life Expectancy |
| IPM | Integrated Pest Management |
| DLS | Dynamic Light Scattering |
| TEM | Transmission Electron Microscopy |
| SEM | Scanning Electron Microscopy |
| BET | Brunauer–Emmett–Teller (Surface Area Analysis) |
| XRD | X-ray Diffraction |
| SPAD | Soil Plant Analysis Development (Chlorophyll Meter Reading) |
| ANOVA | Analysis of Variance |
References
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Campos, M.R.; Rodrigues, A.R.S.; Silva, W.M.; Silva, T.B.M.; Silva, V.R.F.; Guedes, R.N.C.; Siqueira, H.A.A. Spinosad and the tomato borer Tuta absoluta: A bioinsecticide, an invasive pest threat, and high insecticide resistance. PLoS ONE 2014, 9, e103235. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.; Wan, F.H.; Desneux, N. Ecology, Worldwide Spread, and Management of the Invasive South American Tomato Pinworm, Tuta absoluta: Past, Present, and Future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Xian, X.; Han, P.; Wang, S.; Zhang, G.; Liu, W.; Wan, F. The potential invasion risk and preventive measures against the tomato leafminer Tuta absoluta in China. Entomol. Gen. 2017, 36, 319–333. [Google Scholar] [CrossRef]
- Proffit, M.; Birgersson, G.; Bengtsson, M.; Reis Jr, R.; Witzgall, P.; Lima, E. Attraction and oviposition of Tuta absoluta females in response to tomato leaf volatiles. J. Chem. Ecol. 2011, 37, 565–574. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, W.; Song, S.; Wang, Z.; Zhu, X.; Gao, S.; He, W.; Xu, J. The impact of high-temperature stress on the growth and development of Tuta absoluta (Meyrick). Insects 2024, 15, 423. [Google Scholar] [CrossRef]
- Guedes, R.; Picanço, M. The tomato borer Tuta absoluta in South America: Pest status, management and insecticide resistance. EPPO Bull. 2012, 42, 211–216. [Google Scholar] [CrossRef]
- Guedes, R.; Roditakis, E.; Campos, M.; Haddi, K.; Bielza, P.; Siqueira, H.; Tsagkarakou, A.; Vontas, J.; Nauen, R. Insecticide resistance in the tomato pinworm Tuta absoluta: Patterns, spread, mechanisms, management and outlook. J. Pest Sci. 2019, 92, 1329–1342. [Google Scholar] [CrossRef]
- Silva, W.M.; Berger, M.; Bass, C.; Balbino, V.Q.; Amaral, M.H.; Campos, M.R.; Siqueira, H.A. Status of pyrethroid resistance and mechanisms in Brazilian populations of Tuta absoluta. Pestic. Biochem. Physiol. 2015, 122, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.J.; Lorente, M.J.; Stansly, P.A.; Belda, J.E. Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomol. Exp. Appl. 2012, 143, 111–119. [Google Scholar] [CrossRef]
- Zappala, L.; Biondi, A.; Alma, A.; Al-Jboory, I.J.; Arno, J.; Bayram, A.; Chailleux, A.; El-Arnaouty, A.; Gerling, D.; Guenaoui, Y. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J. Pest Sci. 2013, 86, 635–647. [Google Scholar] [CrossRef]
- Hyder, M.; Ul Haq, I.; Younas, M.; Ghafar, M.A.; Akhtar, M.R.; Ahmed, Z.; Bukero, A.; Hou, Y. Floral Resource Integration: Enhancing Biocontrol of Tuta absoluta Within Sustainable IPM Frameworks. Plants 2025, 14, 319. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of knowledge, environmental fate, and exposure modeling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867. [Google Scholar] [CrossRef]
