1. Introduction
Invasive non-native species (invasive species, henceforth) have significant impacts on the global economy, with substantial costs incurred annually worldwide. Recent research indicates they also affect global ecosystems by altering spatial patterns and qualities of cross-ecosystem movements [
1,
2]. In recent years, due to increasing global race size and tighter trade connections, the number of alien invasive species has escalated, exacerbating both current and emerging biological invasions [
3]. Therefore, strengthening the prevention and management of invasive species is crucial. Early Detection and Rapid Response (EDRR) strategies are considered effective in addressing biological invasion issues and minimizing the consequences of invasive species [
4]. Among these strategies, risk assessment and distribution prediction of invasive species are regarded as the initial and most critical steps in invasive species prevention and management [
5]. This approach can identify potential spread areas before species introduction, significantly reducing management costs and allocating resources to the most cost-effective areas, thereby providing a theoretical basis for preventing the spread of invasive species to previously unaffected regions [
6,
7].
The climatic niche, one of the ecological niches, serves as the foundation for risk assessment and distribution prediction of invasive species. It is also a crucial concept in determining biodiversity and biological evolution [
8]. Assessing species’ climatic niches and their dynamics aids in devising effective conservation strategies for invasive species and elucidating species distributions. The dramatic changes in global climate are significantly affecting invasive species, enabling them to expand into new ranges under favorable climatic conditions [
9]. Currently, many invasive species have been reported to varying extents to have expanded beyond their native climatic niches, posing greater risks to additional regions [
10,
11,
12]. This increasingly highlights the necessity of studying the climatic niches of invasive species. Previous studies have utilized three techniques—ordination methods, ecological niche models (ENMs), and univariate methods—to quantify the dynamic changes in species’ climatic niches, with ordination and ENMs methods predominating among these approaches [
13].
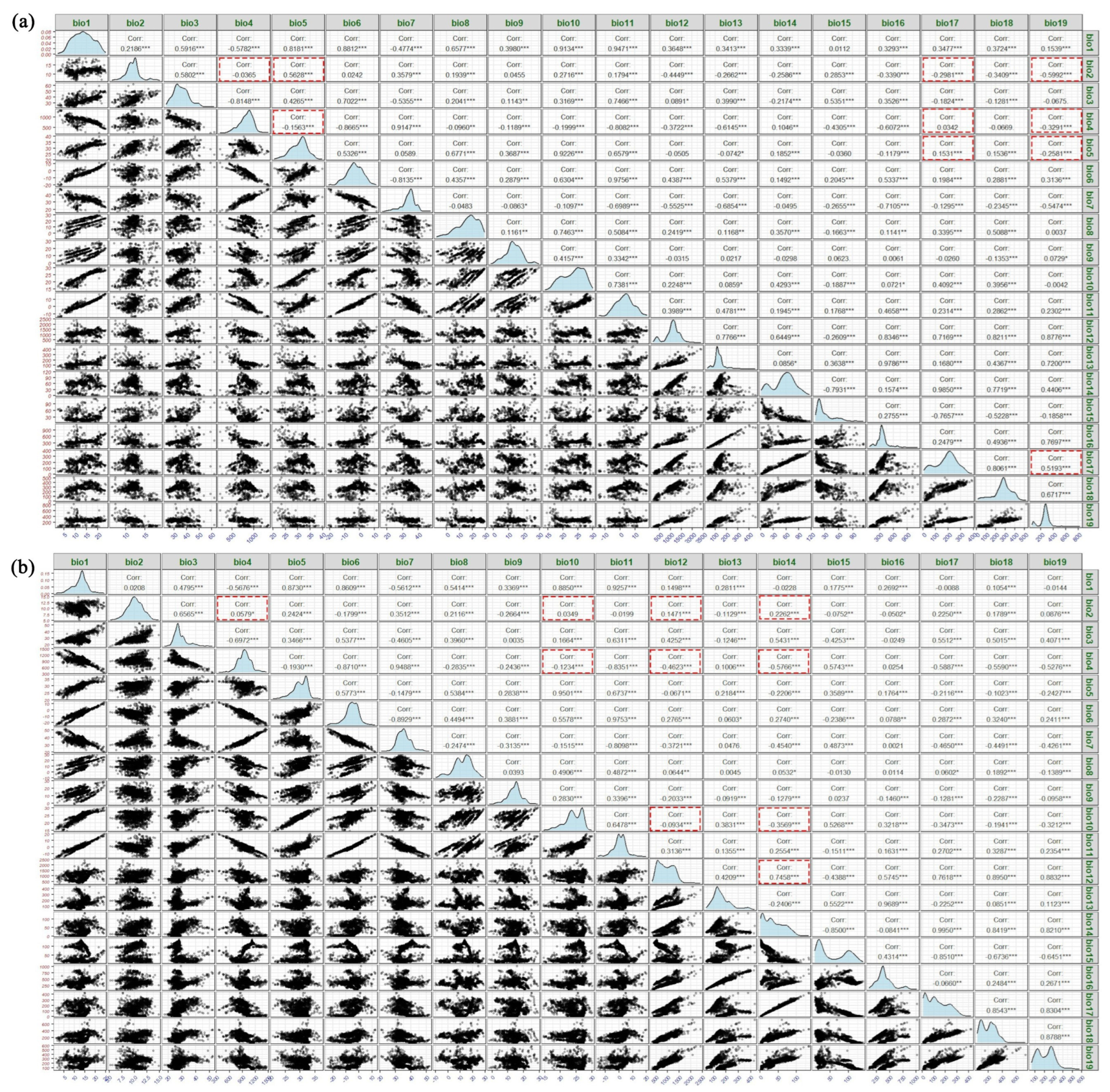
The ordination-based approach simplifies multivariate environmental space into lower-dimensional spaces, enabling the direct quantification of niche differences between native and invasive ranges within the same coordinate system [
13]. Compared to Ecological Niche Models (ENM), its results provide a more intuitive comparison of different niches within the same environmental space and achieve more accurate quantification, albeit being more sensitive to sampling biases [
13]. To address this issue, Olivier Broennimann et al. [
14]. developed a COUE framework widely employed for quantifying and comparing realized niches between invasive and native ranges. This framework illustrates whether niche shifts occur during the invasion process and allows a comparison of different races within the same species [
15,
16]. This new framework, unlike previous methods, alleviates reliance on the fluctuating frequencies of species occurrences across diverse climatic circumstances within a region and guarantees that outcomes are unaffected by sampling attempts and environmental spatial resolution. It represents a robust method for quantifying niche differences currently available [
14].
