Comparative Toxicological Effects of Insecticides and Their Mixtures on Spodoptera littoralis (Lepidoptera: Noctuidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insecticides and Chemicals
2.2. Test Insects
2.3. Bioassay
2.4. Biological Parameters
2.5. Field Experiment Procedure
2.6. Enzyme Preparation
2.6.1. General Esterase Activity Assay
2.6.2. Carboxylesterase Assay
2.6.3. Acetylcholinesterase Assay
2.6.4. Glutathione S-Transferase
2.6.5. Total Protein Assay
2.7. Statistical Analysis
3. Results
3.1. The Toxicity of the Investigated Insecticides Against S. littoralis
3.2. Effect of the Investigated Insecticides and Their Mixtures on Biological Parameters of S. littoralis
3.3. Interactions Between the LC25 and LC50 of Tested Insecticides on the Mortality of Second- and Fourth-Instar Larvae
3.4. Field Efficiency of the Tested Insecticides Against the Cotton Leaf Worm
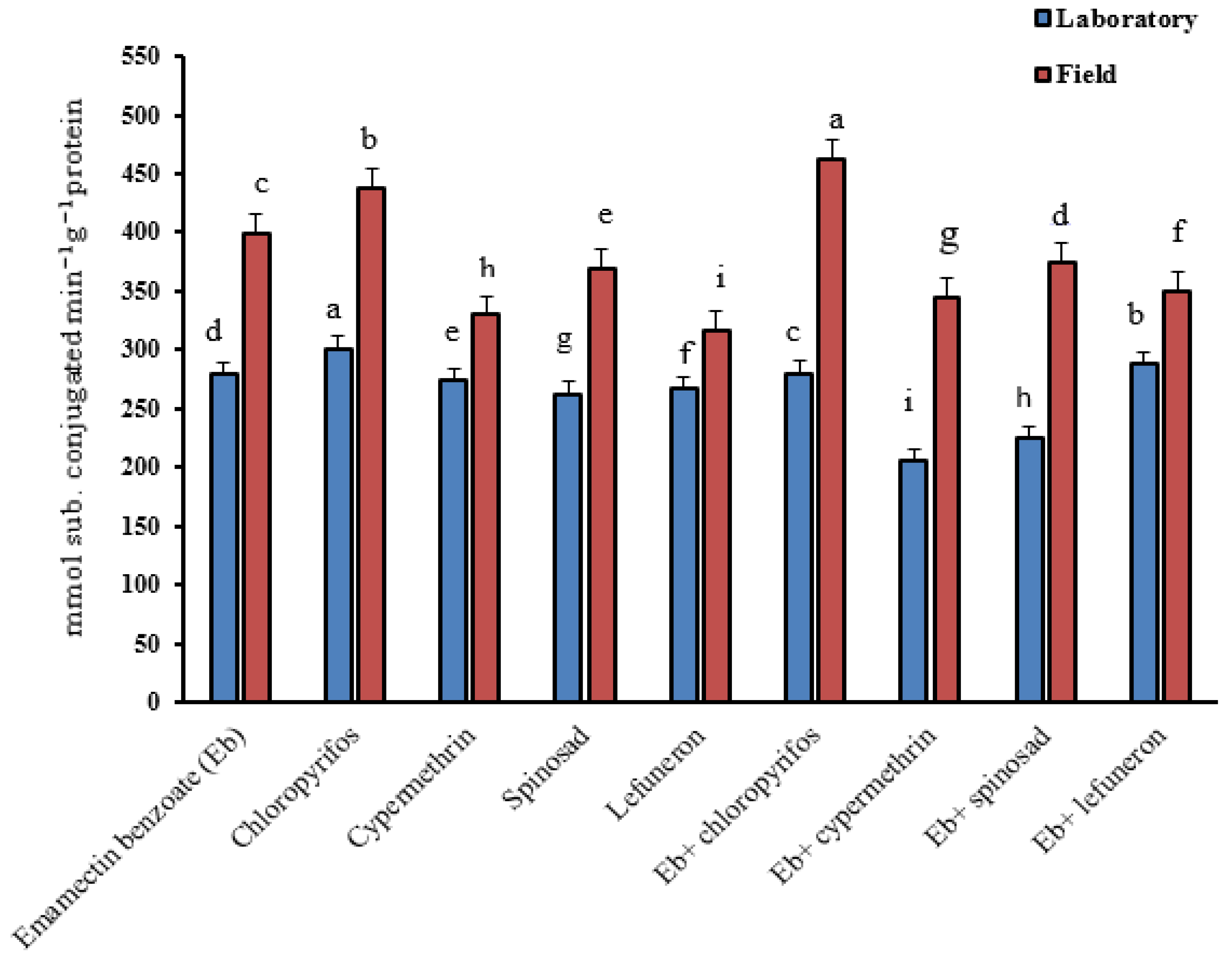
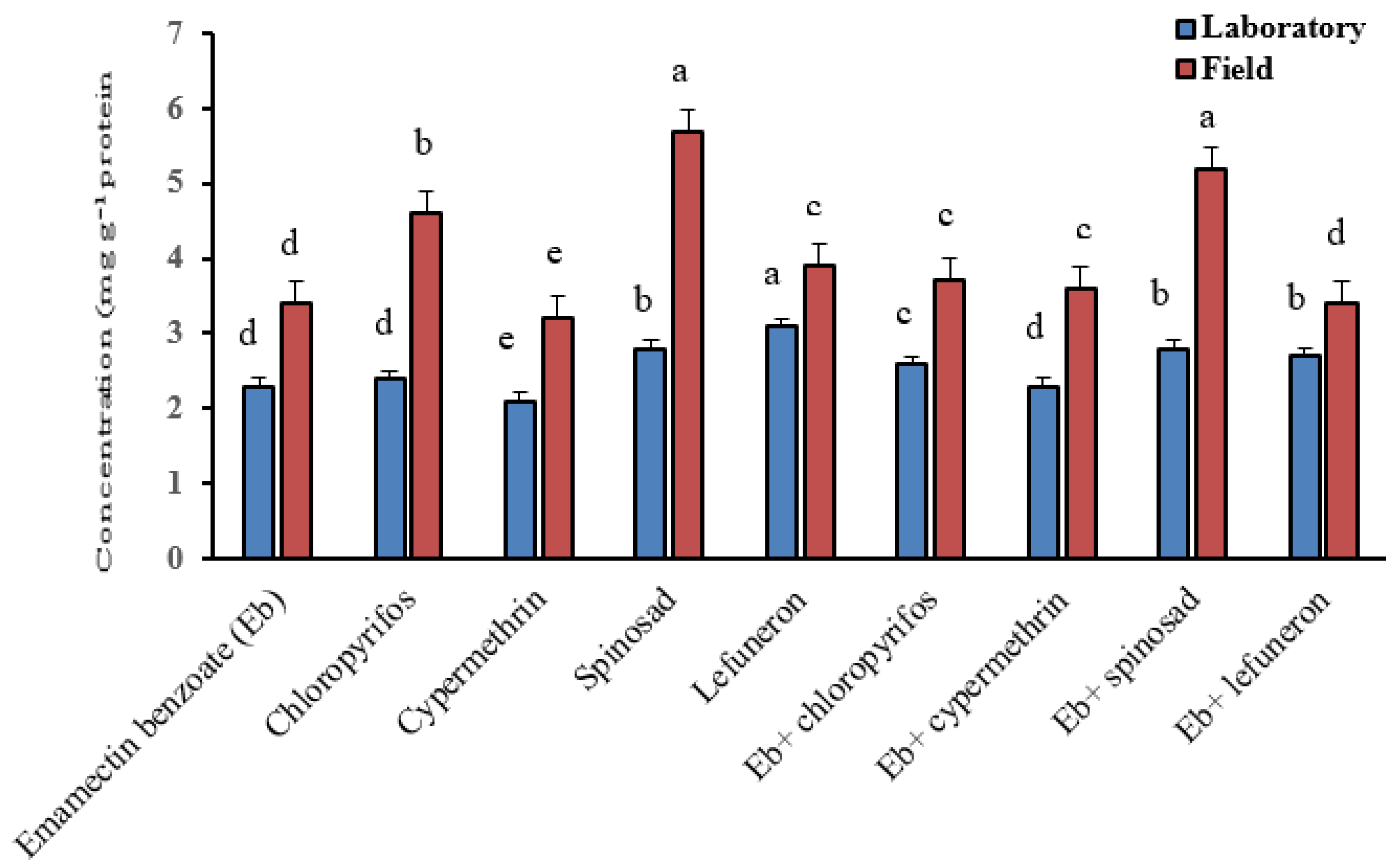
3.5. Biochemical Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kandil, M.; Abdel-Aziz, N.; Sammour, E. Comparative toxicity of chlorofluazron and leufenuron against cotton leaf worm, Spodoptera littoralis (Boisd). Egyp. J. Agric. Res. NRC 2003, 2, 645–661. [Google Scholar]
- Alford, D.V. Pest and Disease Management Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ahmad, M. Potentiation/antagonism of deltamethrin and cypermethrins with organophosphate insecticides in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2004, 80, 31–42. [Google Scholar] [CrossRef]
- Sparks, T.C.; Thompson, G.D.; Kirst, H.A.; Hertlein, M.B.; Larson, L.L.; Worden, T.V.; Thibault, S.T. Biological activity of the spinosyns, new fermentation derived insect control agents, on tobacco budworm (Lepidoptera: Noctuidae) larvae. J. Econ. Entomol. 1998, 91, 1277–1283. [Google Scholar] [CrossRef]
- Osorio, A.; Martínez, A.M.; Schneider, M.I.; Díaz, O.; Corrales, J.L.; Avilés, M.C.; Smagghe, G.; Pineda, S. Monitoring of beet armyworm resistance to spinosad and methoxyfenozide in Mexico. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 1001–1007. [Google Scholar] [CrossRef]
- Terán-Vargas, A.; Garza-Urbina, E.; Blanco-Montero, C.; Perez-Carmona, G.; Pellegaud-Rabago, J. Efficacy of new insecticides to control beet armyworm in northeastern Mexico. In Proceedings of the 1997 Proceedings Beltwide Cotton Conferences, New Orleans, LA, USA, 6–10 January 1997; Volume 2, pp. 1030–1031. [Google Scholar]
- Moustafa, M.A.; El-Said, N.A.; Alfuhaid, N.A.; Abo-Elinin, F.M.; Mohamed, R.M.; Aioub, A.A. Monitoring and detection of insecticide resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae): Evidence for field-evolved resistance in Egypt. Insects 2024, 15, 705. [Google Scholar] [CrossRef]
- Wanwimolruk, S.; Duangsuwan, W.; Phopin, K.; Boonpangrak, S. Food safety in Thailand 5: The effect of washing pesticide residues found in cabbages and tomatoes. J. Consum. Prot. Food Saf. 2017, 12, 209–221. [Google Scholar] [CrossRef]
- Khazri, A.; Sellami, B.; Dellali, M.; Corcellas, C.; Eljarrat, E.; Barceló, D.; Beyrem, H.; Mahmoudi, E. Diastereomeric and enantiomeric selective accumulation of cypermethrin in the freshwater mussel Unio gibbus and its effects on biochemical parameters. Pestic. Biochem. Physiol. 2016, 129, 83–88. [Google Scholar] [CrossRef]
- Narahashi, T.; Frey, J.; Ginsburg, K.; Roy, M. Sodium and GABA-activated channels as the targets of pyrethroids and cyclodienes. Toxicol. Lett. 1992, 64, 429–436. [Google Scholar] [CrossRef]
- Xu, P.; Huang, L. Effects of α-cypermethrin enantiomers on the growth, biochemical parameters and bioaccumulation in Rana nigromaculata tadpoles of the anuran amphibians. Ecotoxicol. Environ. Saf. 2017, 139, 431–438. [Google Scholar] [CrossRef]