- Haq, I.U.; Cai, X.; Ali, H.; Akhtar, M.R.; Ghafar, M.A.; Hyder, M.; Hou, Y. Interactions Between Nanoparticles and Tomato Plants: Influencing Host Physiology and the Tomato Leafminer’s Molecular Response. Nanomaterials 2024, 14, 1788. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Del. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Fatima, R.; Katiyar, P.; Kushwaha, K. Recent advances in mesoporous silica nanoparticle: Synthesis, drug loading, release mechanisms, and diverse applications. Front. Nanotechnol. 2025, 7, 1564188. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-Khaishany, M.Y. Role of nanoparticles in plants. In Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Springer: Berlin/Heidelberg, Germany, 2015; pp. 19–35. [Google Scholar]
- Westwood, J.H.; Roney, J.K.; Khatibi, P.A.; Stromberg, V.K. RNA translocation between parasitic plants and their hosts. Pest Manag. Sci. Former. Pestic. Sci. 2009, 65, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Thakur, S.; Kumari, P.; Shandilya, M.; Sharma, S.; Poczai, P.; Alarfaj, A.A.; Sayyed, R. Nano-insecticide: Synthesis, characterization, and evaluation of insecticidal activity of ZnO NPs against Spodoptera litura and Macrosiphum euphorbiae. Appl. Nanosci. 2022, 12, 3835–3850. [Google Scholar] [CrossRef]
- Asghar, M.S.; Sarwar, Z.M.; Almadiy, A.A.; Shami, A.; El Hadi Mohamed, R.A.; Ahmed, N.; Waghulade, M.S.; Alam, P.; Abd Al Galil, F.M. Toxicological Effects of Silver and Zinc Oxide Nanoparticles on the Biological and Life Table Parameters of Helicoverpa armigera (Noctuidae: Lepidoptera). Agriculture 2022, 12, 1744. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Shende, S.; Gupta, I.; Biswas, J.K.; da Silva, S.S. Copper and copper nanoparticles: Role in management of insect-pests and pathogenic microbes. Nanotechnol. Rev. 2018, 7, 303–315. [Google Scholar] [CrossRef]
- Manjunatha, S.; Biradar, D.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm Sci. 2016, 29, 1–13. [Google Scholar]
- Feng, J.; Liang, Q.; Chen, Z.; Tan, Y.; Jiang, T.; Dong, S. Mesoporous silica nanoparticles based on a dual environmental response corresponding to temperature and α-amylase for the control of Spodoptera litura. Ind. Crops Prod. 2024, 222, 119526. [Google Scholar] [CrossRef]
- Carneiro, A.A.B.; Patekar, S.; Goyal, M.; De Campos, S.B.; Bally, J.; Hassanpour, M.; Zhang, Z. pH-responsive mesoporous silica nanoparticles from rice husk ash for delivering trypsin inhibitor to control cotton bollworm. Ind. Crops Prod. 2025, 228, 120934. [Google Scholar] [CrossRef]
- Fiaboe, K.R.; Khamis, F.M.; Cheseto, X.; Yusuf, A.A.; Torto, B. Nanosilica supplementation in tomato increases oviposition on stems and caterpillar mortality in the tomato pinworm. Proc. Natl. Acad. Sci. USA 2025, 122, e2427314122. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Wang, W.; Ma, Y.; Zhan, X.; Peng, A.; Pu, J.; Yang, J.; Wang, X. Dendritic mesoporous silica-delivered siRNAs nano insecticides to prevent Sogatella furcifera by inhibiting metabolic detoxification and reproduction. J. Nanobiotechnology 2024, 22, 736. [Google Scholar] [CrossRef]