Ecological niche models (ENMs), also known as species distribution models (SDMs), are commonly used to predict the potential invasion ranges of invasive species. They calibrate predictions of potential habitat suitability using environmental data within a species’ range and its distribution [
17]. Many researchers prefer various ENM models for forecasting the current and future potential habitats of pests [
18,
19,
20]. Among these models, the MaxEnt model stands out as one of the most popular tools in the SDM field [
21,
22,
23], widely applied to predict potential distributions of invasive organisms such as animals [
24,
25], and plants [
26,
27]. This approach enables identifying priority areas for future biological invasion management, such as anticipating Africa and Australia as priority regions for combating
Drosophila suzukii (Matsumura) under future climate conditions [
28].
The fall webworm,
Hyphantria cunea (Drury) (Lepidoptera: Erebidae: Arctiidae), is globally recognized as a notorious invasive quarantine pest originating from North America, now extensively invading regions such as Europe and East Asia [
29]. This pest primarily attacks roadside and fruit trees, feeding on over 600 plant species, particularly
Prunus domestica,
Carya illinoinensis, and
Malus domestica [
30,
31]. In North America, there are two races: the black-headed and red-headed, exhibiting distinct food habits, biological characteristics, and ecological characteristic in both adult and larval stages. The larvae of the black-headed race have black head capsules and tend to grow on hosts in low-lying areas, such as the bottom of streams, riverbanks, and the edges of swamps. They mainly attack
Morus alba L and
Salix. There is no clear diurnal feeding rhythm. On the other hand, the larvae of the red-headed race have red head capsules and are more common in areas with good drainage and sparse tree canopies, such as old fields and cleared forests. They mainly attack
Carya cathayensis Sarg.,
Juglans regia L.,
Prunus maximowiczii Rupr., and
Diospyros kaki Thunb., and feed at night. Moreover, there are differences in the life cycles of these two types. The larval stage of the black-headed race is shorter than that of the red-headed race, while the pupal stage of the black-headed race is longer than that of the red-headed race. Their responses to photoperiodic changes are also different; the critical photoperiod for the pupal diapause of the red-headed race is longer than that of the black-headed race [
32,
33,
34,
35]. Reports indicate that the black-headed race has widely invaded Europe and East Asia, causing significant damage primarily in urban areas and feeding on the garden tree species in cities [
36,
37]. The red-headed race, which has not spread beyond North America, has a larger native range compared to the black-headed race, causing severe damage not only in urban areas but also across much of the continental United States [
38]. Hence, we hypothesize that the climatic niche range of the red-headed
H. cunea is broader than that of the black-headed
H. cunea, suggesting that its potential invasion into other countries could result in more severe and extensive damage than currently observed. The current study ignores niche differences between the two races and focuses on a single invasive area compared to a native area, which can have adverse results, it is possible to underestimate or overestimate the risk areas of these two races [
39,
40].
In this study, the authors utilized global occurrence data of the two races of H. cunea as a foundation. They employed the COUE scheme and ENMs to assess niche differences between these races and their potential global distributions. The objectives of this research were: (1) To dissect climatic niche differentiation between the red-headed race and black-headed races in North America. (2) To assess the invasion potential of the Red-headed race in areas where the Black-headed race has already invaded. (3) To determine whether the invasion of the Black-headed race into Europe and Asia has resulted in a change in its climatic niche. (4) To analyze global MaxEnt modeling results for both races, delineating their potential distribution ranges 118 worldwide. These findings underscore the importance of recognizing differences between the two races of H. cunea and provide a scientific basis for effectively controlling the spread of both races.
4. Discussion
In this study, the niche differences between two races of
Hyphantria cunea in their native and invasive ranges were analyzed using the COUE framework [
14] and Maxent model [
69]. Additionally, the global potential distribution ranges of these two races were predicted. Comparisons were made to illustrate the niche differences between the red-headed race and different regional races of the black-headed race, the niche differences between the native range of the black-headed race and various invasive regions, and the potential distribution ranges of both races globally. This analysis aims to elucidate the niche differentiation between the red-headed and black-headed races, the niche shift in the black-headed race during invasion, and the invasive risks and potential distribution ranges of both red-headed and black-headed races globally.