- Parsaeyan, E.; Safavi, S.A.; Saber, M.; Poorjavad, N. Effects of emamectin benzoate and cypermethrin on the demography of Trichogramma brassicae Bezdenko. Crop Prot. 2018, 110, 269–274. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; de Menezes, C.H.M.; dos Santos Cunha, W.; Alvarenga, T.M.; Barbosa, B.F.; Zanuncio, J.C.; Martínez, L.C.; Serrão, J.E. Side-effects caused by chlorpyrifos in the velvetbean caterpillar Anticarsia gemmatalis (Lepidoptera: Noctuidae). Chemosphere 2020, 259, 127530. [Google Scholar] [CrossRef]
- Fergani, Y.A.; Refaei, E.A.E.; Faiz, N.M.; Hamama, H.M. Evaluation of chlorpyrifos and Beauveria bassiana as a strategy in the Egyptian sugar beet fields: Impact on Spodoptera littoralis (Boisduval) and its associated predators populations and the sugar beetroot yield. Egypt. J. Biol. Pest Control 2023, 33, 99. [Google Scholar] [CrossRef]
- Ravi, G.; Verma, S. Persistence and dissipation of insecticides against Heliothis armigera on chickpea. Indian J. Entomol. 1997, 59, 62–68. [Google Scholar]
- El-Aswad, A. Efficiency of certain insecticides and insect growth regulators alone or in mixture with chlorpyrifos for the integrated control of the Egyptian cotton leafworm. J. Pest Control Environ. Sci. 2007, 15, 29–48. [Google Scholar]
- Tunaz, H.; Uygun, N. Insect growth regulators for insect pest control. Turk. J. Agric. For. 2004, 28, 377–387. [Google Scholar]
- Aioub, A.A.; Hashem, A.S.; El-Sappah, A.H.; El-Harairy, A.; Abdel-Hady, A.A.; Al-Shuraym, L.A.; Sayed, S.; Huang, Q.; Abdel-Wahab, S.I. Identification and characterization of glutathione S-transferase genes in Spodoptera frugiperda (Lepidoptera: Noctuidae) under insecticides stress. Toxics 2023, 11, 542. [Google Scholar] [CrossRef]
- El-Sayed, M.H.; Ibrahim, M.M.; Elsobki, A.E.; Aioub, A.A. Enhancing the toxicity of cypermethrin and spinosad against Spodoptera littoralis (Lepidoptera: Noctuidae) by inhibition of detoxification enzymes. Toxics 2023, 11, 215. [Google Scholar] [CrossRef]
- Aioub, A.A.; Moustafa, M.A.; Hashem, A.S.; Sayed, S.; Hamada, H.M.; Zhang, Q.; Abdel-Wahab, S.I. Biochemical and genetic mechanisms in Pieris rapae (Lepidoptera: Pieridae) resistance under emamectin benzoate stress. Chemosphere 2024, 362, 142887. [Google Scholar] [CrossRef]
- Eldefrawi, M.; Toppozada, A.; Salama, A.; Elkishen, S. Toxicological studies on the Egyptian cotton leafworm, Prodenia litura. II. Reversion of toxaphene resistance in the Egyptian cotton leafworm. J. Econ. Entomol. 1964, 57, 593–595. [Google Scholar] [CrossRef]
- El-Ghar, G. Influence of abamectin and juvenile hormone analogues on food utilization, ingestion and larval growth of the cotton leafworm, Spodoptera littoralis (Boisd.). Bull. Ophthalmol. Soc. Egypt 1993, 20, 173–183. [Google Scholar]
- Finney, D.J. Probit Analysis. J. R. Stat. Soc. Ser. D (Stat.) 1972, 135, 148. [Google Scholar]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Sun, Y.-P. Toxicity Index-an improved Method of comparing the relative 378 Toxicity of Insecticides. J. Econ. Entomol. 1950, 43, 45–53. [Google Scholar] [CrossRef]
- Zidan, Z.; Abdel-Megeed, M. New approaches in pesticides and insect control. Sun J. 1988, 3, 78–85. [Google Scholar]
- Henderson, C.F.; Tilton, E.W. Tests with acaricides against the brown wheat mite. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]
- Mohan, M.; Gujar, G. Local variation in susceptibility of the diamondback moth, Plutella xylostella (Linnaeus) to insecticides and role of detoxification enzymes. Crop Prot. 2003, 22, 495–504. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Bonjoch, N.P.; Tamayo, P.R. Protein content quantification by Bradford method. In Handbook of Plant Ecophysiology Techniques; Springer: Berlin/Heidelberg, Germany, 2001; pp. 283–295. [Google Scholar]
