Love in the Time of Pyrethroids: Mating Behavior of Sitophilus zeamais Is Influenced by Sublethal Concentrations of λ-Cyhalothrin and Lateralization
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sitophilus zeamais Colonies and Sex Recognition
2.2. Insecticide
2.3. Contact Toxicity Bioassays
2.4. Mating Behavior Bioassays
2.5. Statistical Analysis
3. Results
3.1. Contact Toxicity of λ-Cyhalothrin on S. zeamais Adults
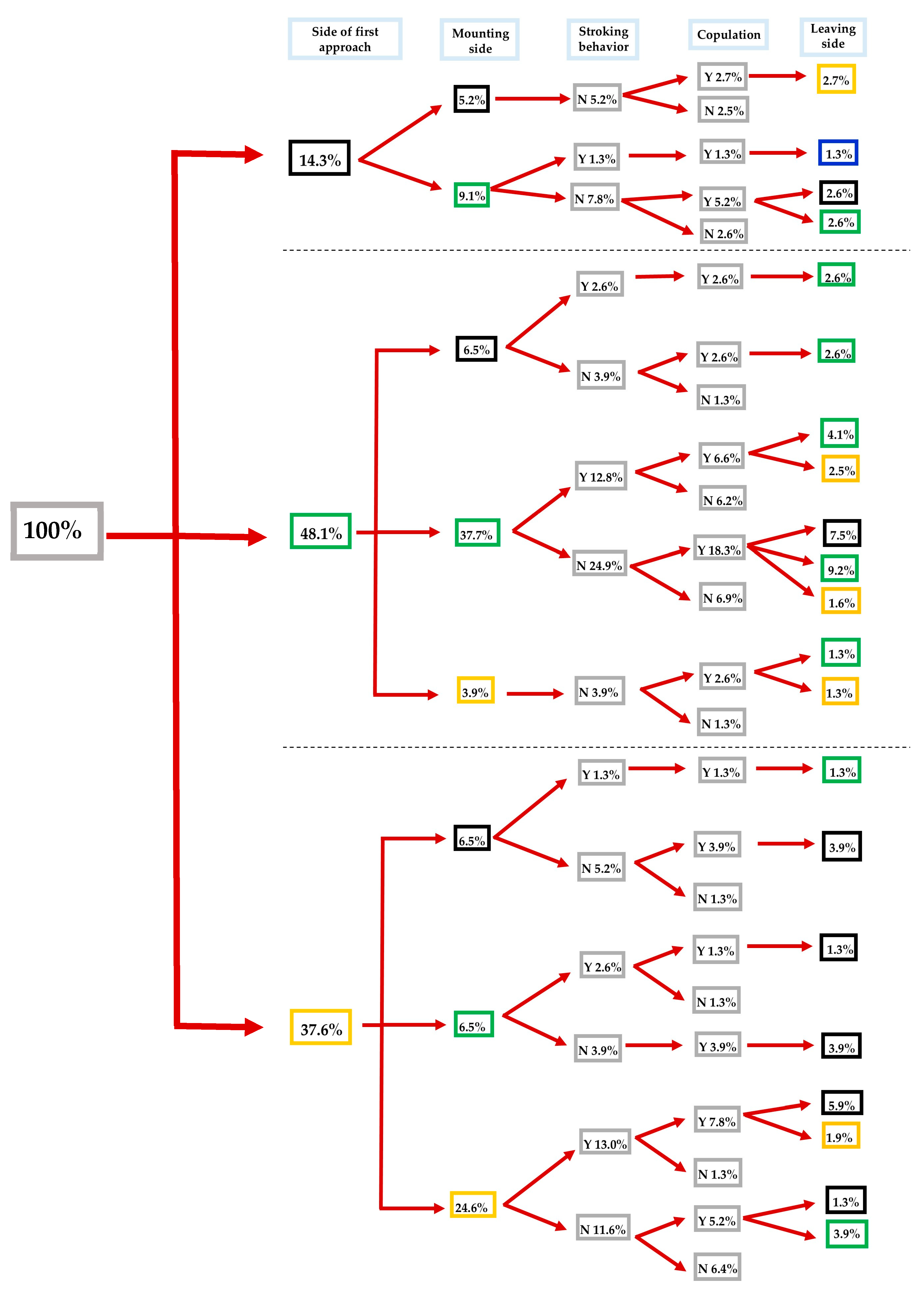
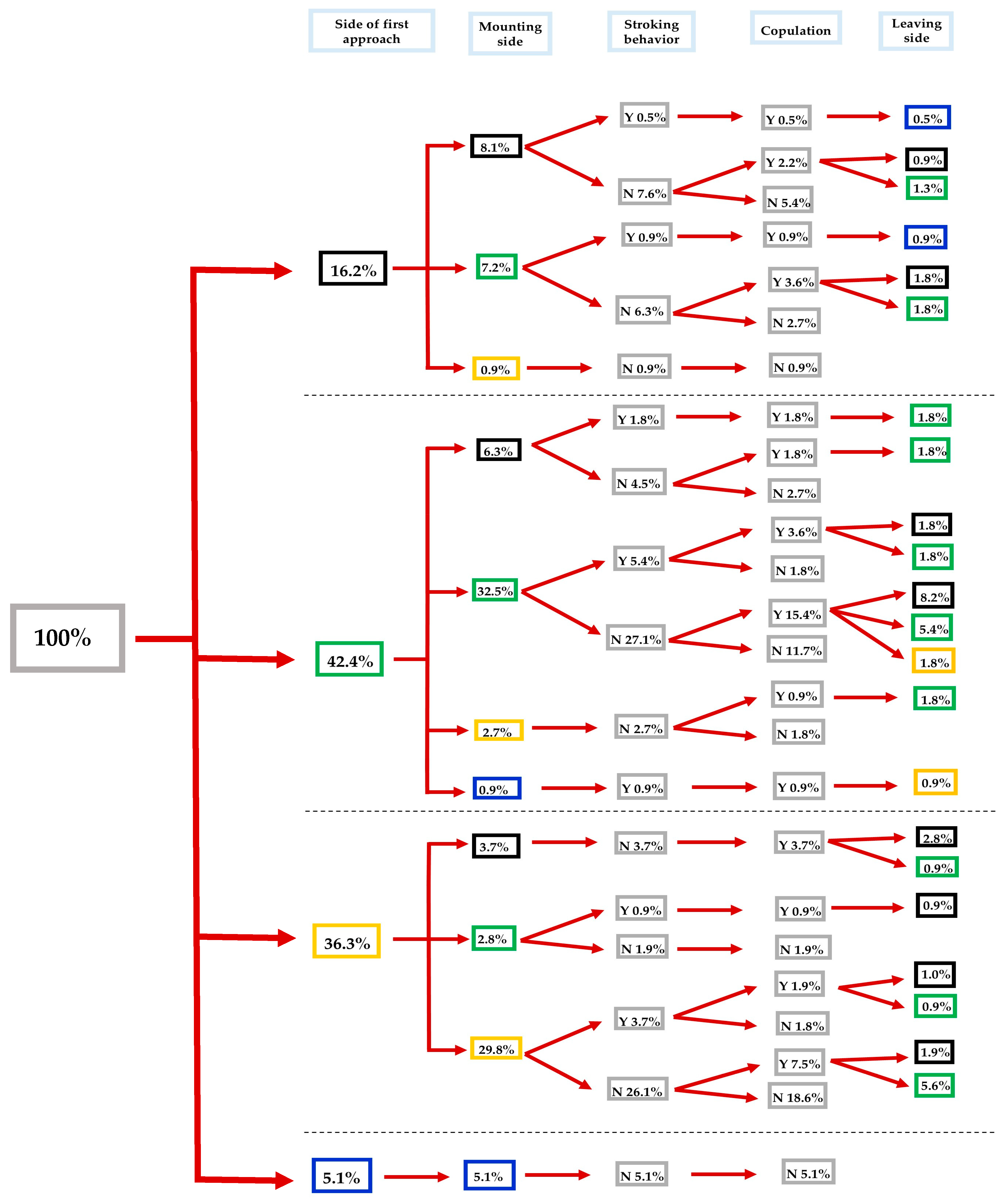
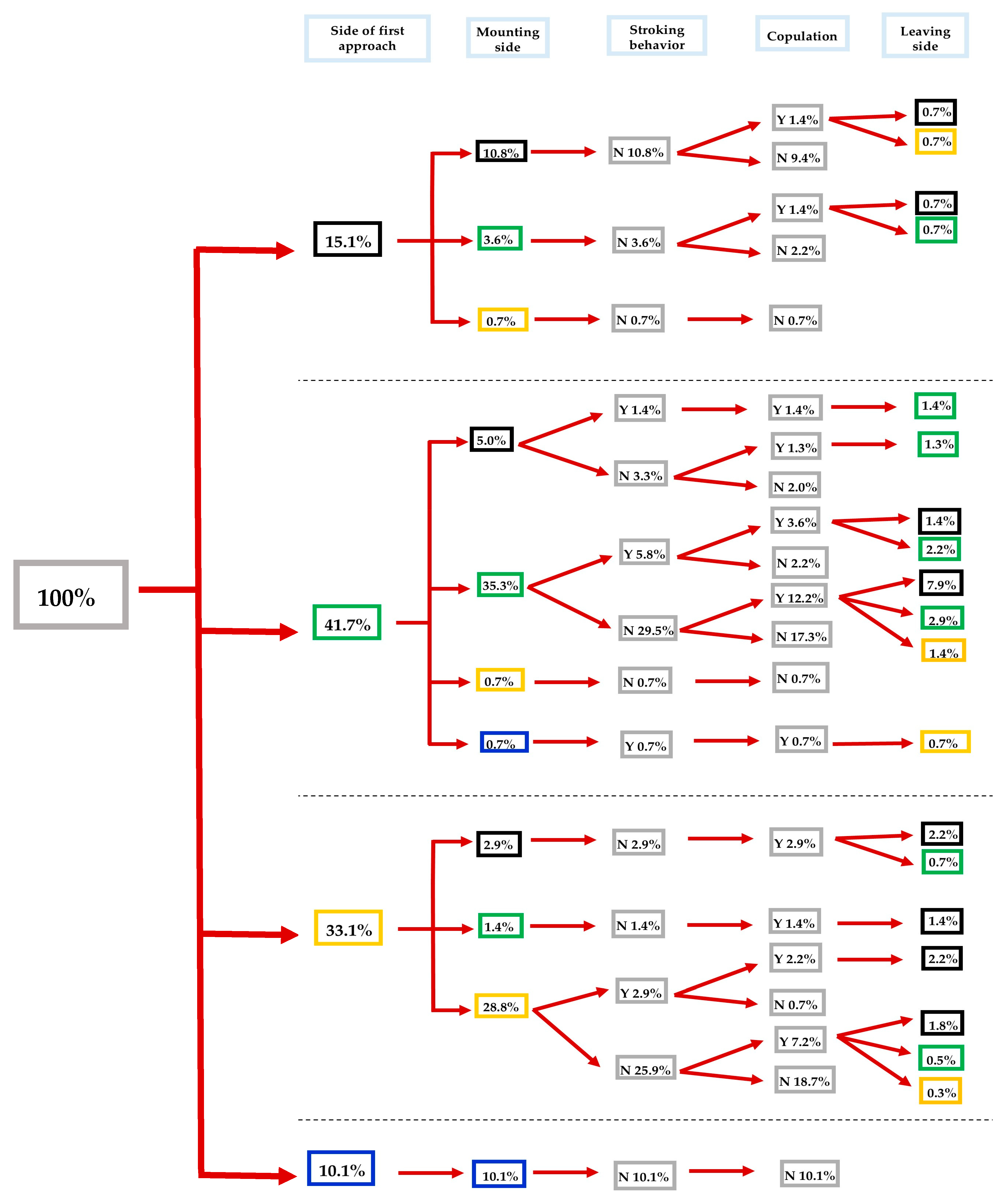
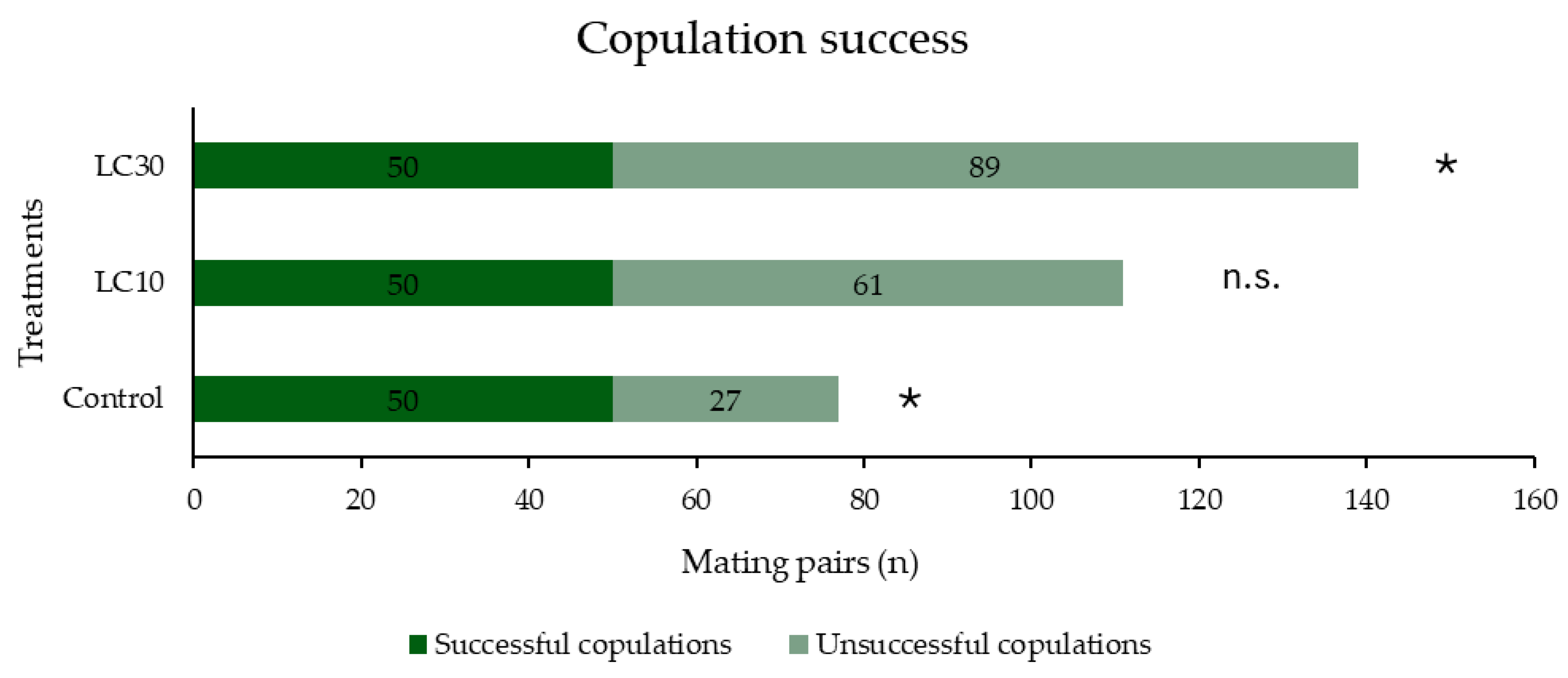
3.2. Impact of λ-Cyhalothrin and Laterality on the Mating Success of S. zeamais
3.3. Impact of λ-Cyhalothrin and Laterality on S. zeamais Mating Time Parameters
3.3.1. Impact of the Direction of the Approach
3.3.2. Impact of the Mounting Direction
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Banga, K.S.; Kumar, S.; Kotwaliwale, N.; Mohapatra, D. Major insects of stored food grains. Int. J. Chem. Stud. 2020, 8, 2380–2384. [Google Scholar] [CrossRef]
- Stathers, T.; Holcroft, D.K.L.; Mvumi, B.; English, A.; Omotilewa, O.; Kocher, M.; Ault, J.; Torero, M. A scoping review of interventions for crop postharvest loss reduction in sub-Saharan Africa and South Asia. Nat. Sustain. 2020, 3, 821–835. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, H.N. Development of microencapsulated vegetable oil powder based cookies and study of its physicochemical properties and storage stability. LWT 2021, 152, 112364. [Google Scholar] [CrossRef]
- Achimón, F.; Peschiutta, M.L.; Brito, V.D.; Beato, M.; Pizzolitto, R.P.; Zygadlo, J.A.; Zunino, M.P. Exploring contact toxicity of essential oils against Sitophilus zeamais through a meta-analysis approach. Plants 2022, 11, 3070. [Google Scholar] [CrossRef]
- Erenso, T.F.; Berhe, D.H. Effect of neem leaf and seed powders against adult maize weevil (Sitophilus zeamais Motschulsky) mortality. Agric. Res. 2016, 2, 90–94. [Google Scholar] [CrossRef]
