Pre-Courtship Behavior of Proholopterus chilensis (Coleoptera: Cerambycidae) in a Nothofagus obliqua (Nothofagaceae) Forest
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
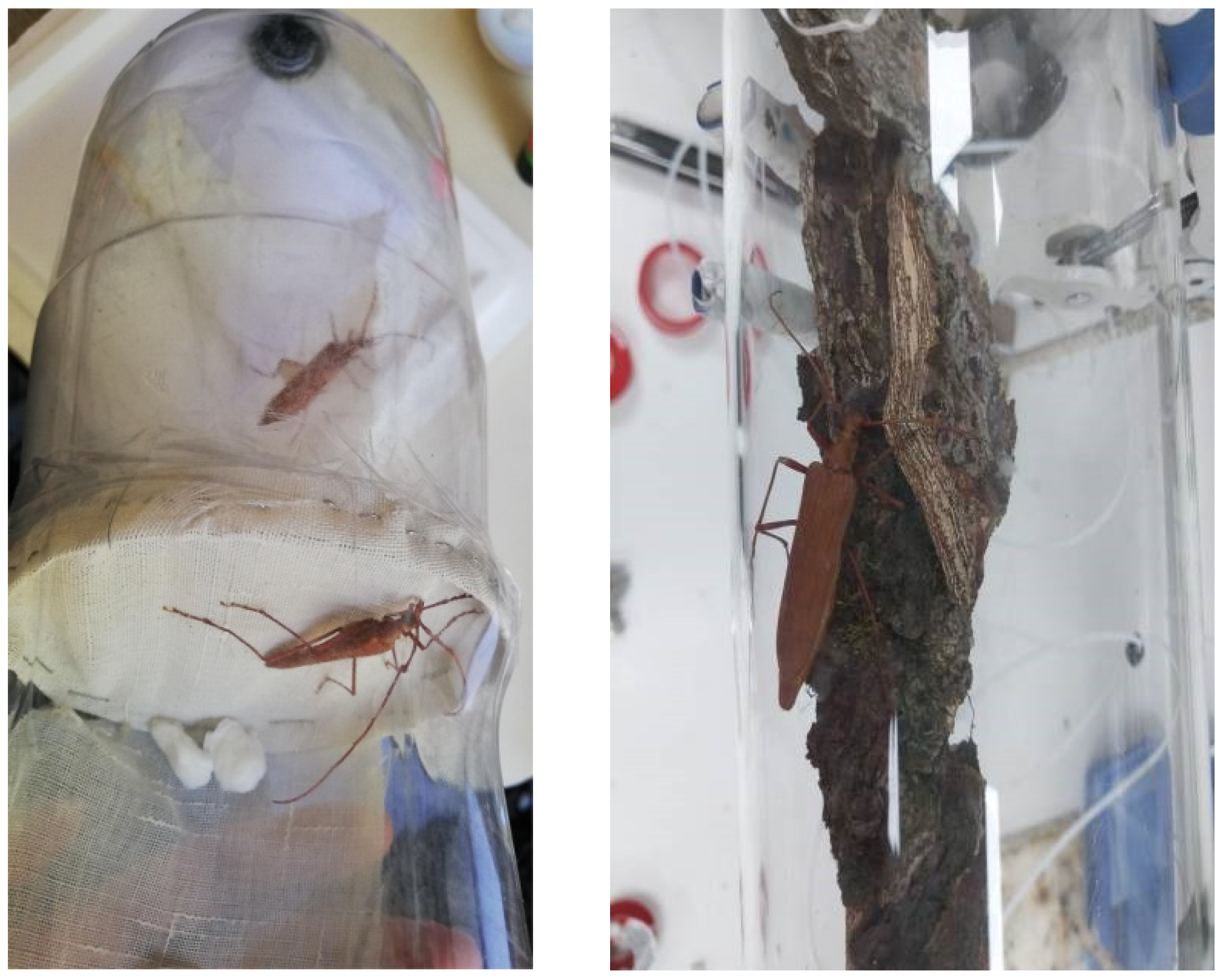
2.1. Collection, Taxonomic Identification, and Pre-Experimental Handling of P. chilensis
2.2. Pre-Courtship Behavioral Sequence Experiments in P. chilensis
2.3. Pre-Courtship Behavioral Units and Field Observations of P. chilensis
2.4. Sequences and Stereotypy of Pre-Courtship Behaviors in P. chilensis
2.5. Ethogram of Pre-Courtship Behavior of P. chilensis
3. Results
3.1. Field Emergence of P. chilensis
3.2. Behavioral Units of Proholopterus chilensis in Observation Arenas and in the Field
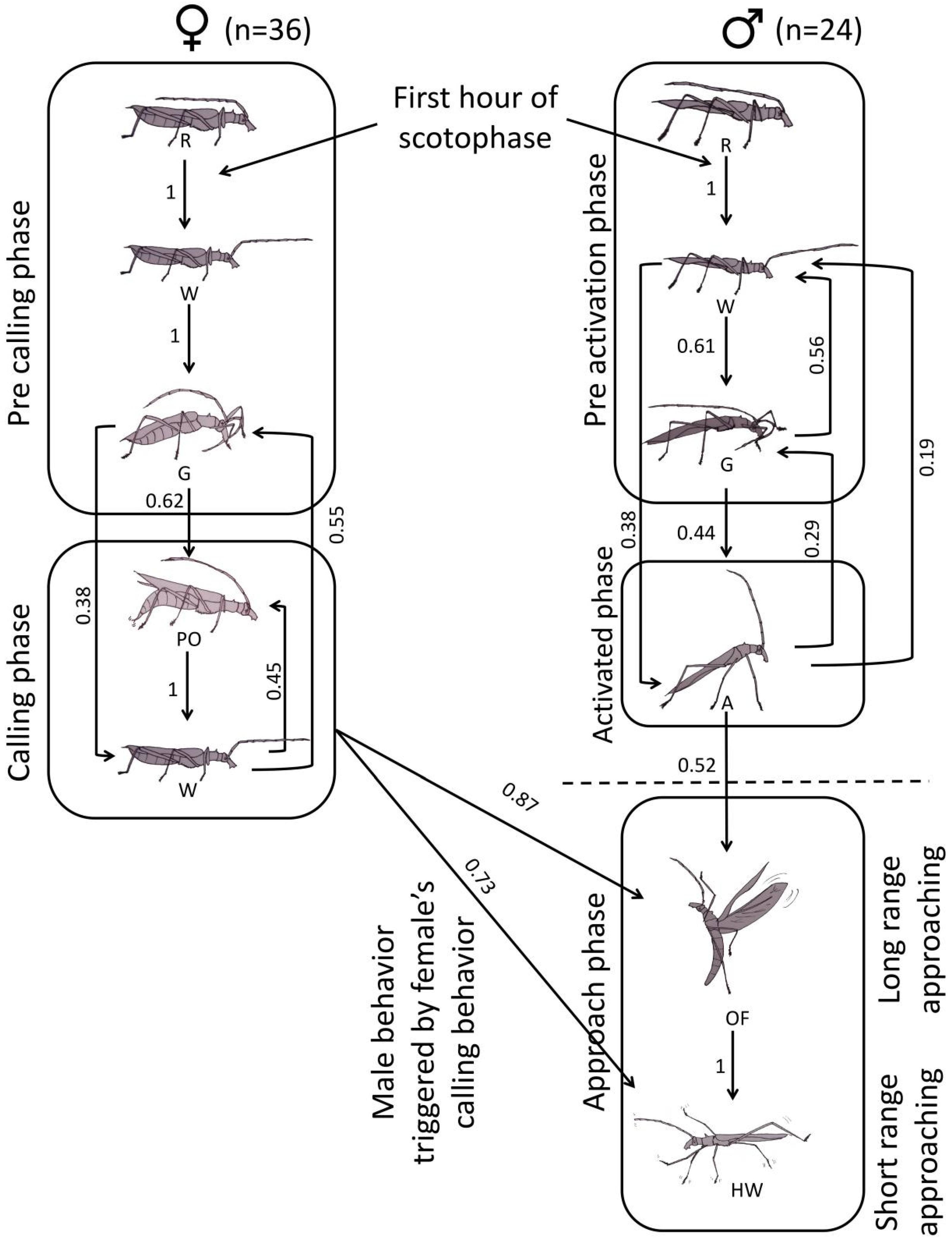
3.3. Pre-Courtship Behavioral Sequences and Stereotypy in P. chilensis
3.4. Ethogram
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matthews, R.W.; Matthews, J.R. Insect Behavior, 2nd ed.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2010; 514p. [Google Scholar]
- Alexander, R.D.; Marshall, D.C.; Cooley, J.R. Evolutionary perspectives in insect mating. In The Evolution of Mating Systems in Insects and Arachnids; Choe, J.C., Crespi, B.J., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 4–31. [Google Scholar]
- Brown, W.D.; Crespi, B.J.; Choe, J. Sexual conflict and the evolution of mating systems. In The Evolution of Mating Systems in Insects and Arachnids; Choe, J.C., Crespi, B.J., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 352–378. [Google Scholar]
- Wyatt, T.D. Pheromones and Animal Behavior: Communication by Smell and Taste; Cambridge University Press: Cambridge, UK, 2008; 391p. [Google Scholar]
- Xu, T.; Teale, S.A. Chemical ecology of the Asian longhorn beetle, Anoplophora glabripennis. J. Chem. Ecol. 2021, 47, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Curkovic, T.; Ferrera, C. Female calling and male flight orientation and searching behaviors in Callisphyris apicicornis: Evidence for a female-produced sex attractant pheromone. Cienc. E Investig. Agrar. 2012, 39, 147–158. [Google Scholar] [CrossRef]
