Toxicity and Detoxification Enzyme Inhibition in the Two-Spotted Spider Mite (Tetranychus urticae Koch) by Artemisia annua L. Essential Oil and Its Major Monoterpenoids
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Cowpea
2.2. Mass Rearing and Synchronization of the Pest
2.3. Essential Oil and Compounds
2.4. Essential Oil Composition
2.5. Fumigant Toxicity
2.6. Determination of Enzyme Activity
2.6.1. General Esterase (EST)
2.6.2. Glutathione S-Transferase (GST)
2.6.3. Cytochrome P450 Monooxygenase Activity
2.7. Statistical Analysis
3. Results
3.1. Chemical Composition of Essential Oil
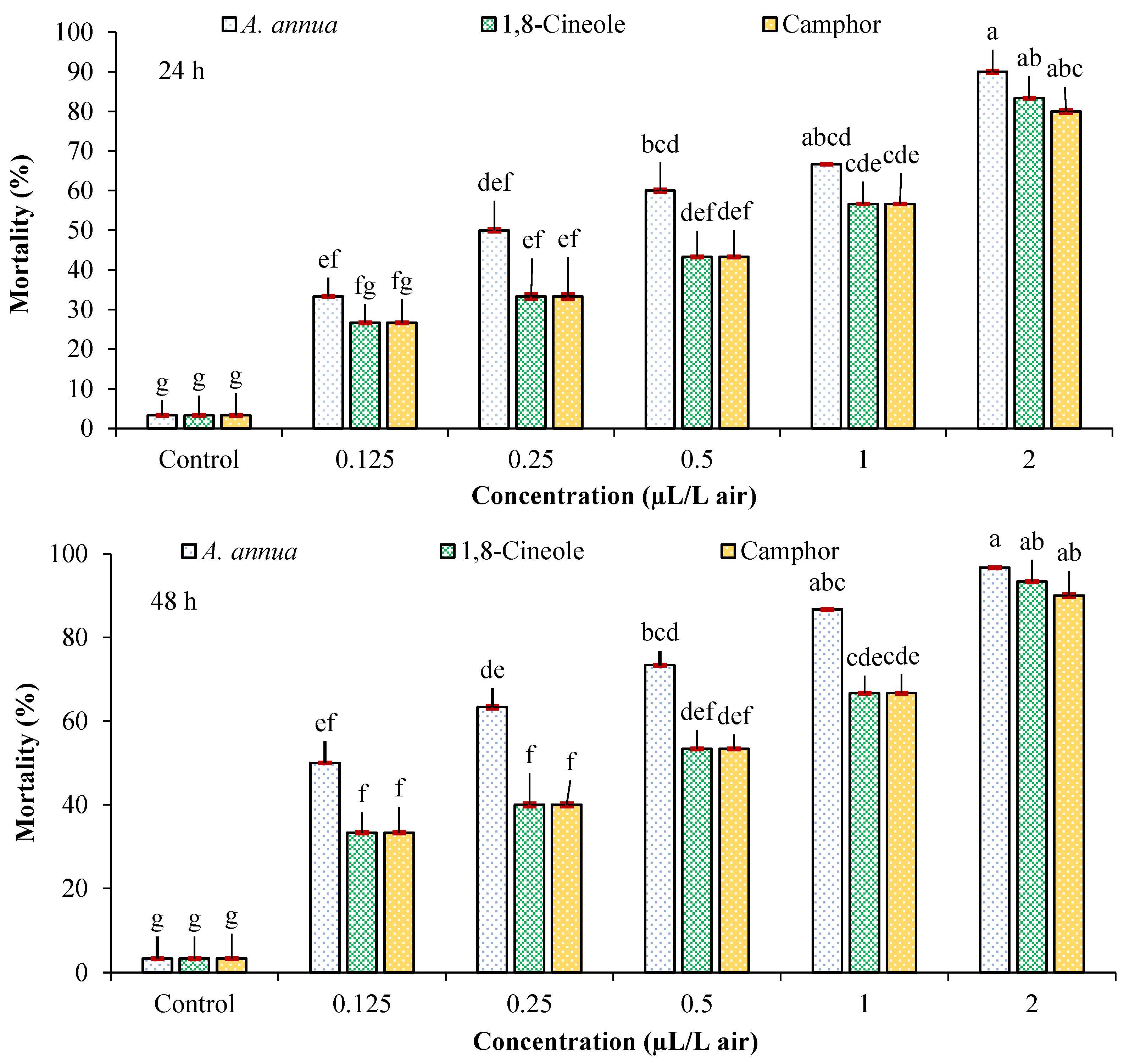
3.2. Fumigant Toxicity
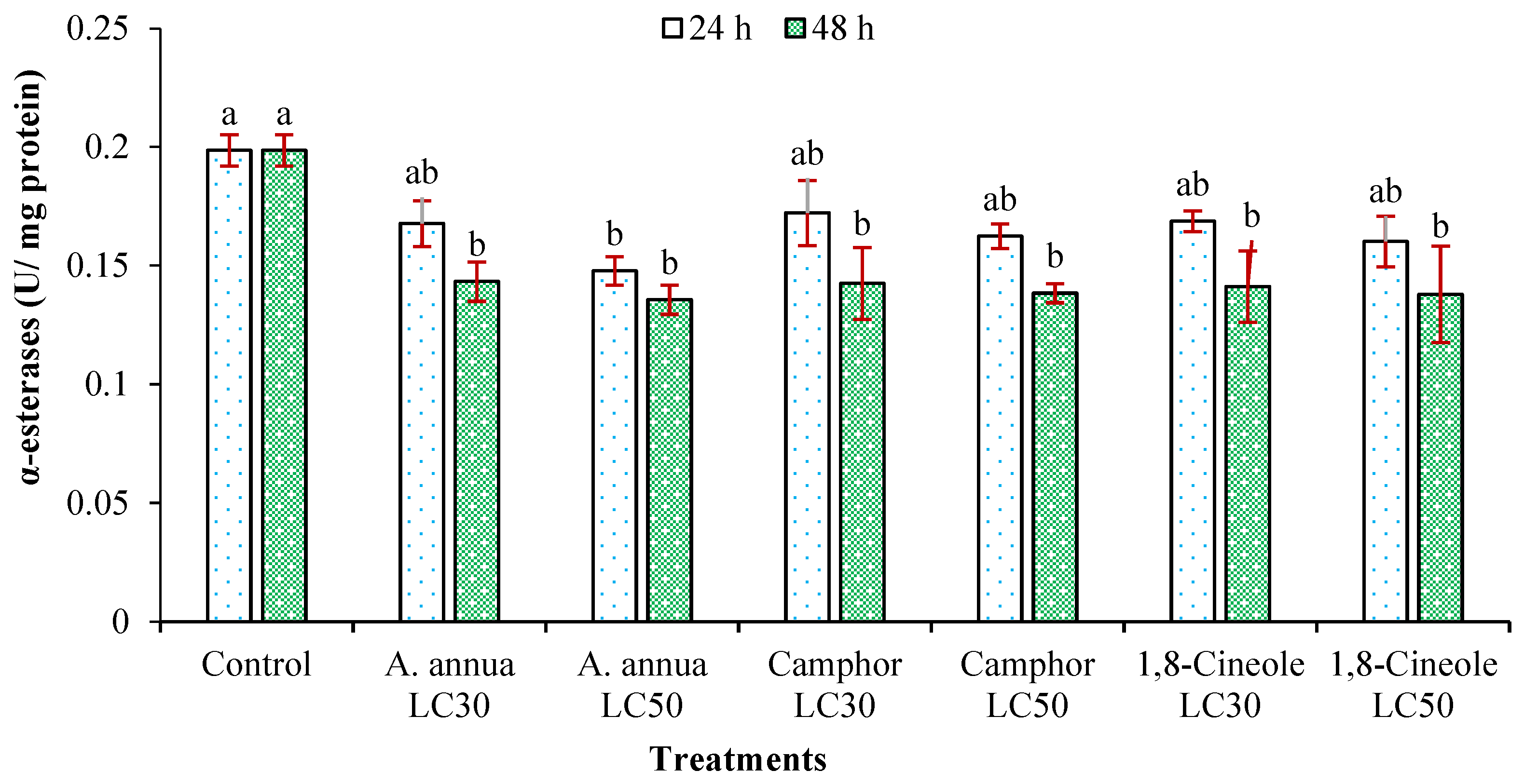
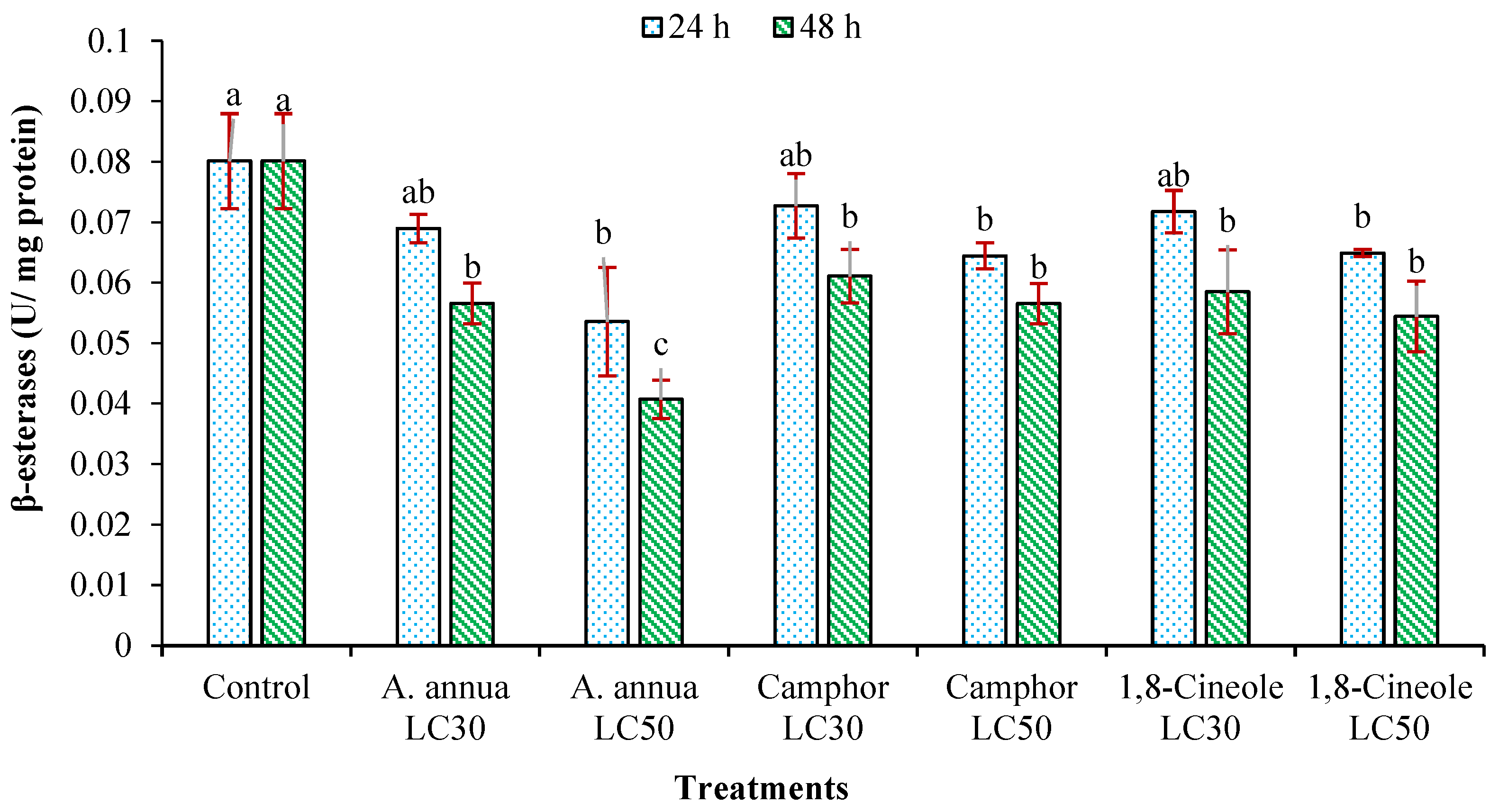
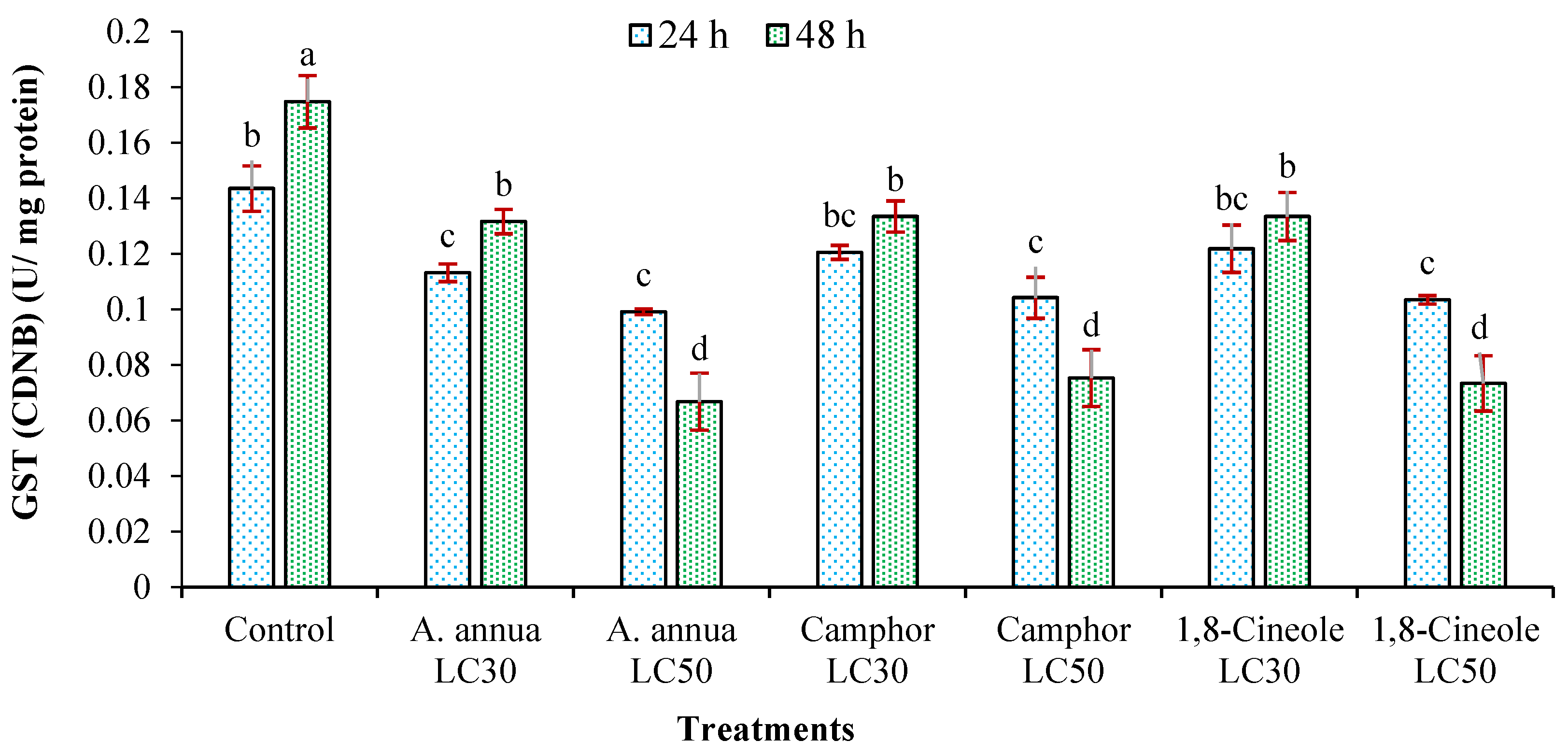
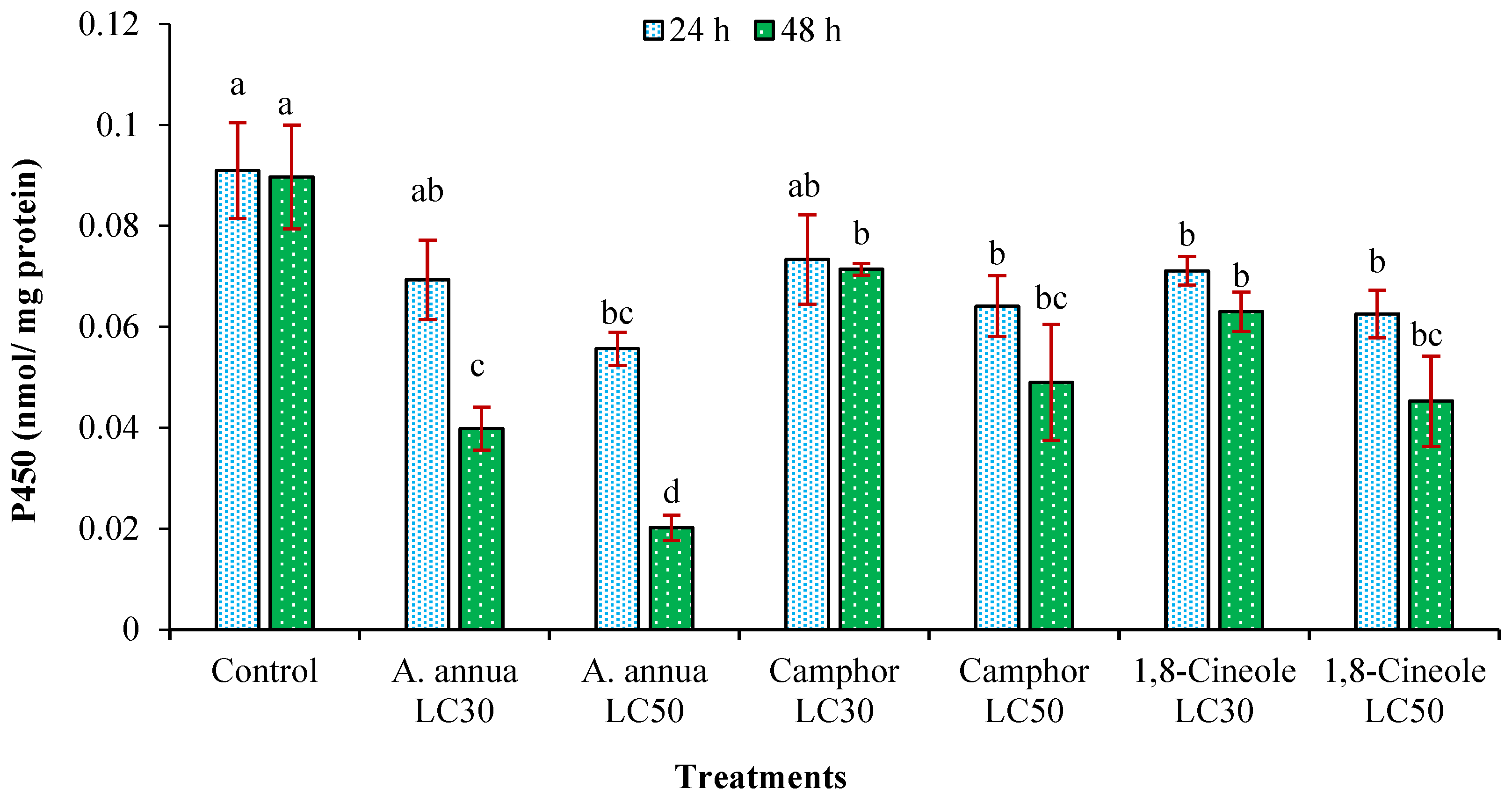
3.3. Effects on Detoxifying Enzymes Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDNB | 1-chloro-2,4-dinitrobenzene |
| CYP450 | cytochrome P450 monooxygenase |
| DCNB | 1,2-Dichloro-4-nitrobenzene |
| df | Degrees of freedom |
| EO | Essential oil |
| EOs | Essential oils |
| ESTs | General esterases |
| GST | Glutathione S-transferase |
| LC30 | Lethal Concentration to kill 30% of tested insects |
| LC50 | Lethal Concentration to kill 50% of tested insects |
| LC90 | Lethal Concentration to kill 90% of tested insects |
| LD50 | Lethal Doses to kill 50% of tested insects |
| r | Intrinsic rate of increase |
| R0 | Net reproductive rate |
| RH | Relative humidity |
| RP | Relative potency |
| SE | Standard error |
| α-NA | α-naphthyl acetate |
| β-NA | β-naphthyl acetate |
| λ | Finite rate of increase |
| χ2 | Chi-square value |
References
- Jakubowska, M.; Dobosz, R.; Zawada, D.; Kowalska, J. A Review of crop protection methods against the twospotted spider mite—Tetranychus urticae Koch (Acari: Tetranychidae)—With special reference to alternative methods. Agriculture 2022, 12, 898. [Google Scholar] [CrossRef]