- Brito, V.D.; Achimón, F.; Pizzolitto, R.P.; Ramírez Sánchez, A.; Gómez Torres, E.A.; Zygadlo, J.A.; Zunino, M.P. An alternative to reduce the use of the synthetic insecticide against the maize weevil Sitophilus zeamais through the synergistic action of Pimenta racemosa and Citrus sinensis essential oils with chlorpyrifos. J. Pest Sci. 2021, 94, 409–421. [Google Scholar] [CrossRef]
- Carvalho, M.O.; Fradinho, P.; Martins, M.J.; Magro, A.; Raymundo, A.; Sousa, I. Paddy rice stored under hermetic conditions: The effect of relative humidity, temperature and storage time in suppressing Sitophilus zeamais and impact on rice quality. J. Stored Prod. Res. 2019, 80, 21–27. [Google Scholar] [CrossRef]
- Zhou, Y.; Hui, Y.B.; Feng, L.F.; Zhou, T.; Wang, Q. A method for reconstructing the internal morphological structure of wheat kernels upon Sitophilus zeamais infestation. J. Stored Prod. Res. 2020, 88, 101676. [Google Scholar] [CrossRef]
- Suleiman, M.; Ibrahim, N.D.; Rugumamu, C.P. Application of some botanicals in the protection of sorghum grains against the maize weevil, Sitophilus zeamais Motsch. (Coleoptera: Curculionidae). Proc. Zool. Soc. 2021, 74, 219–226. [Google Scholar] [CrossRef]
- Bhusal, K.; Khanal, D. Role of maize weevil, Sitophilus zeamais Motsch. on spread of Aspergillus section flavi in different Nepalese maize varieties. Adv. Agric. 2019, 2019, 7584056. [Google Scholar] [CrossRef]
- Sebayang, A.; Rubiana, R.; Sipi, S.; Manwan, S.W.; Fattah, A.; Arrahman, A.; Saenong, M.S. Sitophilus zeamais (Motschulsky): The primary obstacles in the maize quality and quantity. IOP Conf. Ser. Earth Environ. Sci. 2023, 1230, 12089. [Google Scholar] [CrossRef]
- Shakoori, F.R.; Feroz, A.; Gondal, A.; Akram, S.; Riaz, T. Impact of λ-cyhalothrin on carbohydrate metabolizing enzymes and macromolecules of a stored grain pest, Trogoderma granarium. Pak. J. Zool. 2018, 50, 1467–1474. [Google Scholar] [CrossRef]
- Li, X.R.; Li, Y.; Wang, W.; He, N.; Tan, X.L.; Yang, X.Q. LC50 of lambda-cyhalothrin stimulates reproduction on the moth Mythimna separata (Walker). Pestic. Biochem. Phys. 2019, 153, 47–54. [Google Scholar] [CrossRef]