- Allison, J.D.; Borden, J.H.; Seybold, S.J. A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 2004, 14, 123–150. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Graham, E.E.; Wong, J.C.; Reagel, P.F.; Striman, B.L.; Hughes, G.P.; Hanks, L.M. Fuscumol and fuscumol acetate are general attractants for many species of cerambycid beetles in the subfamily Lamiinae. Entomol. Exp. Appl. 2011, 141, 71–77. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Reagel, P.F.; Wong, J.H.C.; Meier, L.R.; Diaz Silva, W.; Mongold-Diers, J.; Millar, J.G.; Hanks, L.M. Cerambycid beetle species with similar pheromones are segregated by phenology and minor pheromone components. J. Chem. Ecol. 2015, 41, 431–440. [Google Scholar] [CrossRef]
- Lacey, E.S.; Ray, A.M.; Hanks, L.M. Calling behavior of the cerambycid beetle Neoclytus acuminatus acuminatus (F.). J. Insect Behav. 2007, 20, 117–128. [Google Scholar] [CrossRef]
- Lemay, M.A.; Silk, P.J.; Sweeney, J. Calling behavior of Tetropium fuscum (Coleoptera: Cerambycidae: Spondylidinae). Can. Entomol. 2010, 142, 256–260. [Google Scholar] [CrossRef]
- Wickham, J.D.; Xu, Z.; Teale, S.A. Evidence for a female-produced, long-range pheromone of Anoplophora glabripennis (Coleoptera: Cerambycidae). Insect Sci. 2012, 19, 355–371. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G. Sex and Aggregation-Sex Pheromones of Cerambycid Beetles: Basic Science and Practical Applications. J. Chem. Ecol. 2016, 42, 631–654. [Google Scholar] [CrossRef]
- Cervantes, D.E.; Hanks, L.; Lacey, E.S.; Barbour, J.D. First documentation of a volatile sex pheromone in a longhorned beetle (Coleoptera: Cerambyicidae) of the primitive subfamily Prioninae. Ann. Entomol. Soc. Am. 2006, 99, 718–722. [Google Scholar] [CrossRef]
- Arraztio, D.; Huerta, A.; Quiroz, A.; Aniñir, W.; Rebolledo, R.; Curkovic, T. Factors to Male-Female Sex Approaches and the Identification of Volatiles and Compounds from the Terminalia of Proholopterus chilensis (Blanchard) (Coleoptera: Cerambycidae) Females in Nothofagus obliqua (Mirb.) Oerst. (Nothofagaceae) Forests in Chile. Insects 2024, 15, 741. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamane, A.; Iwata, R. Mating behavior of the pine sawyer, Monochamus saltuarius (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2003, 38, 141–148. [Google Scholar] [CrossRef]
- Keena, M.A. Factors that influence flight propensity in Anoplophora glabripennis (Coleoptera: Cerambycidae). Environ. Entomol. 2018, 47, 1233–1241. [Google Scholar] [CrossRef]