- Attia, S.; Grissa, K.L.; Lognay, G.; Bitume, E.; Hance, T.; Mailleux, A.C. A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides. J. Pest Sci. 2013, 86, 361–386. [Google Scholar] [CrossRef]
- Meck, E.D.; Kennedy, G.G.; Walgenbach, J.F. Effect of Tetranychus urticae (Acari: Tetranychidae) on yield, quality, and economics of tomato production. Crop Prot. 2013, 52, 84–90. [Google Scholar] [CrossRef]
- Padilha, G.; Fiorin, R.A.; Cargnelutti Filho, A.; Pozebon, H.; Rogers, J.; Marques, R.P.; Castilhos, L.B.; Donatti, A.; Stefanelo, L.; Burtet, L.M.; et al. Damage assessment and economic injury level of the two-spotted spider mite Tetranychus urticae in soybean. Pesqui. Agropecu. Bras. 2020, 55, e01836. [Google Scholar] [CrossRef]
- Dermauw, W.; Wybouw, N.; Rombauts, S.; Menten, B.; Vontas, J.; Grbić, M.; Clark, R.M.; Feyereisen, R.; Van Leeuwen, T. A link between host plant adaptation and pesticide resistance in the polyphagous spider mite Tetranychus urticae. Proc. Natl. Acad. Sci. USA 2013, 110, 113–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, T.; Zhang, K.; Zhang, Y.; Wu, Q.; Xie, W.; Guo, Z.; Wazng, S. Lack of fitness cost and inheritance of resistance to abamectin based on the establishment of a near-isogenic strain of Tetranychus urticae. J. Integr. Agric. 2023, 22, 1809–1819. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Shekari, M.; Jalali Sendi, J.; Etebari, K.; Zibaee, A.; Shadparvar, A. Effects of Artemisia annua L. (Asteracea) on nutritional physiology and enzyme activities of elm leaf beetle. Pestic. Biochem. Physiol. 2008, 91, 66–74. [Google Scholar] [CrossRef]
- Deb, M.; Kumar, D. Bioactivity and efficacy of essential oils extracted from Artemisia annua against Tribolium castaneum (Herbst. 1797) (Coleoptera: Tenebrionidae): An eco-friendly approach. Ecotoxicol. Environ. Saf. 2020, 189, 109988. [Google Scholar] [CrossRef]