- Korrat, E.; Abdelmonem, A.; Helalia, A.; Khalifa, H. Toxicological study of some conventional and nonconventional insecticides and their mixtures against cotton leaf worm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noectudae). Ann. Agric. Sci. 2012, 57, 145–152. [Google Scholar] [CrossRef]
- Deepti Pande, D.P.; Srivastava, R. Toxicity and antifeedant activity of indoxacarb (Avaunt 14.5 SC) against tobacco caterpillar, Spodoptera litura (Fabricius). Insect Environ. 2003, 9, 69–71. [Google Scholar]
- El-Aw, M. Bioassays and sublethal effects of various selected insecticides on some biological aspects of the cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Alex. J. Agric. Res. 2003, 48, 73–82. [Google Scholar]
- Firake, D.; Rachna Pande, R.P. Relative toxicity of Proclaim 5% SG (Emamectin benzoate) and Dipel 8L (Bacillus thuringiensis var. kurstaki) against Spodoptera litura (Fab.) by leaf roll method. Curr. Biot. 2009, 3, 445–449. [Google Scholar]
- Haga, T.; Toki, T.; Koyanagi, T.; Nishiyama, R. Structure-activity relationships of benzoylphenyl ureas. In Chitin and Benzoylphenyl Ureas; Springer: Berlin/Heidelberg, Germany, 1987; pp. 111–129. [Google Scholar]
- Rahman, S.; Abou-Taleb, H. Joint toxic action of spinosad and spinetoram with certain IGR compounds against cotton leafworm. Alex. J. Agric. Res. 2007, 52, 45–51. [Google Scholar]
- El-Din, E.; El-Gahreeb, A.; El-Sayed, A.; Abdu-Allah, G. Toxicity of Spinosad and Abamectin Compared with Some Conventional Insecticides Against Parent Field Strain of Cotton LEAF worm, Spodoptera littoralis (BOISD.). J. Plant Prot. Pathol. 2009, 34, 5221–5229. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Badr, N.A.; Abdel-Hafez, A. Efficacy of two formulations of Pathogenic bacteria Bacillus thurinigiensis against the first instar larvae of Spodoptera littoralis (Boisd.) and Agrotis ipsilon (Hfn.) (Lepidoptera-Noctuidae). Egypt. J. Agric. Res. 2000, 78, 1025–1040. [Google Scholar] [CrossRef]
- Kandil, M.A.; Said, H.K.; Abass, M.; Mahdy, A. The effect of insect growth regulators and their binary mixtures on laboratory strain of Spodoptera littoralis (Lepidoptera: Noctuidae). Bull. Ent. Soc. Egypt Econ. Ser. 2006, 32, 47–63. [Google Scholar]
- Pluschkell, U.; Horowitz, A.R.; Weintraub, P.G.; Ishaaya, I. DPX-MP062—A potent compound for controlling the Egyptian cotton leafworm Spodoptera littoralis (Boisd.). Pestic. Sci. 1998, 54, 85–90. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Zhang, Z.; Ding, X.; Jiang, S.; Huang, J. Enhancing forest insect outbreak detection by integrating tree-ring and climate variables. J. For. Res. 2024, 35, 106. [Google Scholar] [CrossRef]
- Moustafa, O.K.; El-Attal, Z. The joint action of triflumuron and some insecticides against the cotton leafworm, Spodoptera littoralis (Boisd.). Bull. Entomol. Soc. Egypt Econ. Ser. 1984, 14, 161–164. [Google Scholar]
- El-Zahi, E.-Z.S. Field persistence of some novel insecticides residues on cotton plants and their latent effects against Spodoptera littoralis (Boisduval). Alex. Sci. Exch. J. 2013, 34, 37–43. [Google Scholar]
- Putter, I.; Connell, J.M.; Preiser, F.; Haidri, A.; Ristich, S.; Dybas, R. Avermectins: Novel insecticides, acaricides and nematicides from a soil microorganism. Experientia 1981, 37, 963–964. [Google Scholar] [CrossRef]
- Abo El-Ghar, G.; Radwan, H.; El-Bermawy, Z.; Zidan, L. Histopatholgoical effects of abamectin, thuringiensin and diflubenzuron on the midgut of Spodoptera littoralis (Lepidoptera: Noctuidae) larvae. Bull. Ent. Soc. Egypt 1994, 21, 41–52. [Google Scholar]
- El-Sheikh, E.-S.A. Comparative toxicity and sublethal effects of emamectin benzoate, lufenuron and spinosad on Spodoptera littoralis Boisd. (Lepidoptera: Noctuidae). Crop Prot. 2015, 67, 228–234. [Google Scholar] [CrossRef]