- Keyhanian, A.A.; Barari, H.; Mobasheri, M.T. Comparison of the efficacy of insecticides, alphacypermethrin and lambda-cyhalothrin, against canola flea beetles. Appl. Entomol. Phytopathol. 2021, 44, 113–122. [Google Scholar]
- Yeasmin, A.M.; Waliullah, T.M.; Alam, M.A.; Islam, N.; Rahman, A.S. Co-toxicity evaluation of lambda-cyhalothrin and its synergist PBO for susceptibility of Alphitobius diaperinus (Coleoptera: Tenebrionidae). World J. Pharm. Res 2015, 4, 239–253. [Google Scholar]
- Li, W.; Naeem, M.; Cui, J.; Du, G.; Chen, H. Toxicity and sublethal effects of lambda-cyhalothrin insecticide on parent and filial generations of Henosepilachna vigintioctomaculata (Coleoptera: Coccinellidae). Insects 2025, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Lambkin, T.A.; Rice, S. Responses of susceptible and cyfluthrin-resistant broiler house populations of lesser mealworm (Coleoptera: Tenebrionidae) to cyhalothrin. J. Econ. Entomol. 2010, 103, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- He, L.M.; Troiano, J.; Wang, A.; Goh, K. Environmental chemistry, ecotoxicity, and fate of lambda-cyhalothrin. Rev. Environ. Contam. Toxicol. 2008, 195, 71–91. [Google Scholar]
- Ju, D.; Liu, Y.X.; Liu, X.; Dewer, Y.; Mota-Sanchez, D.; Yang, X.Q. Exposure to lambda-cyhalothrin and abamectin drives sublethal and transgenerational effects on the development and reproduction of Cydia pomonella. Ecotoxicol. Environ. Saf. 2023, 252, 114581. [Google Scholar] [CrossRef]
- Kayahan, A. Lethal and sublethal effects of lambda-cyhalothrin on Aphis fabae (Scopoli, 1763), Myzus persicae (Sulzer, 1776) and Acyrthosiphon pisum (Harris, 1776) (Hemiptera: Aphididae). Turk. J. Entomol. 2023, 47, 175–188. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Eleftheriadou, N.; Xefteri, D.N.; Gidari, D.L.S.; Kyrpislidi, V.P.C. The sublethal impacts of five insecticidal formulations on Oryzaephilus surinamensis behavioral traits. Pest Manag. Sci. 2024, 80, 5334–5341. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Z.; Gong, S.; Zhang, Y.; Xue, C. Sublethal concentrations of lambda-cyhalothrin reduce fecundity by affecting the hormone-vitellogenin signaling pathway in Chrysoperla sinica. Entomol. Gen. 2024, 44, 1459–1559. [Google Scholar] [CrossRef]
- De França, S.M.; Breda, M.O.; Barbosa, D.R.S.; Araujo, A.M.N.; Guedes, C.A. The sublethal effects of insecticides in insects. In Biological Control of Pest and Vector Insects; Shields, V.D.C., Ed.; BoD–Books on Demand: London, UK, 2017; pp. 23–39. [Google Scholar]
- Gidari, D.L.S.; Kavallieratos, N.G.; Boukouvala, M.C. Sublethal effects of α-cypermethrin on the behavioral asymmetries and mating success of Alphitobius diaperinus. Insects 2024, 15, 804. [Google Scholar] [CrossRef]
- Campbell, B.; Baldwin, R.; Koehler, P. Locomotion inhibition of Cimex lectularius L. following topical, sublethal dose application of the chitin synthesis inhibitor lufenuron. Insects 2017, 8, 94. [Google Scholar] [CrossRef]
- Vallortigara, G.; Chiandetti, C.; Sovrano, V.A. Brain asymmetry (animal). Wiley Interdiscip. Rev. Cogn. Sci. 2011, 2, 146–157. [Google Scholar] [CrossRef]
- Anfora, G.; Rigosi, E.; Frasnelli, E.; Ruga, V.; Trona, F.; Vallortigara, G. Lateralization in the invertebrate brain: Left-right asymmetry of olfaction in bumble bee, Bombus terrestris. PLoS ONE 2011, 6, e18903. [Google Scholar] [CrossRef]
- Hunt, E.R.; O’Shea-Wheller, T.; Albery, G.F.; Bridger, T.H.; Gumn, M.; Franks, N.R. Ants show a leftward turning bias when exploring unknown nest sites. Biol. Lett. 2014, 10, 20140945. [Google Scholar] [CrossRef] [PubMed]
- Güntürkün, O.; Ströckens, F.; Ocklenburg, S. Brain lateralization: A comparative perspective. Physiol. Rev. 2020, 100, 1019–1063. [Google Scholar] [CrossRef]
- Rogers, L.J. Brain lateralization and cognitive capacity. Animals 2021, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Liga, D.; Stancher, G.; Frasnelli, E. Visuo-motor lateralization in Apis mellifera: Flight speed differences in foraging choices. Sci. Rep. 2024, 14, 660. [Google Scholar] [CrossRef]