- Oftadeh, M.; Sendi, J.J.; Valizadeh, B.; Ebadollahi, A. Hemocytic cell line from the moth Glyphodes pyloalis (Lepidoptera: Crambidae) response to essential oils from Artemisia annua (Asterales: Asteraceae). In Vitro Cell. Dev. Biol. Anim. 2022, 58, 14–20. [Google Scholar] [CrossRef]
- Mojarab-Mahboubkar, M.; Jalali Sendi, J.; Mahmoodi, N. The sweet wormwood essential oil and its two major constituents are promising for a safe control measure against fall webworm. Pestic. Biochem. Physiol. 2022, 184, 105124. [Google Scholar] [CrossRef]
- Oftadeh, M.; Sendi, J.J.; Ebadollahi, A. Toxicity and deleterious effects of Artemisia annua essential oil extracts on mulberry pyralid (Glyphodes pyloalis). Pestic. Biochem. Physiol. 2020, 170, 104702. [Google Scholar] [CrossRef]
- Oftadeh, M.; Sendi, J.J.; Ebadollahi, A. Biologically active toxin identified from Artemisia annua against lesser mulberry pyralid, Glyphodes pyloalis. Toxin Rev. 2021, 40, 953–961. [Google Scholar] [CrossRef]
- Oftadeh, M.; Sendi, J.J.; Ebadollahi, A.; Setzer, W.N.; Krutmuang, P. Mulberry protection through flowering-stage essential oil of Artemisia annua against the lesser mulberry pyralid, Glyphodes pyloalis Walker. Foods 2021, 10, 210. [Google Scholar] [CrossRef]
- Smith, G.H.; Roberts, J.M.; Pope, T.W. Terpene based biopesticides as potential alternatives to synthetic insecticides for control of aphid pests on protected ornamentals. Crop Prot. 2018, 110, 125–130. [Google Scholar] [CrossRef]
- Liu, J.; Hua, J.; Qu, B.; Guo, X.; Wang, Y.; Shao, M.; Luo, S. Insecticidal terpenes from the essential oils of Artemisia nakaii and their inhibitory effects on acetylcholinesterase. Front. Plant Sci. 2021, 12, 720816. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.; Zhang, Y.; Song, X.; Huang, W.; Zhang, R. Identification of terpenoid compounds and toxicity assays of essential oil microcapsules from Artemisia stechmanniana. Insects 2023, 14, 470. [Google Scholar] [CrossRef]