- Radwan, H.; Nassar, M.; El-Sheikh, A.; Abd El-Razik, M.A. Joint action of bio-insecticides and their role in development of resistance in Spodoptera littoralis (Boisd.). Minufiya. J. Agric. Res 2009, 34, 775–788. [Google Scholar]
- Ghoneim, Y.; Singab, M.; Abou-Yousef, H.M.; Abd-El-Hai, N. Efficacy of certain insecticides and their mixtures with the tested IGRs against a field strain of the cotton leaf worm, Spodoptera littoralis (Boisd.) under laboratory conditions. Aust. J. Basic Appl. Sci. 2012, 6, 300–304. [Google Scholar]
- Abou-Taleb, H.; Saad, A.; Mesbah, H.; Abdel-Rahman, S.M.; El-Deeb, D.A. Toxicity of Emamectin benzoate against laboratory and field strains of S. littoralis with reference to its effects on the AST, ALT and ALP activity. Egypt. J. Agric. Res. 2009, 87, 119–133. [Google Scholar]
- Prasad, K.D.; Madhumathi, T.; Rao, P.; Rao, V.S. Toxicity of insecticides to resistant strain of Spodoptera litura (Fab.) on cotton. Ann. Plant Prot. Sci. 2007, 15, 77–82. [Google Scholar]
- Roby, A.E.; Hussein, S. Behavior of bio-and chemical insecticides in tomato ecosystem in Minia Governorate. Acta Ecol. Sin. 2019, 39, 152–156. [Google Scholar] [CrossRef]
- Fouad, E.A.; Ahmed, F.S.; Moustafa, M.A. Monitoring and biochemical impact of insecticides resistance on field populations of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) in Egypt. Pol. J. Entomol. 2022, 91, 109–118. [Google Scholar] [CrossRef]
- Rashwan, M.H. Biochemical impacts of rynaxypyr (Coragen) and spinetoram (Radiant) on Spodoptera littoralis (Boisd.). Nat. Sci. 2013, 11, 40–47. [Google Scholar]
- Ishaaya, I. Biochemical Sites of Insecticide Action and Resistance; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Salgado, V.L.; Sheets, J.J.; Watson, G.B.; Schmidt, A.L. Studies on the mode of action of spinosad: The internal effective concentration and the concentration dependence of neural excitation. Pestic. Biochem. Physiol. 1998, 60, 103–110. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Fan, R.; Naz, H.; Bamisile, B.S.; Hafeez, M.; Ghani, M.I.; Wei, Y.; Xu, Y.; Chen, X. Insights into insecticide-resistance mechanisms in invasive species: Challenges and control strategies. Front. Physiol. 2023, 13, 1112278. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zheng, T.; Chen, W.; Duan, H.; Yuan, Z.; An, J.; Zhang, H.; Liu, X. Genome-wide identification and expression analysis of the GST gene family of Betula platyphylla. J. For. Res. 2024, 35, 123. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Shen, J.; Mao, K.; You, H.; Li, J. Susceptibility of field populations of the diamondback moth, Plutella xylostella, to a selection of insecticides in Central China. Pestic. Biochem. Physiol. 2016, 132, 38–46. [Google Scholar] [CrossRef]
- Gong, Y.-J.; Wang, Z.-H.; Shi, B.-C.; Kang, Z.-J.; Zhu, L.; Jin, G.-H.; Wei, S.-J. Correlation between pesticide resistance and enzyme activity in the diamondback moth, Plutella xylostella. J. Insect Sci. 2013, 13, 135. [Google Scholar] [CrossRef]
- Nehare, S.; Moharil, M.; Ghodki, B.; Lande, G.; Bisane, K.; Thakare, A.; Barkhade, U. Biochemical analysis and synergistic suppression of indoxacarb resistance in Plutella xylostella L. J. Asia-Pac. Entomol. 2010, 13, 91–95. [Google Scholar] [CrossRef]
- Chen, M.; Han, Z.; Qiao, X.; Qu, M. Resistance mechanisms and associated mutations in acetylcholinesterase genes in Sitobion avenae (Fabricius). Pestic. Biochem. Physiol. 2007, 87, 189–195. [Google Scholar] [CrossRef]
- Yu, S.; Nguyen, S.; Abo-Elghar, G. Biochemical characteristics of insecticide resistance in the fall armyworm, Spodoptera frugiperda (JE Smith). Pestic. Biochem. Physiol. 2003, 77, 1–11. [Google Scholar] [CrossRef]
- Hatfield, M.J.; Umans, R.A.; Hyatt, J.L.; Edwards, C.C.; Wierdl, M.; Tsurkan, L.; Taylor, M.R.; Potter, P.M. Carboxylesterases: General detoxifying enzymes. Chem.-Biol. Interact. 2016, 259, 327–331. [Google Scholar] [CrossRef]