- Iwabuchi, K. Mating behavior of Xylotrechus pyrrhoderus Bates (Coleoptera: Cerambycidae) I. Behavioral sequences and existence of the male sex pheromone. Appl. Entomol. Zool. 1982, 17, 494–500. [Google Scholar] [CrossRef]
- Ray, A.M.; Ginzel, M.D.; Hanks, L.M. Male Megacyllene robiniae (Coleoptera: Cerambycidae) Use Multiple Tactics When Aggressively Competing for Mates. Environ. Entomol. 2009, 38, 425–432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiriyama, S.; Iwata, R.; Fukaya, M.; Hoshino, Y.; Yamanaka, Y. Mating behavior of Rosalia batesi (Coleoptera: Cerambycidae) is mediated by male-produced sex pheromones. Insects 2018, 9, 48. [Google Scholar] [CrossRef]
- Švácha, P.; Lawrence, J.F. Volume 3: Morphology and Systematics (Phytophaga). In Handbook of Zoology: Arthropoda: Insecta: Coleoptera, Beetles; Leschen, R.A.B., Beutel, R.G., Eds.; Walter de Gruyter: Berlin, Germany, 2014; pp. 77–177. [Google Scholar]
- Barriga, J.E.; Curkovic, T.; Fichet, T.; Henríquez, J.; Macaya, J. Nuevos antecedentes de coleópteros xilófagos y plantas hospederas en Chile, con una recopilación de citas previas. Rev. Chil. De Entomol. 1993, 20, 65–91. (In Spanish) [Google Scholar][Green Version]
- Baldini, A.; Alvarado, A. Manual de Plagas Y Enfermedades Del Bosque Nativo en Chile, 1st ed.; FAO: Santiago, Chile, 2008; 240p. (In Spanish) [Google Scholar]
- Curkovic, T.; Arraztio, D.; Huerta, A.; Rebolledo, R.; Cheuquel, A.; Contreras, A.; Millar, J.G. Generic pheromones identified from northern hemisphere Cerambycidae (Coleoptera) are attractive to native longhorn beetles from Central-Southern Chile. Insects 2022, 13, 1067. [Google Scholar] [CrossRef]
- Artigas, J. Holopterus chilensis Blanchard, taladrador del roble. In Entomología Económica: Insectos de Interés Agrícola, Forestal, Médico Y Veterinario (Nativos, Introducidos Y Susceptibles de Ser Introducidos); Ediciones Universidad de Concepción: Concepción, Chile, 1994; pp. 146–147. (In Spanish) [Google Scholar]
- Barriga, A. Caracterización Morfológica Y Morfométrica de Proholopterus Chilensis (Blanchard in Gay, 1851) (Col.: Cerambycidae) Y Sus Daños Sobre Nothofagus Obliqua (Mirb.) Oerst. en la Región de la Araucanía; Memoria Ingeniero Forestal, Universidad de Chile: Santiago, Chile, 2021; 58p. (In Spanish) [Google Scholar]