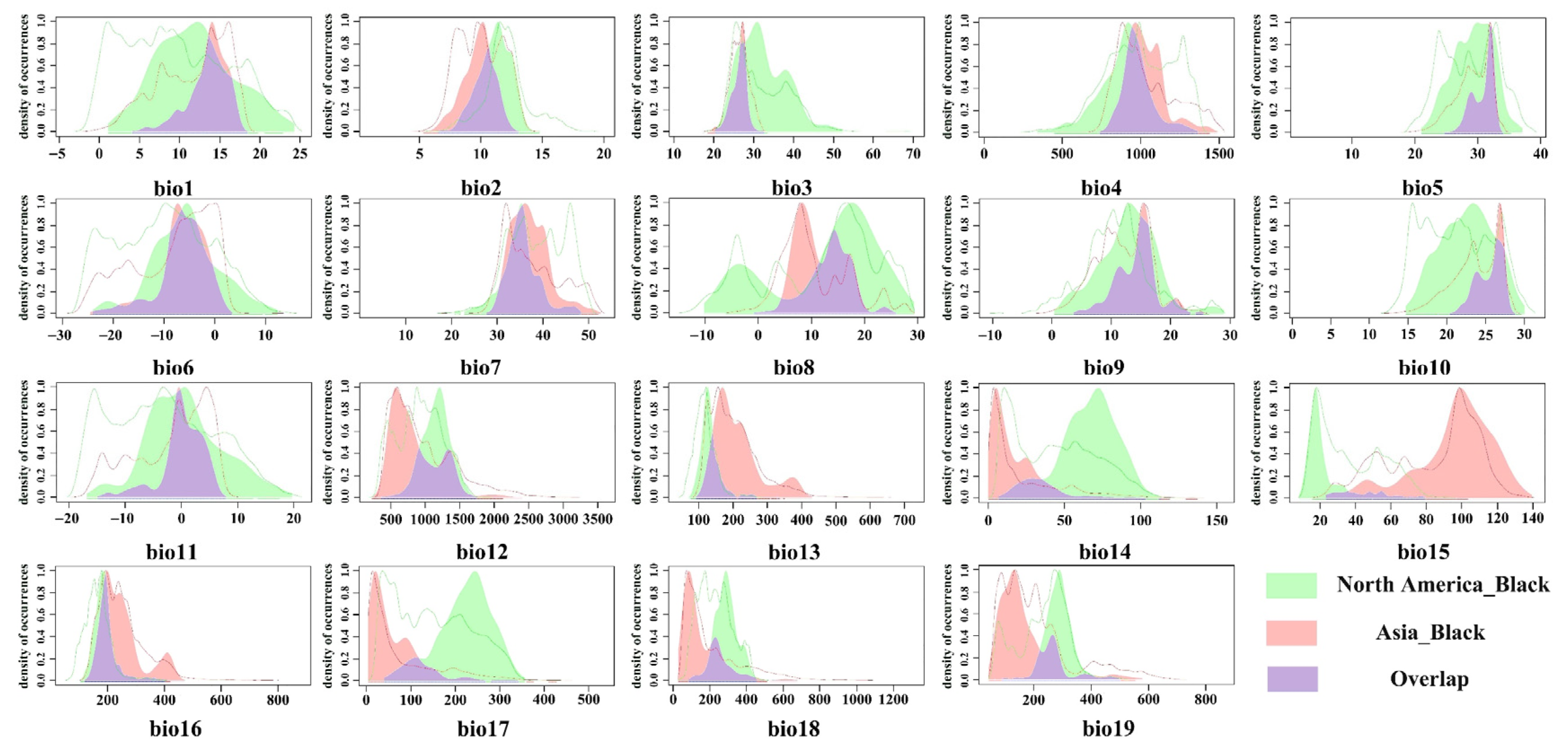
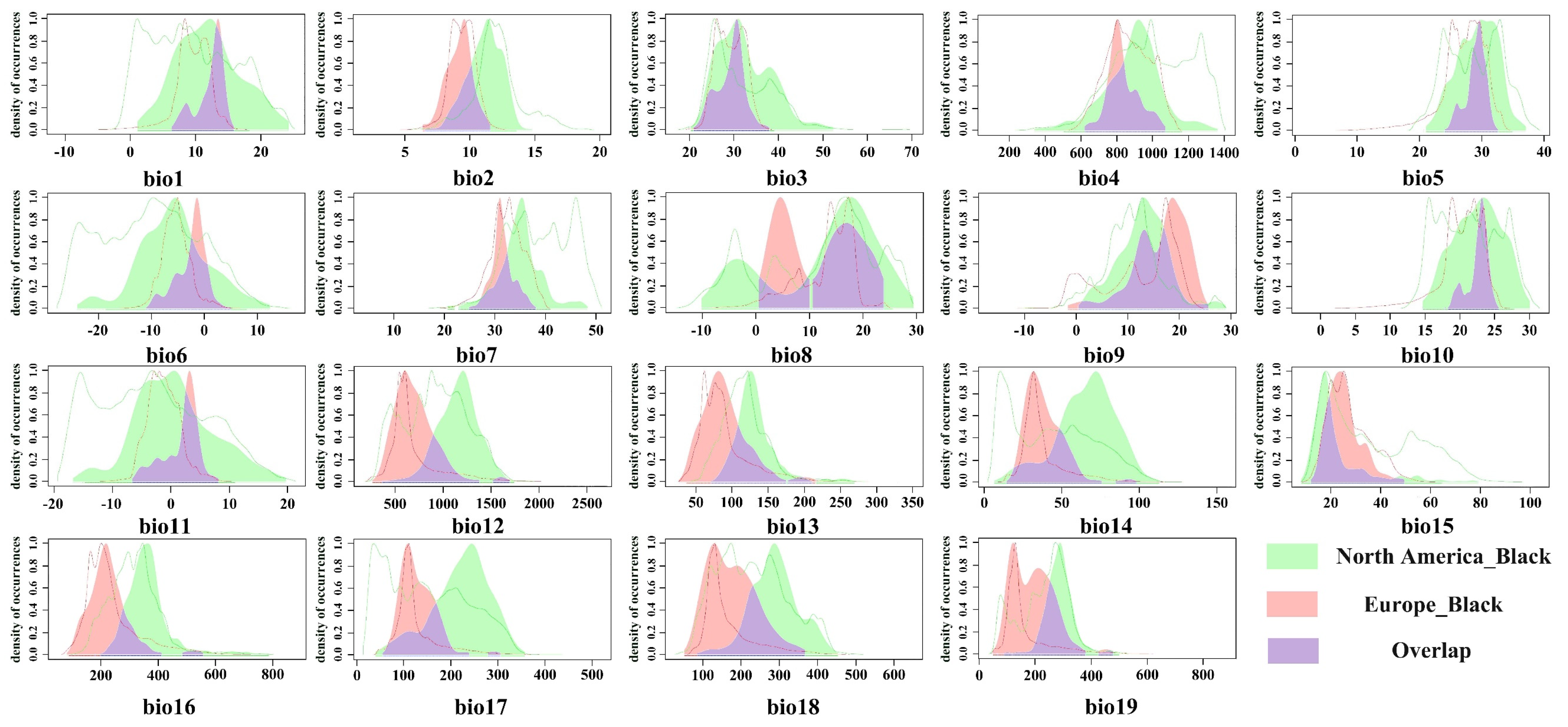
The ecological niches of the red-headed and black-headed races of the fall webworm were compared. It was found that there were subtle but statistically significant differences in their ecological niches, and the ecological niche of the red-headed race was slightly wider than that of the black-headed race. This indicates that, compared to the black-headed race, the red-headed race may cause damage over a wider range. This finding is consistent with previous observations; in North America, the red-headed race is widely distributed from west to east in most areas, spanning all the states of the United States, while the