- Khan, M.R.; Rizvi, T.F. Nanotechnology: Scope and application in plant disease management. Plant Pathol. J. 2014, 13, 214–231. [Google Scholar] [CrossRef]
- Baranwal, A.; Srivastava, A.; Kumar, P.; Bajpai, V.K.; Maurya, P.K.; Chandra, P. Prospects of nanostructure materials and their composites as antimicrobial agents. Front. Microbiol. 2018, 9, 422. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop. Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Landa, P. Positive effects of metallic nanoparticles on plants: Overview of involved mechanisms. Plant Physiol. Biochem. 2021, 161, 12–24. [Google Scholar] [CrossRef]
- Eskin, A.; Nurullahoğlu, Z.U. Effects of zinc oxide nanoparticles (ZnO NPs) on the biology of Galleria mellonella L. (Lepidoptera: Pyralidae). J. Basic Appl. Zool. 2022, 83, 54. [Google Scholar] [CrossRef]
- Ruiz-Aguilar, M.Y.; Aguirre-Uribe, L.A.; Ramírez-Barrón, S.N.; Pérez-Luna, Y.d.C.; Castro-del Ángel, E.; Juárez, A.H. Insecticidal efficacy of zinc oxide and silicon dioxide nanoparticles against larvae of Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae). J. Exp. Nanosci. 2025, 20, 2466532. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Yin, M.; Shen, J.; Yan, S. Recent advances in nanoparticle-mediated co-delivery system: A promising strategy in medical and Agricultural Field. Int. J. Mol. Sci. 2023, 24, 5121. [Google Scholar] [CrossRef]
- Wang, W.; Ghafar, M.A.; Liuyang, L.; Haq, I.U.; Cui, L.; Yuan, H.; Wang, L. Nanoscale Metal–Organic Frameworks for the Co-Delivery of Cycloxaprid and Pooled siRNAs to Enhance Control Efficacy in Diaphorina citri. J. Agric. Food Chem. 2025, 73, 3353–3362. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Yang, W. Synthesis of Mesoporous Silica Using the Sol–Gel Approach: Adjusting Architecture and Composition for Novel Applications. Nanomaterials 2024, 14, 903. [Google Scholar] [CrossRef] [PubMed]
- Noha, K.; Bondok, A.; El-Dougdoug, K. Evaluation of silver nanoparticles as antiviral agent against ToMV and PVY in tomato plants. Sciences 2018, 8, 100–111. [Google Scholar]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Hadwan, M.H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, J.B.; Da Lage, J.L.; Mezdour, S.; Marion-Poll, F.; Terrol, C.; Brouzes, C.M.C.; Schmidely, P. Amylase activity across black soldier fly larvae development and feeding substrates: Insights on starch digestibility and external digestion. Animal 2024, 18, 101337. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.C.; Barbosa, L.A.; Júnior, I.D.B.; Nascimento, T.G.D.; Dornelas, C.B.; Grillo, L.A.M. Lipase Activity in the Larval Midgut of Rhynchophorus palmarum: Biochemical Characterization and the Effects of Reducing Agents. Insects 2017, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Salih Özgökçe, M.; Güncan, A.; Gökçe, A.; Smith, C.L.; Benelli, G.; Guedes, R.N.C.; et al. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–732. [Google Scholar] [CrossRef]