- Seixas, P.T.L.; Demuner, A.J.; Alvarenga, E.S.; Barbosa, L.C.A.; Marques, A.; Farias, E.S.; Picanço, M.C. Bioactivity of essential oils from Artemisia against Diaphania hyalinata and its selectivity to beneficial insects. Sci. Agric. 2018, 75, 519–525. [Google Scholar] [CrossRef]
- Conti, B.; Bocchino, R.; Cosci, F.; Ascrizzi, R.; Flamini, G.; Bedini, S. Essential oils against Varroa destructor: A soft way to fight the parasitic mite of Apis mellifera. J. Apic. Res. 2020, 59, 774–782. [Google Scholar] [CrossRef]
- Caren, J.; Zhu, Y.C.; Read, Q.D.; Du, Y. Risk assessment of effects of essential oils on honey bees (Apis mellifera L.). Insects 2025, 16, 303. [Google Scholar] [CrossRef]
- De Sousa Neto, E.P.; Filgueiras, R.M.C.; de Almeida Mendes, J.; Monteiro, N.V.; de Lima, D.B.; Pallini, A.; da Silva Melo, J.W. A drought-tolerant Neoseiulus idaeus (Acari: Phytoseiidae) strain as a potential control agent of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Biol. Control 2021, 159, 104624. [Google Scholar] [CrossRef]
- Premalatha, K.; Nelson, S.J.; Vishnupriya, R.; Balakrishnan, S.; Santhana Krishnan, V.P. Acaricidal activity of plant extracts on two spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). J. Entomol. Zool. Stud. 2018, 6, 1622–1625. [Google Scholar]
- Wasfy, A.; Torkey, H.; Hagag, H. Chemical composition and evaluation of the acaricidal activity of two essential oils and their formulations against the two-spotted spider mite Tetranychus urticae Koch (Acari: Tetranychidae). J. Appl. Life Sci. Int. 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST. NIST 17; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017. [Google Scholar]