- Eziah, V.Y.; Rose, H.A.; Clift, A.D.; Mansfield, S. Susceptibility of four field populations of the diamondback moth Plutella xylostella L. (Lepidoptera: Yponomeutidae) to six insecticides in the Sydney region, New South Wales, Australia. Aust. J. Entomol. 2008, 47, 355–360. [Google Scholar] [CrossRef]
- Bakr, R.F.; Hafez, J.A.; Khamiss, O.A.; Zyaan, O.H. Biochemical studies on the effect of Chitin synthesis inhibitor,(flufenoxuron) and SpliNPV on the cotton leaf worm Spodopteralittoralis Bosid (Lepidoptera: Noctuidae). Egypt. Acad. J. Biol. Sci. A Entomol. 2013, 6, 29–38. [Google Scholar]
- Siqueira, H.; Guedes, R.; Fragoso, D.D.B.; Magalhaes, L. Abamectin resistance and synergism in Brazilian populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Int. J. Pest Manag. 2001, 47, 247–251. [Google Scholar] [CrossRef]
- Zibaee, I. The expression profile of detoxifying enzyme of tomato leaf miner, Tuta absoluta Meyrik (Lepidoptera: Gelechiidae) to chlorpyrifos. Arthropods 2016, 5, 77. [Google Scholar]
- Badawy, M.E.; Nasr, H.M.; Rabea, E.I. Toxicity and biochemical changes in the honey bee Apis mellifera exposed to four insecticides under laboratory conditions. Apidologie 2015, 46, 177–193. [Google Scholar] [CrossRef]
- Kar, P.; Srivastava, P.; Sinha, R.; Sinha, B. Protein concentration in the pupal haemolymph of different races and F1s of top cross of Antheraea mylitta D. Indian J. Seric. 1994, 33, 174–175. [Google Scholar]
- Awadalla, S.; El-Mezayyen, G.; Bayoumy, M.; EL-Mowafy, N.E. Bioinsecticidal Activity of some Compounds on the Cotton Leafworm, Spodoptera littoralis (Boisd.) under Laboratory Conditions. J. Plant Prot. Pathol. 2017, 8, 525–528. [Google Scholar] [CrossRef]
- Assar, A.; Abo El-Mahasen, M.; Dahi, H.; Amin, H. Biochemical effects of some insect growth regulators and bioinsecticides against cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera Noctuidae). J. Biosci. Appl. Res. 2016, 2, 587–594. [Google Scholar] [CrossRef]
- Hemingway, J.; Karunaratne, S.H. Mosquito carboxylesterases: A review of the molecular biology and biochemistry of a major insecticide resistance mechanism. Med. Vet. Entomol. 1998, 12, 1–12. [Google Scholar] [CrossRef]
| Insecticides | Strain | LC50 (μg/mL) (95%CL) | LC90 (μg/mL) (95%CL) | Slope | Toxicity Index ** | Relative Potency *** | RR * | ||
|---|---|---|---|---|---|---|---|---|---|
| LC50 | LC90 | LC50 | LC90 | ||||||
| 2nd instar larvae | |||||||||
| Emamectin benzoate | Laboratory | 0.01 (0.0001–0.02) | 0.08 (0.07–0.35) | 0.17 | 100 | 100 | 1240 | 513.75 | 1.00 |
| Field | 0.01 (0.01–0.02) | 0.11 (0.03–30.01) | 0.46 | 100 | 100 | 1636 | 770 | 13.6 | |
| Lufenuron | Laboratory | 1.23 (0.71–1.99) | 26.04 (1.90–3.83) | 0.87 | 0.81 | 0.31 | 10.08 | 1.58 | 6.72 |
| Field | 3.06 (1.84–4.96) | 125.68 (21.47–240.20) | 0.90 | 0.12 | 0.09 | 1.98 | 0.67 | 4.83 | |
| Cypermethrin | Laboratory | 3.81 (2.18–6.25) | 47.15 (26.86–116.95) | 0.93 | 0.26 | 0.17 | 2.17 | 0.87 | 1.32 |
| Field | 5.02 (3.42–7.25) | 93.94 (39.17–505.99) | 0.97 | 0.2 | 0.23 | 3.26 | 0.90 | 2.00 | |
| Chlorpyrifos | Laboratory | 12.40 (9.82–15.01) | 41.1 (35.93–79.88) | 0.96 | 0.08 | 0.19 | 1.00 | 1.00 | 1.32 |
| Field | 16.36 (12.49–21.31) | 84.77 (62.99–127.49) | 0.95 | 0.06 | 0.13 | 1.88 | 1.00 | 2.06 | |
| Spinosad | Laboratory | 5.72 (3.30–10.24) | 125.86 (51.49–98.93) | 0.88 | 0.17 | 0.06 | 3.25 | 0.33 | 1.45 |
| Field | 8.27 (5.06–13.59) | 185.29 (63.40–173.0) | 0.84 | 0.12 | 0.06 | 1.98 | 0.46 | 1.47 | |
| 4th instar larvae | |||||||||
| Emamectin benzoate | Laboratory | 0.05 (0.05–0.07) | 0.13 (0.11–0.21) | 0.98 | 100 | 100 | 583.3 | 599 | 1.2 |
| Field | 0.06 (0.05–0.07) | 0.15 (0.11–0.029) | 0.86 | 100 | 100 | 613.83 | 762.47 | 1.15 | |