- Wang, J.; Tian, X.; Wei, S.; Meng, X.; Chen, N.; Shi, D.; Liang, C. Effect of nanoparticles on the growth of okra cultivated in soil affected by rocky desertification. Sci. Rep. 2025, 15, 18930. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Desoky, E.-S.M.; Babalghith, A.O.; El-Tahan, A.M.; Ibrahim, O.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; et al. Role of Nanoparticles in Enhancing Crop Tolerance to Abiotic Stress: A Comprehensive Review. Front. Plant Sci. 2022, 13, 946717. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Gill, R.; Gupta, A.; Taggar, G.; Taggar, M. Review article: Role of oxidative enzymes in plant defenses against insect herbivory. Acta Phytopathol. Èntomol. Hung. 2010, 45, 277–290. [Google Scholar] [CrossRef]
- Waara, E.R.; Iqbal, M.N.; Robert-Nicoud, G.; Benziane, B.; Vallhov, H.; Wasik, A.M.; Lindgren, M.; Hagman, E.; Rinde, M.; Kupferschmidt, N.; et al. Entrapping Digestive Enzymes with Engineered Mesoporous Silica Particles Reduces Metabolic Risk Factors in Humans. Adv. Healthc. Mater. 2020, 9, e2000057. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free. Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef]
- Haq, I.U.; Zhang, K.; Ali, S.; Majid, M.; Ashraf, H.J.; Khurshid, A.; Inayat, R.; Li, C.; Gou, Y.; Al-Ghamdi, A.A. Effectiveness of silicon on immature stages of the fall armyworm [Spodoptera frugiperda (JE Smith)]. J. King Saud Univ. Sci. 2022, 34, 102152. [Google Scholar] [CrossRef]
- Ghafar, M.A.; Feng, Q.; Ul Haq, I.; Hyder, M.; Sufian, M.; Abbas, D.; Haider, K.; Khalid, M.L.; Akhtar, M.R.; Wang, L.; et al. Impact of Silicon-Based Treatments on the Demographic Traits of Spodoptera frugiperda in Maize. J. Crop Health 2025, 77, 119. [Google Scholar] [CrossRef]
- Prudic, K.L.; Oliver, J.C.; Bowers, M.D. Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia 2005, 143, 578–587. [Google Scholar] [CrossRef]
- Benelli, G. Mode of action of nanoparticles against insects. Environ. Sci. Pollut. Res. 2018, 25, 12329–12341. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Li, F.; Gao, D.; Xing, B. Adsorption and inhibition of acetylcholinesterase by different nanoparticles. Chemosphere 2009, 77, 67–73. [Google Scholar] [CrossRef]
- Cruse, C.; Moural, T.W.; Zhu, F. Dynamic Roles of Insect Carboxyl/Cholinesterases in Chemical Adaptation. Insects 2023, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Rahikkala, A.; Pereira, S.A.P.; Figueiredo, P.; Passos, M.L.C.; Araújo, A.R.T.S.; Saraiva, M.L.M.F.S.; Santos, H.A. Mesoporous Silica Nanoparticles for Targeted and Stimuli-Responsive Delivery of Chemotherapeutics: A Review. Adv. Biosyst. 2018, 2, 1800020. [Google Scholar] [CrossRef]
- Li, C.; Hu, C.; Zhi, J.; Yue, W.; Li, H. Effects of Nano-Graphene Oxide on the Growth and Reproductive Dynamics of Spodoptera frugiperda Based on an Age-Stage, Two-Sex Life Table. Insects 2022, 13, 929. [Google Scholar] [CrossRef]
- Abbasi, A.; Sufyan, M.; Ashraf, H.J.; Zaman, Q.u.; Haq, I.U.; Ahmad, Z.; Saleem, R.; Hashmi, M.R.; Jaremko, M.; Abdelsalam, N.R. Determination of silicon accumulation in Non-Bt cotton (Gossypium hirsutum) plants and its impact on fecundity and biology of whitefly (Bemisia tabaci) under controlled conditions. Sustainability 2022, 14, 10996. [Google Scholar] [CrossRef]