black-headed race is limited to the central and eastern regions of the country and is not observed to be distributed in the west [
34]. As early as 1973, Ito, Y. and Warren, L. proved the differences in feeding preference, behavior, and nesting structure between these races [
70]. These results emphasize the importance of conducting separate studies on the red-headed and black-headed races. Therefore, in future analyses of the potential distribution or risk assessment of the fall webworm, it is necessary to independently analyze these two races. Moreover, the ecological niche width of the red-headed race exceeds that of the black-headed race, indicating that if the red-headed race can maintain its ecological niche width to a certain extent, if the red-headed race invades regions such as Europe and Asia, it may occupy a larger environmental space and cause significant damage.
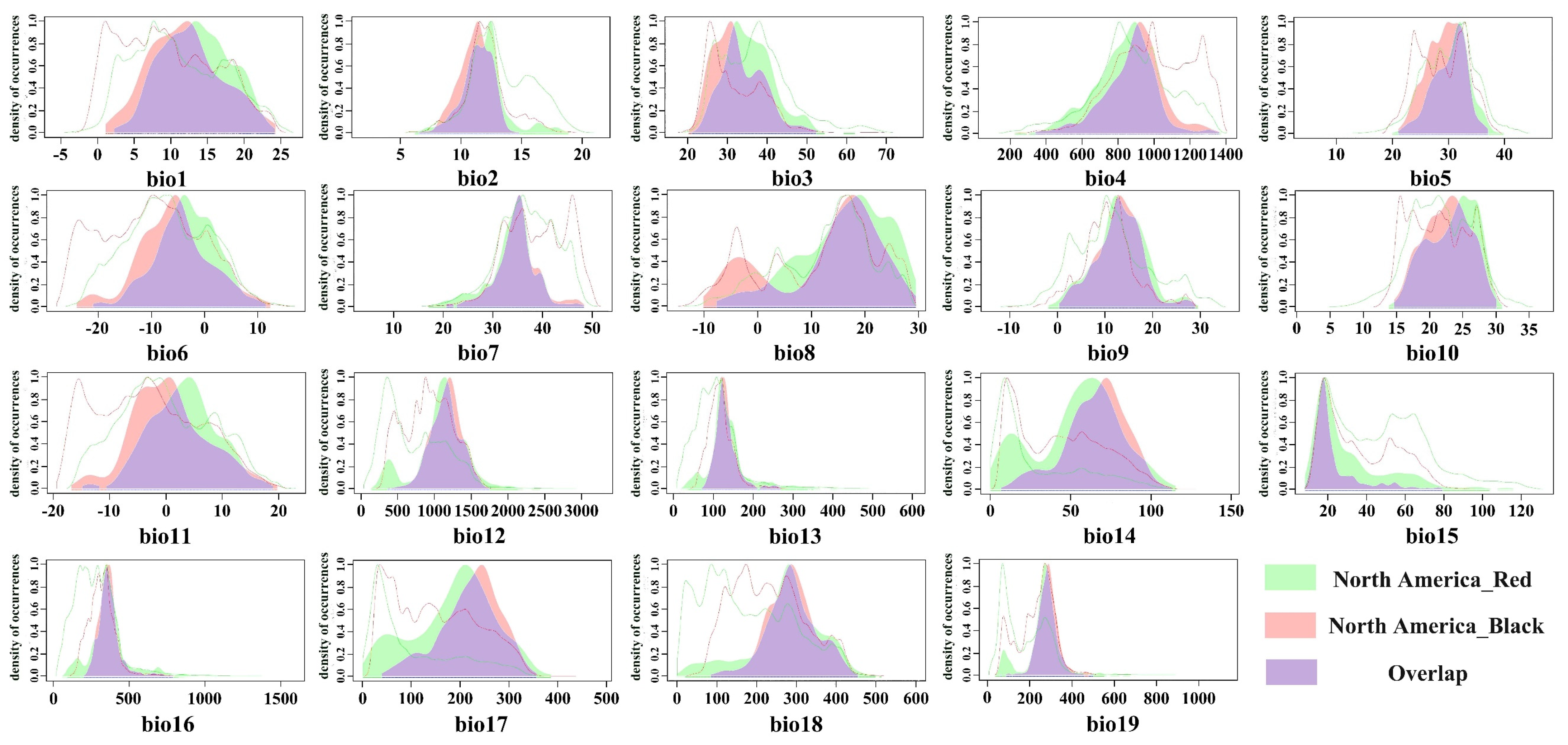
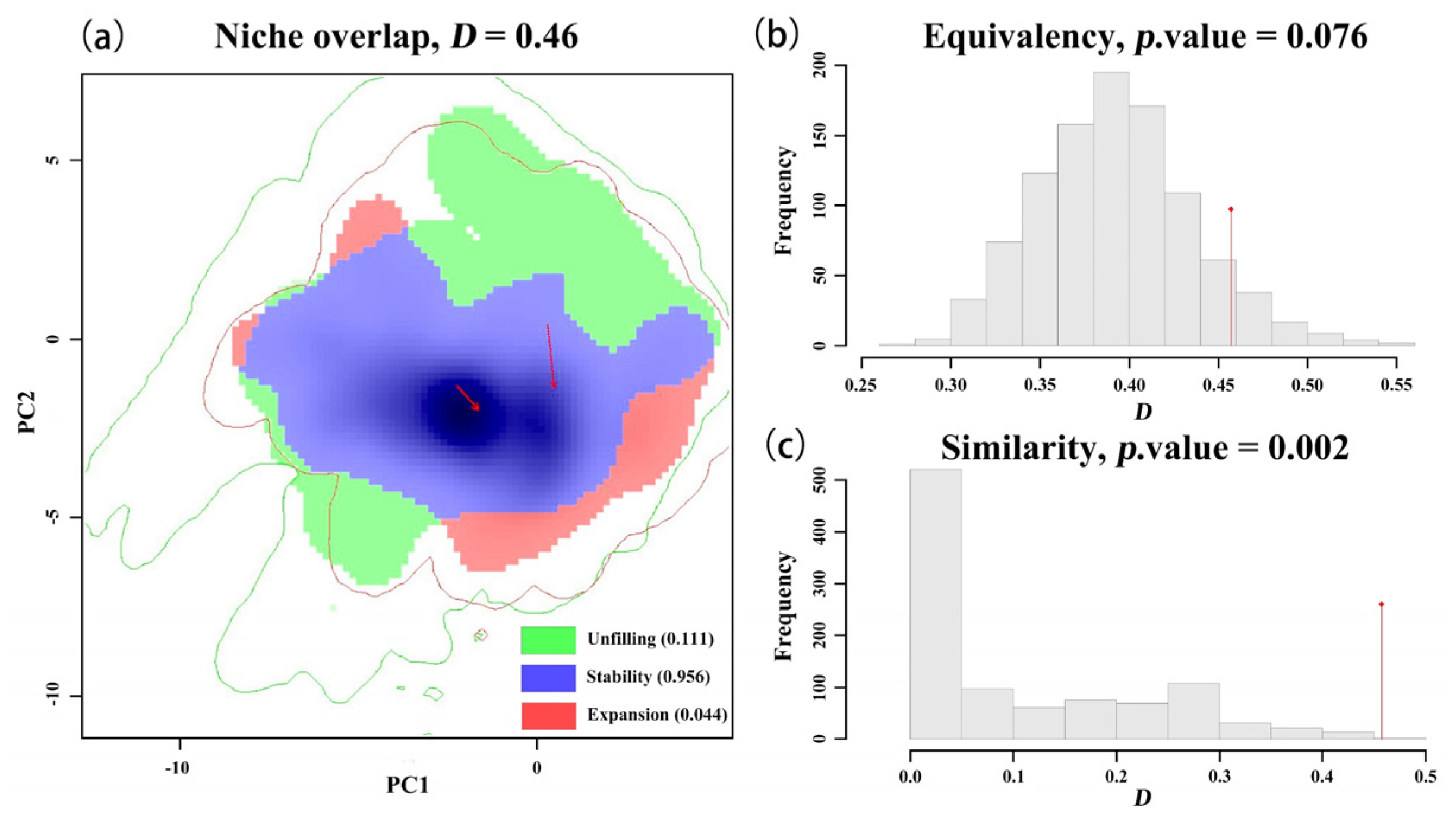
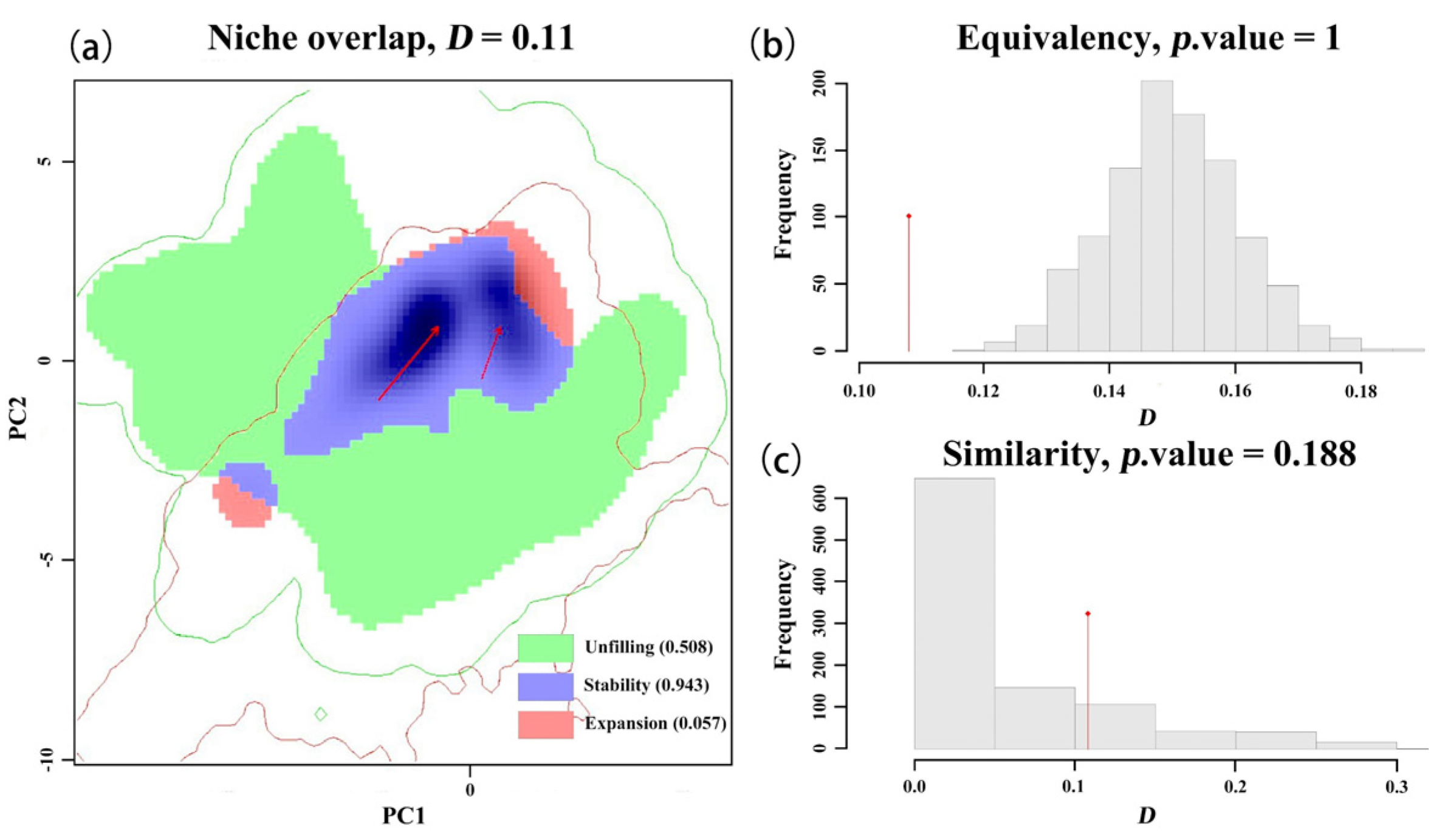
By comparing the climatic ecological niches of the black-headed race in its native habitat with those in different invaded areas, we observed the ecological niche shifts during the invasion process in Asia through single-variable and comprehensive ecological niche difference analyses. During the invasion into Europe, the black-headed race underwent a significant actual ecological niche change (contraction), but no core ecological niche shift occurred. This is consistent with previous research results, namely that the black-headed fall webworm (
H. cunea) in the European invasion retained its native ecological niche, with an extremely small or almost zero expansion index, and exhibited a large number of unfilled areas of the native ecological niche [
39]. Additionally, genomic studies have shown that the fall webworm, due to its strong environmental adaptability and evolutionary capabilities, gradually evolved into different races during its invasion in Asia [
71,
72]. These findings collectively suggest a high likelihood of climatic niche shifts occurring during the invasion of other countries by the black-headed