- Hutcheson, J.A. Arhopalus ferus (Coleoptera: Cerambycidae): Structure and function of the female reproductive system. N. Z. J. Zool. 1980, 7, 417–424. [Google Scholar] [CrossRef]
- Monné, L.; Monne, A.; Wang, Q. General morphology, classification and biology of Cerambycidae. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–70. [Google Scholar]
- Haynes, K.F.; Birch, M.C. Mate-locating and courtship behaviors of the artichoke plume moth, Platyptilia carduidactyla (Lepidoptera: Pterophoridae). Environ. Entomol. 1984, 13, 399–408. [Google Scholar] [CrossRef]
- Hubweber, L.; Schmitt, M. Differences in genitalia structure and function between subfamilies of longhorn beetles (Coleoptera: Cerambycidae). Genetica 2010, 138, 37–43. [Google Scholar] [CrossRef]
- Fagen, R.M.; Young, D.Y. Temporal patterns of behaviors: Durations, intervals, latencies, and sequences. In Quantitative Ethology; Colgan, P.W., Ed.; John Wiley and Son: New York, NY, USA; Chichester, UK; Brisbane, Australia; Toronto, ON, Canada, 1978; pp. 79–114. [Google Scholar]
- Ott, R.L. An Introduction to Statistical Methods and Data Analysis; Duxbury Press: Belmont, CA, USA, 1993; pp. 392–403. [Google Scholar]
- Colgan, P.W.; Smith, J.T. Multidimensional contingency table analysis. In Quantitative Ethology; Colgan, P.W., Ed.; John Wiley and Son: New York, NY, USA; Chichester, UK; Brisbane, Australia; Toronto, ON, Canada, 1978; pp. 145–174. [Google Scholar]
- Liimatainen, J.; Hoikkala, A. Interactions of the males and females of three sympatric Drosophila virilis-group species, D. Montana, D. littoralis, and D. lummei, (Diptera: Drosophilidae) in intra- and interspecific courtships in the wild and in the laboratory. J. Insect Behav. 1998, 11, 399–417. [Google Scholar] [CrossRef]
- Girling, R.D.; Cardé, R.T. Analysis of the courtship behavior of the navel orangeworm, Amyelois transitella (Walker) (Lepidoptera: Pyralidae), with a commentary on methods for the analysis of sequences of behavioral transitions. J. Insect Behav. 2006, 19, 497–520. [Google Scholar] [CrossRef]
- Godínez-Aguilar, J.L.; Macías-Sámano, J.E.; Morón-Ríos, A. Notes on biology and sexual behavior of Tetrasarus plato Bates (Coleoptera: Cerambycidae), a tropical longhorn beetle in coffee plantations in Chiapas, Mexico. Coleopt. Bull. 2009, 63, 311–318. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 23 July 2025).