| Lufenuron | Laboratory | 4.82 (1.81–7.34) | 31.34 (20.65–80.90) | 0.99 | 1.03 | 0.15 | 6.06 | 2.49 | 2.92 |
| Field | 14.08 (9.85–20.82) | 133.38 (68.17–475.69) | 0.86 | 0.34 | 0.11 | 2.61 | 0.86 | 4.26 | |
| Cypermethrin | Laboratory | 8.99 (2.18–6.25) | 113.36 (61.84–604.35) | 0.85 | 0.56 | 0.14 | 3.25 | 0.69 | 1.63 |
| Field | 14.62 (3.42–7.25) | 115.47 (74.11–604.35) | 0.87 | 1.30 | 0.13 | 2.58 | 0.99 | 0.41 | |
| Chlorpyrifos | Laboratory | 29.19 (13.89–19.06) | 77.90 (65.65–121.19) | 0.98 | 0.17 | 0.12 | 1.00 | 1.0 | 1.26 |
| Field | 36.80 (30.80–45.04) | 114.37 (103.66–211.44) | 0.94 | 0.65 | 0.13 | 1.00 | 1.0 | 0.37 | |
| Spinosad | Laboratory | 25.08 (18.80–32.88) | 118.06 (73.21–330.53) | 0.87 | 0.20 | 0.11 | 1.16 | 0.67 | 1.16 |
| Field | 29.09 (21.58–40.62) | 163.32 (90.06–684) | 0.99 | 0.24 | 0.09 | 1.0 | 4.56 | 0.21 | |
| Treatment | %Larval Mortality | Larval Duration (Days) | Pupal Duration (Days) | Pupation% | Pupal Mean Weight (mg) | Adult Emergence (%) | Fecundity (No. Eggs Laid/Female) | Fertility (%Egg Hatched) | Incubation Period (Days) | Adult Longevity (Days) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0 ± 0 | 19 ± 0.90 ab | 9.7 ± 0.70 b | 100 a | 2.71 ± 0.63 a | 100 a | 1125 ± 82.91 a | 98.67 a | 2.75 ± 0.43 b | 8 ± 0.70 ab |
| Emamectin benzoate | 27.5 | 16.79 ± 0.16 c | 9.7 ± 0.10 b | 72.5 b | 1.93 ± 0.15 b | 70 b | 862.5 ± 96.01 b | 75.36 c | 3.0 ± 0.71 b | 8 ± 0.71 ab |
| Chlorpyrifos | 50 | 18.6 ± 0.14 b | 9.6 ± 0.14 b | 50 c | 1.55 ± 0.21 c | 50 c | 637.5 ± 119.24 c | 70.58 e | 3.5 ± 0.5 b | 7 ± 0.70 c |
| Emamectin + chlorpyrifos | 80 | 17.63 ± 0.22 b | 10.88 ± 0.21 a | 20 g | 0.544 ± 0.06 c | 20 g | 500 ± 40.82 b | 48 f | 3.67 ± 0.47 a | 6 ± 82 b |
| Cypermethrin | 47.5 | 18.67 ± 0.13 b | 9.77 ± 0.23 b | 47.5 d | 0.94 ± 0.11 e | 47.5 d | 600 ± 35.35 c | 75 c | 3.25 ± 0.0.43 a | 7.25 ± 0.43 b |
| Emamectin + cypermethrin | 75.5 | 18.00 | 10.00 ± 0.31 a | 50 c | 0.96 ± 0.12 e | 50 c | 650.5 ± 55.0 c | 50 f | 2.75 ± 0.43 b | 7.0 ± 0.5 ab |
| Spinosad | 45 | 19.55 ± 0.05 a | 9.56 ± 0.13 b | 45 e | 1.02 ± 0.06 d | 45 e | 737.5 ± 96.01 bc | 77.28 b | 3.0 ± 0.70 b | 8.5 ± 0.5 a |
| Emamectin + spinosad | 60 | 17.71 ± 0.32 ab | 9.5 ± 0.18 c | 40 f | 1.09 ± 0.07 d | 40 b | 516 ± 62.63 b | 38.75 c | 3.3 ± 0.47 ab | 7.3 ± 0.47 ab |
| Lufenuron | 50 | 19.6 ± 0.14 a | 10.25 ± 0.31 a | 50 c | 1.23 ± 1.19 d | 42.5 f | 625 ± 55.90 c | 72 d | 2.75 ± 0.43 b | 7.5 ± 0.5 ab |
| Emamectin + lufenuron | 87.5 | 18.25 ± 0.43 ab | 9.75 ± 0.43 b | 12.5 h | 0.225 ± 0.19 f | 12.5 h | 450 ± 40.82 d | 42.1 g | 2.67 ± 0.47 ab | 7.0 ± 0.82 ab |
| Combinations | % Mortality | Co-Toxicity Factor | Effect |
|---|---|---|---|
| 2nd instar larvae | |||
| Emamectin benzoate LC25 + chlorpyrifos LC25 | 43.33 | −18.75 | Additive |
| Emamectin benzoate LC25 + lufenuron LC25 | 56.67 | 6.26 | Additive |
| Emamectin benzoate LC25 + cypermthrin LC25 | 46.67 | −6.66 | Additive |
| Emamectin benzoate LC25 + spinosad LC25 | 30 | −40 | Antagonism |
| Emamectin benzoate LC25 + chlorpyrifosLC50 | 83.33 | 24.99 | potentiation |
| Emamectin benzoate LC25 + lufenuron LC50 | 70 | 5.01 | Additive |
| Emamectin benzoate LC25 + cypermthrin LC50 | 80 | 21.06 | Potentiation |
| Emamectin benzoate LC25 + spinosad LC50 | 65 | −35 | Antagonism |
| 4th instar larvae | |||
| Emamectin benzoate LC25 + spinosad LC50 | 33.33 | −33.34 | Antagonism |
| Emamectin benzoate LC25 + chlorpyrifos LC25 | 53.33 | 6.66 | Additive |
| Emamectin benzoate LC25 + lufenuron LC25 | 50 | 7.13 | Additive |
| Emamectin benzoate LC25 + cypermthrin LC25 | 43.33 | −13.34 | Additive |
| Emamectin benzoate LC25 + spinosad LC25 | 40 | −25 | Antagonism |
| Emamectin benzoate LC25 + chlorpyrifos LC50 | 76.67 | 21 | Potentiation |