H. cunea.
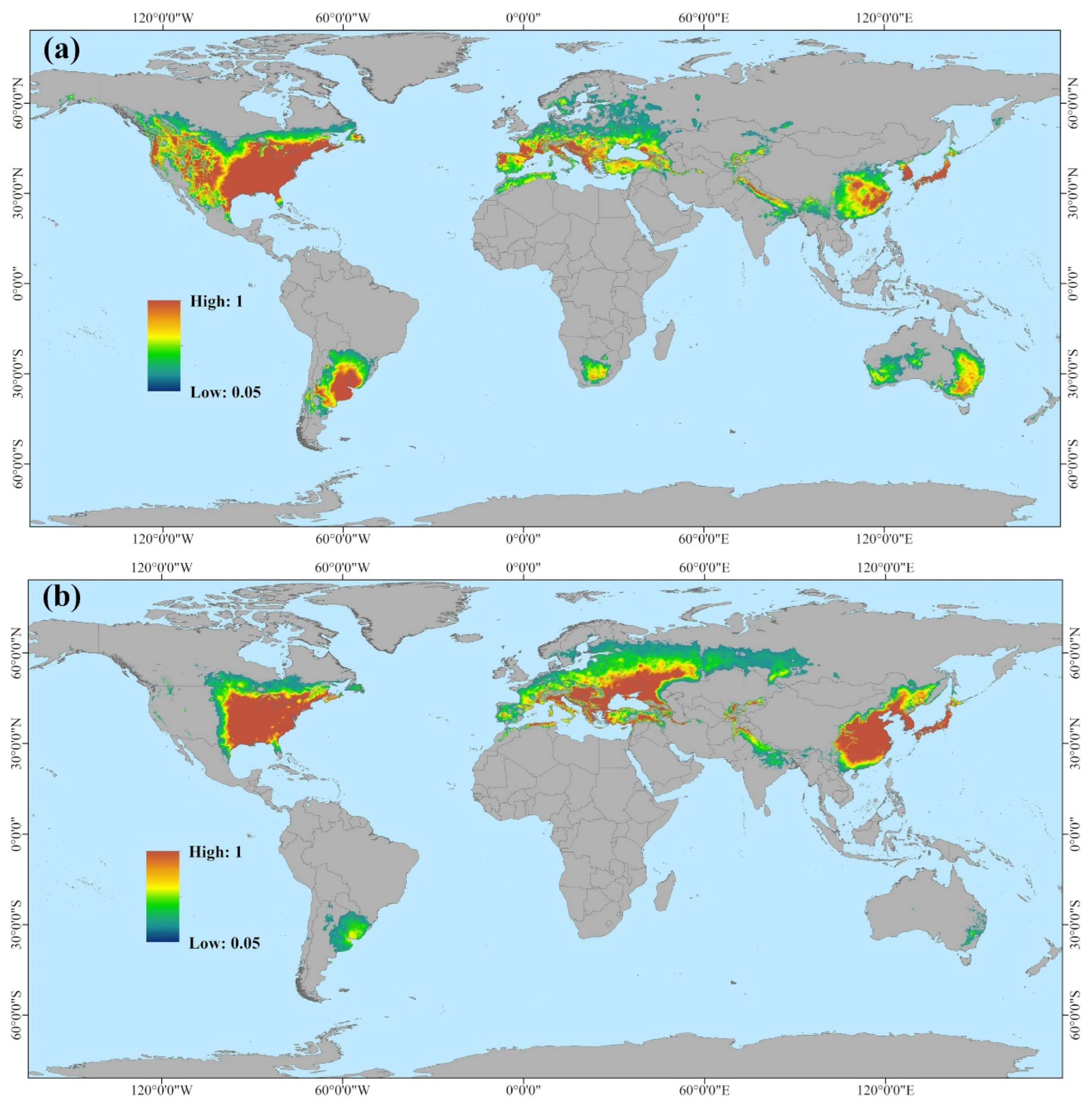
Based on Maxent modeling, we further conducted a global-scale prediction of potentially suitable ranges for two races of
H. cunea. According to
Figure 6,
Figure 7 and
Figure 8, both races of
H. cunea exhibit broader potential suitable ranges globally relative to current distributions, with the red-headed race generally posing greater potential risks than the black-headed race in certain regions. In comparison with Ge et al.’s use of Climex to predict the global suitable ranges of
H. cunea, our predicted suitable ranges were less extensive [
73]. This difference may stem from the differing principles underlying the two models. Several studies indicate that Climex consistently produces broader predictions than Maxent [
74,
75,
76]. Climex operates by simulating species survival under appropriate climatic conditions given a set of parameters, potentially reflecting the subjective biases of the modelers and leading to predictions that exceed those of the Maxent model. Maxent, in contrast, predicts species probability distributions based on the principle of maximum entropy, providing more objective results but susceptible to biases from distributional data points. To minimize subjective influences, we ultimately opted to use Maxent for separate prediction analyses of these two races [
77].