- Sarto, V.; Torras, G. A new alien invasive longhorn beetle, Xylotrechus chinensis (Cerambycidae), is infesting mulberries in Catalonia (Spain). Insects 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Royzenblat, S.; Kulacic, J.; Friedrich, M. Evidence of ancestral nocturnality, locomotor clock regression, and cave zone-adjusted sleep duration modes in a cave beetle. Subterr. Biol. 2023, 45, 75–94. [Google Scholar] [CrossRef]
- Jiang, X.L.; Ren, Z.; Hai, X.X.; Zhang, L.; Wang, Z.G.; Lyu, F. Exposure to artificial light at night mediates the locomotion activity and oviposition capacity of Dastarcus helophoroides (Fairmaire). Front. Physiol. 2023, 14, 1063601. [Google Scholar] [CrossRef]
- Ferreira, H.; Goncalvez, J. Forrageamento em Achaearanea cinnabarina Levi, 1963 (Araneae: Theriididae) e evolucao da caca em aranhas de teia irregular. Biota Neotrop. 2005, 5, 53–67. (In Portuguese) [Google Scholar]
- Facundo, H.T.; Linn, C.E.; Villani, M.G.; Roelofs, W.L. Emergence, mating, and postmating behaviors of the Oriental beetle (Coleoptera: Scarabaeidae). J. Insect Behav. 1999, 12, 175–192. [Google Scholar] [CrossRef]
- Charlton, R.E.; Cardé, R.T. Behavioral interactions in the courtship of Lymantria dispar (Lepidoptera: Lymantriidae). Ann. Entomol. Soc. Am. 1990, 83, 89–96. [Google Scholar] [CrossRef]
- Iwabuchi, K. Mating Behavior of Xylotrechus pyrrhoderus Bates (Coleoptera: Cerambycidae). VI Mating System. J. Ethol. 1988, 6, 69–76. [Google Scholar] [CrossRef]
- Haack, R.A.; Keena, M.A.; Eyre, D. Life history and population dynamics of Cerambycidae. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 71–103. [Google Scholar]
- Barbour, J.D.; Cervantes, D.E.; Lacey, E.S.; Hanks, L.M. Calling behavior in the primitive longhorned beetle Prionus californicus Mots. J. Insect Behav. 2006, 19, 623–629. [Google Scholar] [CrossRef]
- Xu, T.; Hansen, L.; Teale, S.A. Female calling behaviour in the Asian longhorned beetle (Coleoptera: Cerambycidae). Can. Entomol. 2019, 151, 600–607. [Google Scholar] [CrossRef]
- Cardé, R.T. Navigation along windborne plumes of pheromone and resource-linked odors. Annu. Rev. Entomol. 2021, 66, 317–336. [Google Scholar] [CrossRef]
- Curkovic, T.; Muñoz, J. Characterization of courtship and mating in Callisphyris apicicornis: Tool to define the viability to develop management strategies. Agrociencia 2011, 45, 453–464. [Google Scholar]
- Yasui, H.; Fujiwara-Tsujii, N. The effects of foods consumed after adult eclosion on the mate-searching behavior and feeding preferences of the white-spotted longicorn beetle Anoplophora malasiaca (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2013, 48, 181–188. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, W.; Chen, L.; Li, J.; Yin, X. Circadian reproductive rhythms, pair-bonding, and evidence for sex-specific pheromones in Nadezhdiella cantori (Coleoptera: Cerambycidae). J. Insect Behav. 2002, 15, 527–539. [Google Scholar] [CrossRef]