- Romano, D.; Kavallieratos, N.G.; Athanassiou, C.G.; Stefanini, C.; Canale, A.; Benelli, G. Impact of geographical origin and rearing medium on mating success and lateralization in the rice weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae). J. Stored Prod. Res. 2016, 69, 106–112. [Google Scholar] [CrossRef]
- Benelli, G.; Romano, D.; Kavallieratos, N.G.; Conte, G.; Stefanini, C.; Mele, M.; Athanassiou, C.G.; Canale, A. Multiple behavioural asymmetries impact male mating success in the khapra beetle, Trogoderma granarium. J. Pest Sci. 2017, 90, 901–909. [Google Scholar] [CrossRef]
- Boukouvala, M.C.; Romano, D.; Kavallieratos, N.G.; Stefanini, C.; Canale, A.; Benelli, G. Behavioral asymmetries affecting male mating success in Tenebrio molitor (Coleoptera: Tenebrionidae), an important edible species. J. Econ. Entomol. 2021, 114, 454–461. [Google Scholar] [CrossRef]
- Obata, H.; Manabe, A.; Nakamura, N.; Onishi, T.; Senba, Y. A new light on the evolution and propagation of prehistoric grain pests: The World’s oldest maize weevils found in Jomon potteries, Japan. PLoS ONE 2011, 6, e14785. [Google Scholar] [CrossRef]
- Cordeiro, E.M.C.; Correa, A.S.; Rosi-Denadai, C.A.; Tome, H.V.V.; Guedes, R.N.C. Insecticide resistance and size assortative mating in females of the maize weevil (Sitophilus zeamais). Pest Manag. Sci. 2017, 73, 823–829. [Google Scholar] [CrossRef]
- Arrahman, A.; Mirsam, H.; Djaenuddin, N.; Suriani; Pakki, S.; Saenong, M.S.; Sebayang, A. An in-depth study on Sitophilus zeamais Motsch (Coleoptera: Curculionidae) pests on corn plants. IOP Conf. Ser. Earth Environ. Sci. 2022, 1107, 012060. [Google Scholar] [CrossRef]
- Asgher, S.; Avasthi, S. Infestation and growth index on Zea mays by Sitophilus zeamais affected by temperature and humidity; created in in-vitro conditions. Int. J. Entomol. Res. 2024, 9, 74–79. [Google Scholar]