- Ul Haq, I.; Idrees, A.; Abbasi, A.; Ali, S.; Asad, M.; Li, C.; Liu, C.-Z.; Zhang, K.-X.; Yasin, M.; Asghar, M.A. Silicon Accumulation in Maize and its Effects on Demographical Traits of Fall armyworm, [Spodoptera frugiperda (JE Smith)]. Silicon 2023, 15, 3269–3281. [Google Scholar] [CrossRef]
- English, S.; Barreaux, A.M.G. The evolution of sensitive periods in development: Insights from insects. Curr. Opin. Behav. Sci. 2020, 36, 71–78. [Google Scholar] [CrossRef]
- Abarca, M.; Parker, A.L.; Larsen, E.A.; Umbanhowar, J.; Earl, C.; Guralnick, R.; Kingsolver, J.; Ries, L. How development and survival combine to determine the thermal sensitivity of insects. PLoS ONE 2024, 19, e0291393. [Google Scholar] [CrossRef]
- Zong, S.; Xu, D.; Jiang, Y.; Wang, X.; Zhu-Salzman, K.; Zhang, X.; Zhao, J.; Xiao, L.; Zhang, L.; Xu, G.; et al. Mesoporous silica nanoparticles enhance the toxicity of chlorantraniliprole to Spodoptera frugiperda by possibly inhibiting energy metabolism and chitin protein synthesis. Insect Sci. 2025; Online Early. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Ali, A.M. Silver and zinc oxide nanoparticles induce developmental and physiological changes in the larval and pupal stages of Spodoptera littoralis (Lepidoptera: Noctuidae). J. Asia Pac. Entomol. 2018, 21, 1373–1378. [Google Scholar] [CrossRef]
- Yasur, J.; Usha Rani, P. Lepidopteran insect susceptibility to silver nanoparticles and measurement of changes in their growth, development and physiology. Chemosphere 2015, 124, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, N.; Li, X.; Yue, L.; Wang, X.; Liao, H.; Xiao, Z. Trophic Transfer of Metal Oxide Nanoparticles in the Tomato-Helicoverpa armigera Food Chain: Effects on Phyllosphere Microbiota, Insect Oxidative Stress, and Gut Microbiome. ACS Nano 2024, 18, 26631–26642. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Ramírez, J.A.; Betancourt-Galindo, R.; Aguirre-Uribe, L.A.; Cerna-Chávez, E.; Sandoval-Rangel, A.; Ángel, E.C.-d.; Chacón-Hernández, J.C.; García-López, J.I.; Hernández-Juárez, A. Insecticidal Effect of Zinc Oxide and Titanium Dioxide Nanoparticles against Bactericera cockerelli Sulc. (Hemiptera: Triozidae) on Tomato Solanum lycopersicum. Agronomy 2021, 11, 1460. [Google Scholar] [CrossRef]
- Elqady, E.M.; El-said, E.; Tharwat, A.A.; El-Khashab, L.A.A.; Mostafa, I.M.Y.; Hamed, F.Z.; Morsi, W.M.; Rezk, M.M.; El-Enain, I.M.A. Biogenic synthesis of titanium nanoparticles by Streptomyces rubrolavendulae for sustainable management of Icerya aegyptiaca (Douglas). Sci. Rep. 2025, 15, 1380. [Google Scholar] [CrossRef]
- Ding, R.; Li, Y.; Yu, Y.; Sun, Z.; Duan, J. Prospects and hazards of silica nanoparticles: Biological impacts and implicated mechanisms. Biotechnol. Adv. 2023, 69, 108277. [Google Scholar] [CrossRef] [PubMed]
- Tuncsoy, B.; Idikut, M.; Tuncsoy, M. Investigation of the Effects of Silicon Dioxide Nanoparticles and Environmental Contaminants on Immunocytotoxic and Antioxidant Defense Systems in Model Organism Galleria mellonella L. Biol. Trace Elem. Res. 2025, 203, 4887–4904. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yang, Y.; Xia, D.; Meng, L.; He, M.; Liu, C.; Zhang, Z. Silica Nanoparticles Promote α-Synuclein Aggregation and Parkinson’s Disease Pathology. Front. Neurosci. 2022, 15, 807988. [Google Scholar] [CrossRef] [PubMed]