- Yasui, H.; Yasuda, T.; Fukaya, M.; Akino, T.; Wakamura, S.; Hirai, Y.; Kawasaki, K.; Ono, H.; Narahara, M.; Kousa, K.; et al. Host plant chemicals serve intraspecific communication in the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2007, 42, 255–268. [Google Scholar] [CrossRef]
- Sabri, M.S.; Abdullah, F. Mating behaviour and evidence of a female sex pheromone in Rytidodera simulans White (Coleoptera: Cerambycidae). J. Entomol. Res. 2016, 40, 313–326. [Google Scholar] [CrossRef]
- Farrell, S.L.; Andow, D.A. Highly variable male courtship behavioral sequences in a crambid moth. J. Ethol. 2017, 35, 221–236. [Google Scholar] [CrossRef]
- Fukaya, M.; Kiriyama, S.; Yasui, H. Mate-location flight of the red-necked longicorn beetle, Aromia bungii (Coleoptera: Cerambycidae): An invasive pest lethal to Rosaceae trees. Appl. Entomol. Zool. 2017, 52, 559–565. [Google Scholar] [CrossRef]
- Jung, J.K.; Lee, C.; Jang, B.; Nam, Y. Effects of Sex, Age, and Body Size on Flight Performance of Monochamus alternatus (Coleoptera: Cerambycidae), a Vector of Pine Wood Nematodes, Using Flight Mills. Insects 2025, 16, 444. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vila, L.M.; Mendiola-Diaz, F.J.; Sánchez-González, Á. Dispersal differences of a pest and a protected C erambyx species (Coleoptera: Cerambycidae) in oak open woodlands: A mark–recapture comparative study. Ecol. Entomol. 2017, 42, 18–32. [Google Scholar] [CrossRef]
- Gray, B. Observations on insect flight in a tropical forest plantation: II. Flight activity of Syllitus sp. nov.(Col. Cerambycidae). Z. Für Angew. Entomol. 1973, 74, 282–286. [Google Scholar] [CrossRef]
- Sheehan, T.N.; Ulyshen, M.D.; Horn, S.; Hoebeke, E.R. Vertical and horizontal distribution of bark and woodboring beetles by feeding guild: Is there an optimal trap location for detection? J. Pest Sci. 2019, 92, 327–341. [Google Scholar] [CrossRef]
- Wermelinger, B.; Flückiger, P.F.; Obrist, M.K.; Duelli, P. Horizontal and vertical distribution of saproxylic beetles (Col., Buprestidae, Cerambycidae, Scolytinae) across sections of forest edges. J. Appl. Entomol. 2007, 131, 104–114. [Google Scholar] [CrossRef]
- Miller, G.L.; Loudon, C.; Freed, S. Position around a tree: Consequences for pheromone detection. J. Chem. Ecol. 2007, 33, 541–554. [Google Scholar] [CrossRef]



| Behavioral Units | Definitions |
|---|---|
| Rest | Individuals remain immobile and in the same location, with the antennae directed backward and elytra resting over the dorsum of the body from the last hour of the previous scotophase until the second hour of the following one. |