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Thesis, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]
- Ebadollahi, A.; Jalali-Sendi, J.; Razmjou, J. Toxicity and phytochemical profile of essential oil from Iranian Achillea mellifolium L. against Tetranychus urticae Koch (Acari: Tetranychidae). Toxin Rev. 2016, 35, 24–28. [Google Scholar] [CrossRef]
- Han, Q.P.; Zhuang, Z.; Tang, A. The mechanism of resistance to fenitrothion in Chilo suppressalis Walker. Acta Entomol. Sin. 1995, 38, 266–272. [Google Scholar]
- Oppenorth, F.J. Glutathione S-transferase and hydrolytic activity in a tetrachlorvinphos-resistant strain of housefly and their influence on resistance. Pestic. Biochem. Physiol. 1979, 11, 176–178. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.; Dong, K.; Elzen, P.J.; Pettis, J.; Huang, Z. Association of novel mutations in a sodium channel gene whit fluvalinate resistance in the mite, Varroa destructor. J. Apic. Res. 2002, 41, 17–25. [Google Scholar] [CrossRef]
- Martin, T.; Ochou, O.G.; Vaissayre, M.; Fournier, D. Oxides responsible for resistance to pyrethroides sensitize Helicoverpa armigera (Hubner) to triazophos in West Africa. Insect Biochem. Mol. Biol. 2003, 33, 883–887. [Google Scholar] [CrossRef]
- LeOra Software. Polo Plus, a User’s Guide to Probit or Logit Analysis; LeOra Software: Berkeley, CA, USA, 2002. [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide for Personal Computers; SAS Institute: Cary, NC, USA, 1997. [Google Scholar]
- Ebadollahi, A.; Davari, M.; Razmjou, J.; Naseri, B. Separate and combined effects of Mentha piperata and Mentha pulegium essential oils and a pathogenic fungus Lecanicillium muscarium against Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 2017, 110, 1025–1030. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Jalali Sendi, J.; Maroufpoor, M.; Rahimi-Nasrabadi, M. Acaricidal potentials of the terpene-rich essential oils of two Iranian Eucalyptus species against Tetranychus urticae Koch. J. Oleo Sci. 2017, 66, 307–314. [Google Scholar] [CrossRef][Green Version]