- Agathokleous, E.; Blande, J.D.; Calabrese, E.J.; Guedes, R.N.C.; Benelli, G. Stimulation of insect vectors of pathogens by sublethal environmental contaminants: A hidden threat to human and environmental health? Environ. Pollut. 2023, 336, 122422. [Google Scholar] [CrossRef]
- Qu, Y.; Xiao, D.; Liu, J.; Chen, Z.; Song, L.; Desneux, N.; Benelli, G.; Xiwu, G.; Song, D. Sublethal and hormesis effects of beta-cypermethrin on the biology, life table parameters and reproductive potential of soybean aphid Aphis glycines. Ecotoxicology 2017, 26, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Trematerra, P.; Ianiro, R.; Athanassiou, C.G.; Kavallieratos, N.G. Behavioral interactions between Sitophilus zeamais and Tribolium castaneum: The first colonizer matters. J. Pest Sci. 2015, 88, 573–581. [Google Scholar] [CrossRef]
- Suleiman, M.; Ibrahim, N.D.; Majeed, Q. Control of Sitophilus zeamais (Motsch) (Coleoptera: Curculionidae) on sorghum using some plant powders. Int. J. Agric. For. 2012, 2, 53–57. [Google Scholar] [CrossRef]
- Halstead, D. External sex differences in stored-products Coleoptera. Bull. Entomol. Res. 1963, 54, 119–134. [Google Scholar] [CrossRef]
- Tolpo, N.C.; Morrison, E.O. Sex determination by snout characteristics of Sitophilus zeamais Motschulsky. Tex. J. Sci. 1965, 7, 122–124. [Google Scholar]
- Boukouvala, M.C.; Romano, D.; Kavallieratos, N.G.; Athanassiou, C.G.; Stefanini, C.; Canale, A.; Benelli, G. Does geographical origin affect lateralization and male mating success in Rhyzopertha dominica beetles? J. Stored Prod. Res. 2020, 88, 101630. [Google Scholar] [CrossRef]
- Guedes, N.M.P.; Guedes, R.N.C.; Campbell, J.F.; Throne, J.E. Mating behaviour and reproductive output in insecticide-resistant and-susceptible strains of the maize weevil (Sitophilus zeamais). Ann. Appl. Biol. 2017, 170, 415–424. [Google Scholar] [CrossRef]
- Finney, D.J. Statistical Methods in Biological Assay; Charles Griffin: London, UK, 1978. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.Rproject.org/ (accessed on 30 June 2025).