| Grooming | Individuals bend their antennae with the aid of forelegs, directing them toward the mouthparts passing the medial and apical antennal segments between the mandibles. Both sexes performed this behavior for 3–10 min; females repeated it up to 40 times, while males did so 1 to 6 times, per night. |
| Walk | Individuals walk slowly, at a constant pace, in a marching-like fashion, with the antennae directed forward or sideways and the legs barely surpassing the height of the elytra, which remains flat over the dorsum of the abdomen. Each walk lasts 1–10 min in males and 5–10 min in females. Females tend to walk continuously, stopping only to groom. |
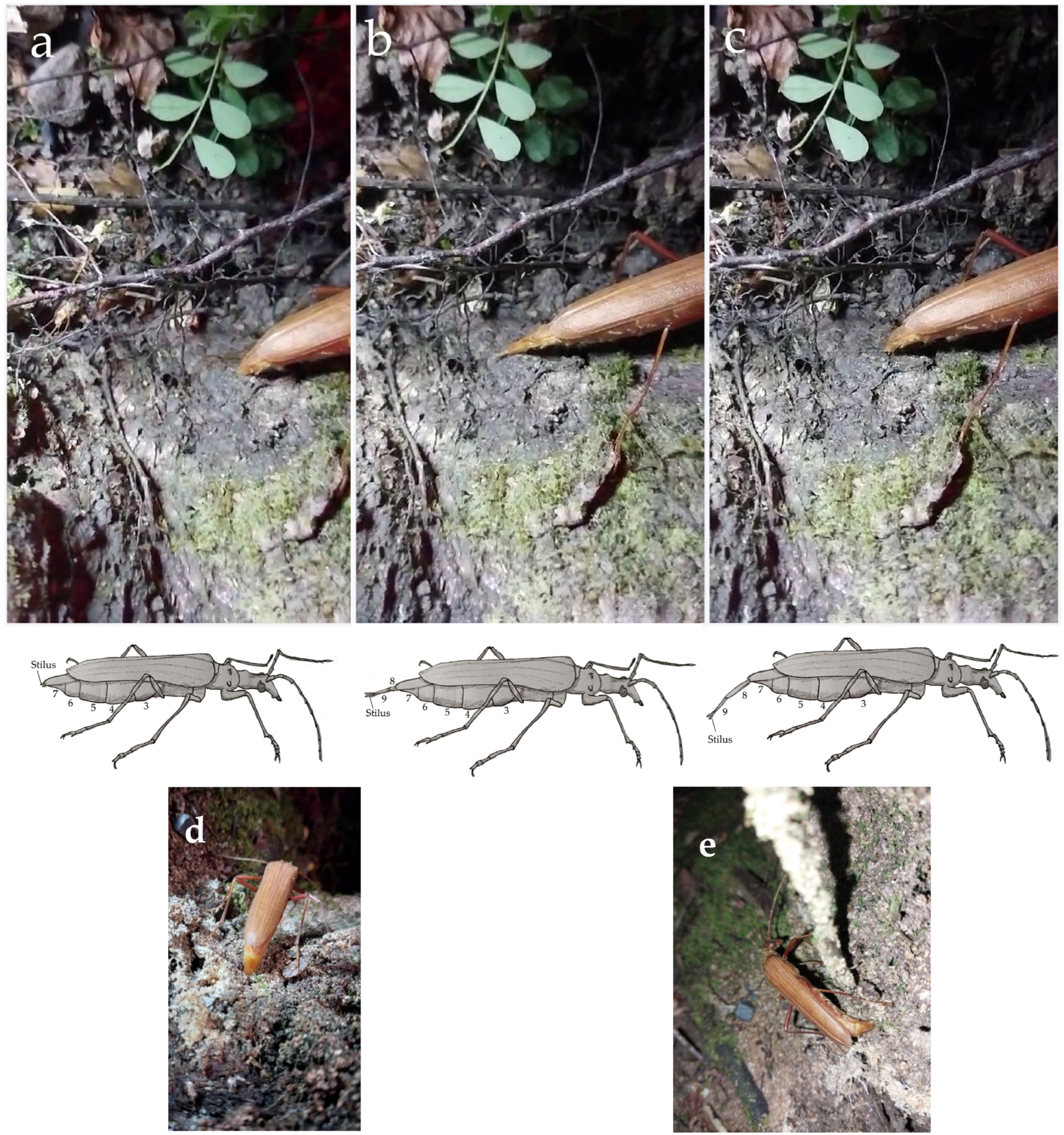
| Projection of the ovipositor | Females project segments VIII and IX posteriorly. These telescoping segments form the final part of the ovipositor and remain invaginated at rest. The y-shaped styli is located at the distal end of it. During this phase, the female walks slowly and alternately moves the tip of the ovipositor in a particular sequence: first backward (parallel to the substrate), then upward, and finally downward, while briefly dragging the styli over the substrate, resembling a “brushstroke.” Each “brushstroke” lasted an average of 7 s and was repeated for 50 to 70 min each night in the absence of males. This female movement and putative marking behavior ceased due to unfavorable environmental conditions (wind, rain), the circadian cycle, or an encounter with a male. During this behavior, there is no ovipositor penetration into the trunk or the epiphyte layer covering the bark surface, nor any change in ovipositor width. After the brushstroke, the ovipositor is retracted back into the abdomen, and the sequence is repeated multiple times throughout the night. During the projection of the ovipositor, when females were on trunks, they placed themselves vertically, with their antennae directed perpendicularly or forward relative to the substrate and the elytra slightly raised. This behavior is assumed to represent the putative calling in P. chilensis. |
| Activation | The male, while stationary, raises their antennae perpendicularly to the longitudinal axis of the body and maintains the position. The male also extends their forelegs forward, lifting the anterior part of the body at a 45° angle relative to the horizontal. Sometimes, the orientation shifts, but the male remains in the same location. This posture can be held for 10–40 min. This behavior was not observed in females. |
| Oriented flight | The male opens its elytra, unfolds the membranous wings, and takes off; during flight, the body maintains a semi-vertical position relative to the substrate, with the antennae slightly projected forward. The flight is relatively linear when the male is released 2–3 m from the female but becomes pendular-zigzagging (casting) near (final 20–40 cm) the tree where she is located before landing. This behavior was not observed in females. |