- Esmaeily, M.; Bandani, A.; Zibaee, I.; Sharifian, I.; Zare, S. Sublethal effects of Artemisia annua L. and Rosmarinus officinalis L. essential oils on life table parameters of Tetranychus urticae (Acari: Tetranychidae). Persian J. Acarol. 2017, 6, 39–52. [Google Scholar] [CrossRef]
- Liang, J.Y.; Guo, S.S.; You, C.X.; Zhang, W.J.; Wang, C.F.; Geng, Z.F.; Deng, Z.W.; Du, S.S.; Zhang, J. Chemical constituents and insecticidal activities of Ajania Fruticulosa essential oil. Chem. Biodiv. 2016, 13, 1053–1057. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Y.; Yang, C.; Liu, T.; Liu, X.; Yuan, H. Isolation and insecticidal activity of essential oil from Artemisia lavandulaefolia DC. against Plutella xylostella. Toxins 2021, 13, 842. [Google Scholar] [CrossRef]
- You, C.X.; Guo, S.S.; Zhang, W.J.; Yang, K.; Geng, Z.F.; Du, S.S.; Wang, C.F.; Deng, Z.W. Identification of repellent and insecticidal constituents from Artemisia mongolica essential oil against Lasioderma serricorne. J. Chem. 2015, 1, 549057. [Google Scholar] [CrossRef]
- Kordali, S.; Aslan, I.; Calmasur, O.; Cakir, A. Toxicity of essential oils isolated from three Artemisia species and some of their major components to granary weevil, Sitophilus granarius (L.) (Coleoptera: Curculionidae). Ind. Crops Prod. 2006, 23, 162–170. [Google Scholar] [CrossRef]
- Gao, S.; Guo, M.; Yin, Y.; Zhang, X.; Zhang, Y.; Zhang, K. Bioactivity of Artemisia vulgaris essential oil and two of its constituents against the red flour beetle (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2023, 58, 355–369. [Google Scholar] [CrossRef]