| Emamectin benzoate LC25 + lufenuron LC50 | 70 | 10.53 | Additive |
| Emamectin benzoate LC25 + cypermthrin LC50 | 83.33 | 31.58 | Potentiation |
| Treatments | Pre-Count | % Mortality | Residual Effect | Residual Mean of % Reduction | Annual Mean% of Reduction | |||
|---|---|---|---|---|---|---|---|---|
| 1 Day | 3 Days | 7 Days | 10 Days | |||||
| 2023 | ||||||||
| Emamectin benzoate (EB) | No | 153.33 ± 1.53 | 22 ± 2.00 | *** | 19 ± 1.00 | 13.33 ± 1.53 | 89.91 ± 0.70 c | 88.57 ± 0.85 d |
| % | 85.90 ± 1.15 | *** | 88.90 ± 0.54 | 91.80 ± 0.90 | ||||
| Chlorpyrifos | No | 181.33 ± 1.53 | 30.67 ± 1.53 | *** | 28 ± 2.00 | 24.67 ± 1.53 | 86.12 ± 0.86 g | 85.21 ± 0.81 e |
| % | 83.37 ± 0.37 | *** | 85.08 ± 0.96 | 87.17 ± 0.76 | ||||
| Cypermethrin | No | 158.00 ± 2.00 | 28 ± 2.00 | *** | 24 ± 1.00 | 21.67 ± 1.53 | 86.19 ± 0.64 g | 85.00 ± 0.87 e |
| % | 82.59 ± 1.03 | *** | 85.32 ± 0.46 | 87.07 ± 0.82 | ||||
| Spinosad | No | 158.00 ± 2.00 | 29 ± 2.00 | *** | 25.67 ± 1.53 | 22.67 ± 1.53 | 85.39 ± 0.80 g | 84.24 ± 0.87 e |
| % | 81.96 ± 1.02 | *** | 84.30 ± 0.81 | 86.47 ± 0.81 | ||||
| Lufenuron | No | 163.33 ± 2.52 | ** | 23 ± 2.00 | 22 ± 1.00 | 19 ± 1.00 | 88.00 ± 0.43 f | 87.44 ± 0.62 d |
| % | ** | 86.30 ± 0.99 | 86.98 ± 0.43 | 89.03 ± 0.47 | ||||
| EB + chlorpyrifos | No | 166.00 ± 1.00 | 10 ± 1.00 | 8 ± 1.00 | 6 ± 1.00 | 4.76 ± 0.58 | 96.84 ± 0.28 a | 95.98 ± 0.47 a |
| % | 94.08 ± 0.56 | 59.29 ± 0.56 | 95.49 ± 0.53 | 97.18 ± 0.03 | ||||
| EB + cypermethrin | No | 155.67 ± 1.53 | 20.33 ± 1.53 | 17.33 ± 2.08 | 14 ± 1.00 | 11.67 ± 1.53 | 92.12 ± 0.71 d | 90.47 ± 0.36 c |
| % | 87.16 ± 1.83 | 89.16 ± 1.21 | 91.31 ± 0.53 | 92.93 ± 0.89 | ||||
| EB + spinosad | No | 169.00 ± 1.00 | 18.33 ± 1.53 | 16 ± 1.0 | 13 ± 1.00 | 9 ± 1.00 | 93.75 ± 0.54 c | 92.28 ± 0.63 b |
| % | 89.34 ± 0.81 | 90.78 ± 0.52 | 92.56 ± 0.53 | 94.94 ± 0.56 | ||||
| EB + lufenuron | No | 151.00 ± 2.00 | 15.33 ± 1.53 | 11 ± 1.08 | 9 ± 1.00 | 6 ± 1.00 | 92.25 ± 0.58 b | 93.51 ± 0.67 b |
| % | 90.02 ± 0.85 | 89.25 ± 0.47 | 94.25 ± 0.57 | 96.25 ± 0.60 | ||||
| Control | No | 173.67 ± 1.53 | 176.33 ± 1.53 | 178.33 ± 1.52 | 180.33 ± 1.52 | 183.67 ± 1.52 | ||
| LSD 0.05 | 1.09 | 1.24 | ||||||
| 2024 | ||||||||
| Emamectin benzoate (EB) | No | 96.67 ± 6.03 | 13.33 ± 1.53 | *** | 10.67 ± 2.08 | 8.67 ± 0.58 | 91.08 ± 1.51 b | 89.57 ± 1.65 c |
| % | 86.57 ± 1.91 | *** | 89.94 ± 3.23 | 92.22 ± 0.97 | ||||
| Chlorpyrifos | No | 100.67 ± 2.52 | 20 ± 1.00 | *** | 15 ± 1.00 | 12.67 ± 0.64 | 87.82 ± 0.82 c | 85.45 ± 0.46 d |
| % | 80.73 ± 0.63 | *** | 86.46 ± 0.64 | 89.14 ± 0.34 | ||||
| Cypermethrin | No | 95.33 ± 3.51 | 15 ± 1.00 | *** | 14.00 ± 1.00 | 13.33 ± 1.51 | 87.31 ± 0.62 c | 86.46 ± 0.50 d |
| % | 84.75 ± 0.43 | *** | 86.69 ± 0.54 | 87.93 ± 0.76 | ||||
| Spinosad | No | 106 ± 2.65 | 20 ± 1.00 | *** | 17.00 ± 1.00 | 14.67 ± 0.58 | 86.75 ± 0.53 c | 85.07 ± 0.26 d |
| % | 81.70 ± 0.58 | *** | 85.46 ± 0.67 | 88.04 ± 0.76 | ||||
| Lufenuron | No | 98.00 ± 2.00 | ** | 15.33 ± 0.58 | 12.33 ± 0.58 | 10.33 ± 0.58 | 89.72 ± 0.74 b | 88.27 ± 0.77 c |
| % | ** | 85.33 ± 0.89 | 88.58 ± 0.78 | 90.89 ± 0.64 | ||||
| EB + chlorpyrifos | No | 102.67 ± 2.52 | 9 ± 1.00 | 7.67 ± 1.53 | 6.67 ± 1.53 | 6.00 ± 1.00 | 94.55 ± 0.99 a | 93.54 ± 0.98 a |
| % | 91.51 ± 0.69 | 92.49 ± 0.61 | 94.13 ± 1.42 | 94.97 ± 0.75 | ||||
| EB + cypermethrin | No | 97.67 ± 0.58 | 13 ± 2.00 | 10.33 ± 1.53 | 8.00 ± 1.00 | 7.67 ± 0.58 | 92.90 ± 0.70 a | 91.19 ± 1.11 b |
| % | 87.10 ± 1.85 | 89.90 ± 0.85 | 92.57 ± 0.93 | 93.22 ± 0.50 | ||||
| EB + spinosad | No | 95.33 ± 2.08 | 14.67 ± 0.58 | 13 ± 1.00 | 10.33 ± 0.58 | 8.33 ± 0.58 | 90.80 ± 0.87 b | 89.17 ± 0.07 c |
| % | 84.88 ± 0.32 | 84.88 ± 1.20 | 90.18 ± 0.37 | 91.42 ± 1.44 | ||||
| EB + lufenuron | No | 101 ± 2.65 | 9.67 ± 0.58 | 10.67 ± 1.15 | 8.00 ± 1.00 | 6.33 ± 1.15 | 93.70 ± 0.85 a | 92.76 ± 0.74 a |