- SAS Institute Inc. Using JMP 16.2; SAS Institute Inc.: Cary, NC, USA, 2021. [Google Scholar]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries, 1st ed.; Cambridge UP: Cambridge, UK, 2013. [Google Scholar]
- Boukouvala, M.C.; Kavallieratos, N.G.; Maggi, F.; Angeloni, S.; Ricciutelli, M.; Spinozzi, E.; Ferrati, M.; Petrelli, R.; Canale, A.; Benelli, G. Being exposed to Acmella oleracea-based insecticide extract reduces mobility and mating success in Prostephanus truncatus, the major pest of maize in storages. J. Stored Prod. Res. 2023, 104, 102151. [Google Scholar] [CrossRef]
- Walgenbach, C.A.; Burkholder, W.E. Mating behavior of the maize weevil, Sitophilus zeamais (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 1987, 80, 578–583. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Guedes, R.N.; Drempela, J.D.; Boukouvala, M.C. Invader competition with local competitors: Displacement or coexistence among the invasive khapra beetle, Trogoderma granarium Everts (Coleoptera: Dermestidae), and two other major stored-grain beetles? Front. Plant Sci. 2017, 8, 1837. [Google Scholar] [CrossRef]
- Hoppe, K.R.; Roush, R.T. Mate finding, dispersal, number released, and the success of biological control introductions. Ecol. Entomol. 1993, 18, 321–331. [Google Scholar] [CrossRef]
- Hardy, I.C.; Ode, P.J.; Siva-Jothy, M. Mating behavior. In Insects as Natural Enemies: A Practical Perspective; Jervis, M.A., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 219–260. [Google Scholar]
- Sallam, M.N. Insect Damage: Damage on Post-Harvest; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Volume 38, p. 1. [Google Scholar]
- Rosentrater, K.A. Insects in grains: Identification, damage, and detection. In Storage of Cereal Grains and their Products; Rosentrater, K.A., Ed.; Elsevier: Duxford, UK, 2022; pp. 261–292. [Google Scholar]
- Ojo, J.A.; Omoloye, A.A. Rearing the maize weevil, Sitophilus zeamais, on an artificial maize cassava diet. J. Insect Sci. 2012, 12, 69. [Google Scholar] [CrossRef]
- Chidege, M.Y.; Venkataramana, P.B.; Ndakidemi, P.A. Enhancing food grains storage systems through insect pest detection and control measures for maize and beans: Ensuring food security post-COVID-19 Tanzania. Sustainability 2024, 16, 1767. [Google Scholar] [CrossRef]
| Active Ingredient | Unit | LC10 (95% CI) | LC30 (95% CI) | LC50 (95% CI) | χ2 (df = 23) | p |
|---|---|---|---|---|---|---|
| λ-cyhalothrin | mg a.i./cm2 | 0.000212 (0.000177–0.000241) | 0.000282 (0.000250–0.000308) | 0.000344 (0.000317–0.000366) | 30.3 | 1.00 |
| Precopula | |||||||
|---|---|---|---|---|---|---|---|
| Traits | Direction of Approach | Mate Recognition (min) | Interaction (min) | Walking (min) | Mounting Attempts (Number) | Copulation (min) | |
| Treatment | |||||||
| control | Back | 2.8 ± 0.1 D | 6.7 ± 0.5 BC | 7.2 ± 1.2 A | 1.7 ± 0.2 C | 268.1 ± 13.6 A | |
| Left | 1.8 ± 0.1 F | 3.8 ± 1.0 D | 4.8 ± 0.3 B | 1.6 ± 0.1 C | 276.9 ± 13.9 A | ||
| Right | 2.2 ± 0.1 E | 6.2 ± 0.6 C | 5.1 ± 0.4 B | 1.6 ± 0.1 C | 274.1 ± 14.2 A | ||
| Tested beetles (number = back + left + right) | 11 + 37 + 29 = 77 | 11 + 37 + 29 = 77 | 11 + 37 + 29 = 77 | 11 + 37 + 29 = 77 | 7 + 25 + 18 = 50 | ||
| LC10 | Back | 2.8 ± 0.2 CD | 7.2 ± 0.6 BC | 3.6 ± 0.3 D | 4.2 ± 0.8 B | 109.2 ± 28.0 C | |
| Left | 2.7 ± 0.1 D | 5.9 ± 0.4 C | 4.2 ± 0.2 C | 3.9 ± 0.5 B | 160.4 ± 19.2 B | ||
| Right | 3.0 ± 0.1 C | 6.5 ± 0.5 C | 3.7 ± 0.2 D | 3.9 ± 0.5 B | 82.4 ± 21.4 C | ||
| Front | 3.4 ± 0.3 BC | 8.9 ± 1.2 AB | 4.1 ± 0.4 BCD | 5.8 ± 1.2 AB | 46.8 ± 6.7 D | ||
| Tested beetles (number = back+ left + right + front) | 18 + 47 + 39 + 7 = 111 | 18 + 47 + 39 + 7 = 111 | 18 + 47 + 39 + 7 = 111 | 18 + 47 + 39 + 7 = 111 | 6 + 27 + 10 + 7= 50 | ||
| LC30 | Back | 3.9 ± 0.2 A | 8.5 ± 0.6 AB | 3.1 ± 0.2 D | 4.8 ± 0.6 B | 95.1 ± 24.2 C | |