| 0 mg L−1 | 3 mg L−1 | 30 mg L−1 | 300 mg L−1 | |
|---|---|---|---|---|
| Intrinsic rate of increase (r) | 0.21 ± 0.01 | 0.14 ± 0.01 | 0.09 ± 0.02 | 0.07 ± 0.02 |
| Finite rate of increase (λ) | 1.23 ± 0.02 | 1.16 ± 0.02 | 1.09 ± 0.02 | 1.07 ± 0.006 |
| Net reproduction rate (R0) | 93.7 ± 23.18 | 33.15 ± 8.89 | 14.35 ± 6.28 | 7.1 ± 3.57 |
| Mean generation time (T) | 21.76 ± 0.32 | 24.23 ± 0.32 | 30.24 ± 0.41 | 28.83 ± 0.41 |
| Gross reproduction rate (GRR) | 98.93 ± 24.06 | 45.09 ± 12.95 | 29.51 ± 11.03 | 18.49 ± 7.80 |
| Fecundity per female (F) | 0.208.22 ± 3.43 | 97.71 ± 4.32 | 71.75 ± 1.22 | 47.33 ± 1.11 |
| Female percentage (Nf/F) | 0.45 ± 0.11 | 0.35 ± 0.11 | 0.2 ± 0.09 | 0.15 ± 0.08 |
| 0 mg L−1 | 3 mg L−1 | 30 mg L−1 | 300 mg L−1 | |
|---|---|---|---|---|
| Egg (n) | 3.4 ± 0.11 | 3.60 ± 0.15 | 3.75 ± 0.14 | 3.70 ± 0.10 |
| Larva (days) | 7.4 ± 0.11 | 8.45 ± 0.11 | 10.90 ± 0.19 | 11.80 ± 0.19 |
| Pupa (days) | 5.58 ± 0.11 | 6.40 ± 0.13 | 7.50 ± 0.16 | 6.62 ± 0.18 |
| Adult (days) | 13.63 ± 0.66 | 11.87 ± 0.23 | 13.10 ± 0.22 | 9.50 ± 0.30 |
| Pre-adult (days) | 16.37 ± 0.21 | 18.53 ± 0.21 | 22.00 ± 0.35 | 22.62 ± 0.31 |
| 0 mg L−1 | 3 mg L−1 | 30 mg L−1 | 300 mg L−1 | |
|---|---|---|---|---|
| Fn | 9.00 ± 2.22 | 7.00 ± 2.13 | 4.00 ± 1.75 | 3.00 ± 1.51 |
| RepF | 9.00 ± 2.22 | 7.00 ± 2.13 | 4.00 ± 1.75 | 3.00 ± 1.51 |
| Male | 10.00 ± 2.23 | 1.00 ± 2.19 | 6.00 ± 2.03 | 5.00 ± 1.90 |
| APOP | 2.11 ± 0.11 | 1.14 ± 0.14 | 3.75 ± 0.24 | 3.00 ± 0.00 |
| TPOP | 18.44 ± 0.33 | 19.71 ± 0.28 | 26.00 ± 0.40 | 25.67 ± 0.31 |
| Sa | 0.95 ± 0.05 | 0.75 ± 0.10 | 0.50 ± 0.11 | 0.40 ± 0.11 |
| Ovi-days | 6.56 ± 0.17 | 9.14 ± 0.26 | 8.00 ± 0.00 | 5.67 ± 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, I.U.; Liu, H.; Ghafar, M.A.; Zafar, S.; Subhan, M.; Abbasi, A.; Hyder, M.; Basit, A.; Rebouh, N.Y.; Hou, Y. Mesoporous Silica Nanoparticles Impair Physiology and Reproductive Fitness of Tuta absoluta Through Plant-Mediated Oxidative Stress and Enzymatic Disruption. Insects 2025, 16, 877. https://doi.org/10.3390/insects16090877
Haq IU, Liu H, Ghafar MA, Zafar S, Subhan M, Abbasi A, Hyder M, Basit A, Rebouh NY, Hou Y. Mesoporous Silica Nanoparticles Impair Physiology and Reproductive Fitness of Tuta absoluta Through Plant-Mediated Oxidative Stress and Enzymatic Disruption. Insects. 2025; 16(9):877. https://doi.org/10.3390/insects16090877
Chicago/Turabian StyleHaq, Inzamam Ul, Huiping Liu, Muhammad Adeel Ghafar, Saba Zafar, Mishal Subhan, Asim Abbasi, Moazam Hyder, Abdul Basit, Nazih Y. Rebouh, and Youming Hou. 2025. "Mesoporous Silica Nanoparticles Impair Physiology and Reproductive Fitness of Tuta absoluta Through Plant-Mediated Oxidative Stress and Enzymatic Disruption" Insects 16, no. 9: 877. https://doi.org/10.3390/insects16090877
APA StyleHaq, I. U., Liu, H., Ghafar, M. A., Zafar, S., Subhan, M., Abbasi, A., Hyder, M., Basit, A., Rebouh, N. Y., & Hou, Y. (2025). Mesoporous Silica Nanoparticles Impair Physiology and Reproductive Fitness of Tuta absoluta Through Plant-Mediated Oxidative Stress and Enzymatic Disruption. Insects, 16(9), 877. https://doi.org/10.3390/insects16090877