- Soni, R.; Shankar, G.; Mukhopadhyay, P.; Gupta, V. A concise review on Artemisia annua L.: A major source of diverse medicinal compounds. Ind. Crops Prod. 2022, 184, 115072. [Google Scholar] [CrossRef]
- Hong, M.; Kim, M.; Jang, H.; Bo, S.; Deepa, P.; Sowndhararajan, K.; Kim, S. Multivariate analysis of essential oil composition of Artemisia annua L. collected from different locations in Korea. Molecules 2023, 28, 1131. [Google Scholar] [CrossRef]
- Omer, E.A.; Abou Hussein, E.A.; Hendawy, S.F.; Ezz, E.D.; Azza, A.; El Gendy, A.G. Effect of soil type and seasonal variation on growth, yield, essential oil and artemisinin content of Artemisia annua L. Int. Res. J. Hortic. 2013, 1, 15–27. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Horgan, T.; Schlegel, V.; Simonnet, X. Distillation time effect on essential oil yield, composition, and antioxidant capacity of sweet sagewort (Artemisia annua L.) oil. HortScience 2013, 48, 1288–1292. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Salimov, A.; Numonov, S.; Safomuddin, A.; Bakri, M.; Salimov, T.; Setzer, W.N.; Habasi, M. Chemical composition, antioxidant, and antimicrobial activities of the essential oils from Аrtemisia annua L. growing wild in Tajikistan. Nat. Prod. Commun. 2020, 15, 1934578X20927814. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Arami, S.A.A.; Abdelgaleil, S.A.M. Acaricidal and quantitative structure activity relationship of monoterpenes against the two-spotted spider mite, Tetranychus urticae. Exp. Appl. Acarol. 2010, 52, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.H.; Isman, M.B. Acaricidal and repellent activity of plant essential oil-derived terpenes and the effect of binary mixtures against Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crops Prod. 2017, 108, 786–792. [Google Scholar] [CrossRef]