| % | 90.72 ± 0.54 | 90.08 ± 1.21 | 92.74 ± 0.90 | 94.60 ± 0.88 | ||||
| Control | No | 95 ± 1.00 | 98.33 ± 1.53 | 178.33 ± 1.52 | 104.33 ± 0.58 | 183.67 ± 1.52 | ||
| LSD 0.05 | 1.47 | 1.46 | ||||||
| Insecticides | Strains | Esterases Activity (µg α/β naphthol/min/g protein) | Carboxylesterase Activity (µg product/min/g protein) | AChE Activity (µg product/min/g protein) | ||
|---|---|---|---|---|---|---|
| α-NA | β-NA | α-NA | β-NA | |||
| Emamectin benzoate (EB) | Laboratory | 153.93 ± 2.3 d | 129.94 ± 1.3 b | 543.5 ± 2.8 b | 27.3 ± 1.3 g | 914.2 ± 4.1 f |
| Field | 471.99 ± 3.1 d | 190.14 ± 1.5 g | 758.8 ± 3.1 c | 49.4 ± 1.4 h | 1315.9 ± 2.7 b | |
| Chlorpyrifos | Laboratory | 154.97 ± 1.1 d | 135.16 ± 1.81 a | 230.7 ± 1.4 f | 36.4 ± 0.3 d | 1076.0 ± 3.8 a |
| Field | 614.15 ± 3.4 b | 239.80 ± 1.7 c | 689.3 ± 1.3 e | 76.3 ± 0.3 d | 1346.8 ± 1.4 b | |
| Cypermethrin | Laboratory | 157.05 ± 2.2 c | 129.24 ± 2.3 b | 151.6 ± 2.3 h | 45.9 ± 0.7 b | 1073.9 ± 2.8 b |
| Field | 795.97 ± 1.2 c | 130.97 ± 2.3 h | 255.4 ± 1.8 i | 89.6 ± 0.6 c | 1047.9 ± 3.3 g | |
| Spinosad | Laboratory | 156.09 ± 1.7 c | 126.28 ± 1.5 c | 590.0 ± 2.5 a | 22.3 ± 1.7 h | 1017.8 ± 2.6 e |
| Field | 506.55 ± 3.4 e | 247.58 ± 1.3 b | 884.7 ± 3.4 a | 56.4 ± 1.3 f | 1428.7 ± 3.6 a | |
| Lufenuron | Laboratory | 155.32 ± 3.6 c | 119.84 ± 1.8 e | 267.6 ± 1.2 e | 32.5 ± 1.6 e | 863.0 ± 4.7 g |
| Field | 371.79 ± 4.1 b | 232.71 ± 1.4 f | 839.7 ± 1.3 b | 98.6 ± 1.4 b | 1127.5 ± 3.9 f | |
| EB + chlorpyrifos | Laboratory | 143.67 ± 1.9 f | 118.60 ± 1.6 e | 298.8 ± 1.5 d | 57.4 ± 0.7 a | 1037.8 ± 3.8 c |
| Field | 485.49 ± 2.5 a | 337.85 ± 1.7 d | 319.5 ± 2.4 h | 106.7 ± 0.8 a | 1189.1 ± 1.9 e | |
| EB + cypermethrin | Laboratory | 144.84 ± 1.8 e | 118.65 ± 1.9 e | 129.6 ± 2.4 i | 29.3 ± 1.2 f | 1007.8 ± 1.7 c |
| Field | 379.41 ± 1.4 b | 233.44 ± 1.7 e | 596.7 ± 3.1 f | 72.7 ± 1.4 e | 1298.9 ± 2.3 c | |
| EB + spinosad | Laboratory | 161.69 ± 1.6 b | 122.44 ± 1.6 d | 426.3 ± 3.2 c | 41.3 ± 1.3 c | 784.1 ± 3.2 i |
| Field | 282.31 ± 2.5 c | 189.71 ± 1.1 g | 698.1 ± 2.6 d | 69.1 ± 1.1 g | 1038.4 ± 1.6 h | |
| EB + lufenuron | Laboratory | 163.65 ± 2.6 a | 127.32 ± 1.3 c | 192.9 ± 3.1 g | 23.6 ± 0.6 h | 855.1 ± 1.6 h |
| Field | 215.81 ± 3.4 d | 253.44 ± 1.4 a | 321.7 ± 2.4 g | 55.7 ± 0.9 f | 1262.3 ± 4.3 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Saleh, M.A.; Aioub, A.A.; El-Sheikh, E.-S.A.; Desuky, W.M.H.; Alkeridis, L.A.; Al-Shuraym, L.A.; Farag, M.M.A.; Sayed, S.; Aioub, A.A.A.; Hamed, I.A. Comparative Toxicological Effects of Insecticides and Their Mixtures on Spodoptera littoralis (Lepidoptera: Noctuidae). Insects 2025, 16, 821. https://doi.org/10.3390/insects16080821
El-Saleh MA, Aioub AA, El-Sheikh E-SA, Desuky WMH, Alkeridis LA, Al-Shuraym LA, Farag MMA, Sayed S, Aioub AAA, Hamed IA. Comparative Toxicological Effects of Insecticides and Their Mixtures on Spodoptera littoralis (Lepidoptera: Noctuidae). Insects. 2025; 16(8):821. https://doi.org/10.3390/insects16080821
Chicago/Turabian StyleEl-Saleh, Marwa A., Ali A. Aioub, El-Sayed A. El-Sheikh, Wahied M. H. Desuky, Lamya Ahmed Alkeridis, Laila A. Al-Shuraym, Marwa M. A. Farag, Samy Sayed, Ahmed A. A. Aioub, and Ibrahim A. Hamed. 2025. "Comparative Toxicological Effects of Insecticides and Their Mixtures on Spodoptera littoralis (Lepidoptera: Noctuidae)" Insects 16, no. 8: 821. https://doi.org/10.3390/insects16080821
APA StyleEl-Saleh, M. A., Aioub, A. A., El-Sheikh, E.-S. A., Desuky, W. M. H., Alkeridis, L. A., Al-Shuraym, L. A., Farag, M. M. A., Sayed, S., Aioub, A. A. A., & Hamed, I. A. (2025). Comparative Toxicological Effects of Insecticides and Their Mixtures on Spodoptera littoralis (Lepidoptera: Noctuidae). Insects, 16(8), 821. https://doi.org/10.3390/insects16080821