| Hasty walking | The male lands on the plant where the calling female is located and projects its antennae forward, touching the substrate with their tips while walking rapidly, lifting its legs exaggeratedly above the dorsal level of the elytra. Males follow the route previously traveled by the female. This behavior was not observed in females. |
| Oviposition | Stationary females project the terminal ovipositor segments posteriorly for several minutes and insert them into crevices in the wood or within the epiphyte layer covering the tree trunk. During this phase, the flow of eggs (or ovules) significantly increases the ovipositor width. |
| Next step (→) Previous Step (↓) | Grooming | Walk | Projection of the Ovipositor | ∑ *→ |
|---|---|---|---|---|
| Grooming | ** | 35 | 57 | 92 |
| Walk | 71 | ** | 59 | 130 |
| Projection of the ovipositor | 0 | 59 | ** | 59 |
| ∑↓ | 71 | 94 | 116 | ∑total: 281 |
| Next Step (→) Previous Step (↓) | Grooming | Activation | Walk | Oriented Flight | Hasty Walking | ∑ *→ |
|---|---|---|---|---|---|---|
| Grooming | ** | 15 | 19 | 0 | 0 | 34 |
| Activation | 6 | ** | 4 | 11 | 0 | 21 |
| Walk | 19 | 12 | ** | 0 | 0 | 31 |
| Oriented flight | 0 | 0 | 0 | ** | 12 | 12 |
| ∑↓ | 25 | 27 | 23 | 11 | 12 | ∑total: 98 |
| Categories in Rows (Behaviors) | Categories in Columns (Behaviors) | Couples (♀♂) (n) | χ2 * | CC ** | CI *** | SP **** |
|---|---|---|---|---|---|---|
| ♀ does/does not project the ovipositor | ♂ does/does not perform oriented flight | 8 | 28.48 | 0.79 | 0.81 | 0.99 |
| ♂ does/does not perform hasty walking | 12 | 57.735 | 0.84 | 0.73 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arraztio, D.; Huerta, A.; Rebolledo, R.; Contreras, A.; Curkovic, T. Pre-Courtship Behavior of Proholopterus chilensis (Coleoptera: Cerambycidae) in a Nothofagus obliqua (Nothofagaceae) Forest. Insects 2025, 16, 847. https://doi.org/10.3390/insects16080847
Arraztio D, Huerta A, Rebolledo R, Contreras A, Curkovic T. Pre-Courtship Behavior of Proholopterus chilensis (Coleoptera: Cerambycidae) in a Nothofagus obliqua (Nothofagaceae) Forest. Insects. 2025; 16(8):847. https://doi.org/10.3390/insects16080847
Chicago/Turabian StyleArraztio, Diego, Amanda Huerta, Ramón Rebolledo, Americo Contreras, and Tomislav Curkovic. 2025. "Pre-Courtship Behavior of Proholopterus chilensis (Coleoptera: Cerambycidae) in a Nothofagus obliqua (Nothofagaceae) Forest" Insects 16, no. 8: 847. https://doi.org/10.3390/insects16080847
APA StyleArraztio, D., Huerta, A., Rebolledo, R., Contreras, A., & Curkovic, T. (2025). Pre-Courtship Behavior of Proholopterus chilensis (Coleoptera: Cerambycidae) in a Nothofagus obliqua (Nothofagaceae) Forest. Insects, 16(8), 847. https://doi.org/10.3390/insects16080847