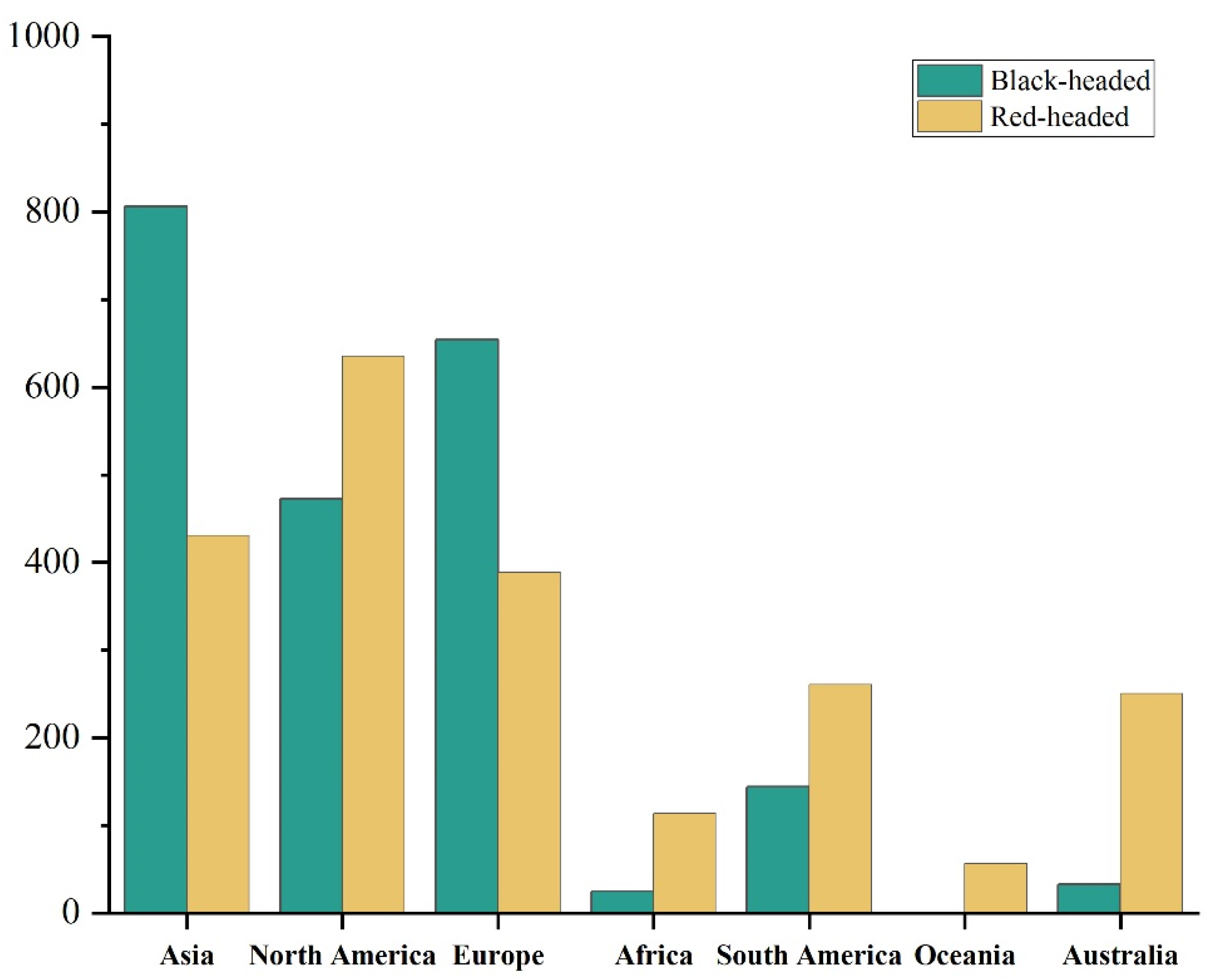
One interesting extension of this study is that the red-headed race’s slightly wider ecological niche corresponds to a much larger potential suitable range in North America, South America, and Australia compared to the black-headed race. Logically, this should cause greater and more extensive harm than the black-headed race. However, the red-headed race is only distributed in North America, and there are no relevant records in other regions of the world. Only the black-headed race has widely invaded regions such as Europe and Asia. This might be due to the wide spread of the black-headed fall webworm (
H. cunea), which might have been affected, prompting an increase in global management efforts for this species, regardless of its races. Additionally, the netting of the black-headed race is thinner and it spreads harm to trees after the fifth instar, while the netting of the red-headed race is more obvious. The larvae of the red-headed race stay in the netting during the day and leave it at night to forage [
33,
78]. The larval period of the black-headed race is shorter than that of the red-headed race, and the pupal period of the black-headed race is longer than that of the red-headed race [
34]. This makes the black-headed race more likely to be a main carrier of human transmission and less likely to be detected, making it easier to spread to other regions with goods. The red-headed race is more likely to be detected, which is not conducive to its spread. However, regions such as South America, Africa, and Australia that have not experienced the spread of the fall webworm yet might face a greater invasion risk due to the lack of prior prevention experience for this pest. Therefore, it is necessary to strengthen the monitoring of the fall webworm in the already-invaded Asian and European regions and other potential suitable areas to prevent the red-headed race from causing harm in these potential suitable areas. Future efforts should focus on strengthening the monitoring of both races of fall webworm to mitigate their further global expansion and related risks.
Our study was also limited by only considering climatic niche differences and employed the Maxent model exclusively for predicting suitable habitat ranges. Factors such as plant species closely associated with insect growth were not incorporated into our analysis, particularly concerning folivorous pests. Additionally, integrated ecological niche modeling is widely recognized for yielding more accurate predictive outcomes [
79,
80]. Future research could encompass multiple models and factors related to the pest, including climate and plant variables, utilizing diverse ecological niche modeling approaches. This approach could provide decision-makers with more detailed and effective strategies for preventing and managing invasive outbreaks of
H. cunea.