| Left | 3.5 ± 0.1 B | 6.2 ± 0.4 C | 3.7 ± 0.2 D | 4.6 ± 0.5 B | 119.1 ± 17.4 C | ||
| Right | 3.5 ± 0.1 B | 7.6 ± 0.5 B | 3.3 ± 0.1 D | 4.1 ± 0.5 B | 80.4 ± 19.1 C | ||
| Front | 3.7 ± 0.2 B | 9.7 ± 0.7 A | 3.32 ± 0.3 D | 6.5 ± 0.6 A | 18.6 ± 18.6 E | ||
| Tested beetles (number = back+ left + right + front) | 21 + 58 + 46 + 14 = 139 | 21 + 58 + 46 + 14 = 139 | 21 + 58 + 46 + 14 = 139 | 21 + 58 + 46 + 14 = 139 | 4 + 27 + 18 + 1 = 50 | ||
| χ2, df, p | 170.0, 10, <0.01 | 81.6, 10, <0.01 | 63.7, 10, <0.01 | 96.7, 10, <0.01 | 140.5, 10, <0.01 | ||
| Precopula | |||||||
|---|---|---|---|---|---|---|---|
| Traits | Direction of Mounting Side | Mate Recognition (min) | Interaction (min) | Walking (min) | Mounting Attempts (Number) | Copulation (min) | |
| Treatment | |||||||
| control | Back | 2.1 ± 0.2 E | 5.4 ± 0.7 C | 5.3 ± 0.6 A | 1.7 ± 0.2 D | 248.4 ± 10.8 B | |
| Left | 1.9 ± 0.1 E | 4.5 ± 0.3 D | 5.3 ± 0.4 A | 1.7 ± 0.1 D | 269.0 ± 10.1 B | ||
| Right | 2.0 ± 0.2 E | 6.2 ± 0.8 BC | 5.2 ± 0.5 A | 1.6 ± 0.1 D | 309.7 ± 24.1 A | ||
| Tested beetles (number = back + left + right) | 11 + 37 + 29 = 77 | 11 + 37 + 29 = 77 | 11 + 37 + 29 = 77 | 11 + 37 + 29 = 77 | 7 + 25 + 18 = 50 | ||
| LC10 | Back | 2.8 ± 0.2 CD | 7.2 ± 0.6 BC | 3.8 ± 0.3 BC | 4.4 ± 1.0 B | 115.5 ± 8.2 D | |
| Left | 2.7 ± 0.1 D | 5.9 ± 0.4 C | 4.1 ± 0.3 B | 3.6 ± 0.5 C | 133.9 ± 5.3 C | ||
| Right | 3.0 ± 0.1 C | 6.5 ± 0.5 BC | 3.6 ± 0.2 BC | 4.1 ± 0.6 C | 103.2 ± 5.0 D | ||
| Front | 3.4 ± 0.3 ABC | 8.9 ± 1.2 AB | 4.1 ± 0.4 B | 5.3 ± 1.1 AB | 61.8 ± 14.9 E | ||
| Tested beetles (number = back+ left + right + front) | 20 + 47 + 36 + 8 = 111 | 20 + 47 + 36 + 8 = 111 | 20 + 47 + 36 + 8 = 111 | 20 + 47 + 36 + 8 = 111 | 9 + 29 + 11 + 1 = 50 | ||
| LC30 | Back | 3.9 ± 0.2 A | 7.8 ± 0.5 B | 3.3 ± 0.2 C | 4.8 ± 0.6 B | 104.3 ± 6.8 D | |
| Left | 3.5 ± 0.1 B | 6.2 ± 0.4 C | 3.6 ± 0.2 BC | 4.6 ± 0.5 B | 121.0 ± 2.0 D | ||
| Right | 3.5 ± 0.1 B | 7.6 ± 0.6 B | 3.3 ± 0.1 C | 4.1 ± 0.5 BC | 51.8 ± 6.7 E | ||
| Front | 3.7 ± 0.2 AB | 9.9 ± 0.7 A | 3.3 ± 0.3 C | 6.5 ± 0.6 A | 44.6 ± 26.0 E | ||
| Tested beetles (number = back+ left + right + front) | 26 + 56 + 42 + 15 = 139 | 26 + 56 + 42 + 15 = 139 | 26 + 56 + 42 + 15 = 139 | 26 + 56 + 42 + 15 = 139 | 10 + 26 + 13 + 1 = 50 | ||
| χ2, df, p | 170.5, 10, <0.01 | 59.5, 10, <0.01 | 59.4, 10, <0.01 | 126.8, 10, <0.01 | 129.6, 10, <0.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukouvala, M.C.; Kavallieratos, N.G.; Gidari, D.L.S.; Filintas, C.S.; Skourti, A.; Kyrpislidi, V.P.C.; Skordos, D.P. Love in the Time of Pyrethroids: Mating Behavior of Sitophilus zeamais Is Influenced by Sublethal Concentrations of λ-Cyhalothrin and Lateralization. Insects 2025, 16, 865. https://doi.org/10.3390/insects16080865
Boukouvala MC, Kavallieratos NG, Gidari DLS, Filintas CS, Skourti A, Kyrpislidi VPC, Skordos DP. Love in the Time of Pyrethroids: Mating Behavior of Sitophilus zeamais Is Influenced by Sublethal Concentrations of λ-Cyhalothrin and Lateralization. Insects. 2025; 16(8):865. https://doi.org/10.3390/insects16080865
Chicago/Turabian StyleBoukouvala, Maria C., Nickolas G. Kavallieratos, Demeter Lorentha S. Gidari, Constantin S. Filintas, Anna Skourti, Vasiliki Panagiota C. Kyrpislidi, and Dionysios P. Skordos. 2025. "Love in the Time of Pyrethroids: Mating Behavior of Sitophilus zeamais Is Influenced by Sublethal Concentrations of λ-Cyhalothrin and Lateralization" Insects 16, no. 8: 865. https://doi.org/10.3390/insects16080865
APA StyleBoukouvala, M. C., Kavallieratos, N. G., Gidari, D. L. S., Filintas, C. S., Skourti, A., Kyrpislidi, V. P. C., & Skordos, D. P. (2025). Love in the Time of Pyrethroids: Mating Behavior of Sitophilus zeamais Is Influenced by Sublethal Concentrations of λ-Cyhalothrin and Lateralization. Insects, 16(8), 865. https://doi.org/10.3390/insects16080865