- Xu, Z.B.; Zou, X.P.; Zhang, N.; Feng, Q.L.; Zheng, S.C. Detoxification of insecticides, allechemicals and heavy metals by glutathione S-transferase SlGSTE1 in the gut of Spodoptera litura. Insect Sci. 2015, 22, 503–511. [Google Scholar] [CrossRef]
- Bhatt, P.; Zhou, X.; Huang, Y.; Zhang, W.; Chen, S. Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. J. Hazard. Mater. 2021, 411, 125026. [Google Scholar] [CrossRef]
- Scott, J.G.; Liu, N.; Wen, Z. Insect cytochromes P450: Diversity, insecticide resistance and tolerance to plant toxins. Comp. Biochem. Physiol. C. Pharmacol. Toxicol. Endocrinol. 1998, 121, 147–155. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Chrzanowski, G.; Sprawka, I.; Sytykiewicz, H. Aphicidal activity of selected Asteraceae essential oils and their effect on enzyme activities of the green peach aphid, Myzus persicae (Sulzer). Pestic. Biochem. Physiol. 2018, 145, 84–92. [Google Scholar] [CrossRef]
- Chauhan, N.; Kashyap, U.; Dolma, S.K.; Reddy, S.G.E. Chemical composition, insecticidal, persistence and detoxification enzyme inhibition activities of essential oil of Artemisia maritima against the pulse beetle. Molecules 2022, 27, 1547. [Google Scholar] [CrossRef]

| RIcalc | RIdb | Compound | % | RIcalc | RIdb | Compound | % |
|---|---|---|---|---|---|---|---|
| 925 | 926 | Tricyclene | 0.1 | 1431 | 1432 | β-Copaene | 0.5 |
| 939 | 939 | α-Pinene | 7.5 | 1445 | 1451 | iso-Germacrene D | 0.2 |
| 953 | 950 | Camphene | 2.1 | 1455 | 1454 | α-Humulene | 0.7 |
| 978 | 979 | β-Pinene | 3.1 | 1478 | 1477 | γ-Gurjunene | 2.4 |
| 995 | 990 | Myrcene | 0.2 | 1485 | 1485 | Germacrene D | 3.3 |
| 1005 | 999 | Yomogi alcohol | 0.1 | 1493 | 1490 | β-Selinene | 5.9 |
| 1020 | 1025 | p-Cymene | 0.6 | 1500 | 1493 | Capillene | 2.9 |
| 1028 | 1031 | 1,8-Cineole | 7.5 | 1516 | 1513 | γ-Cadinene | 0.2 |
| 1060 | 1062 | Artemisia ketone | 4.2 | 1524 | 1523 | δ-Cadinene | 0.4 |
| 1085 | 1083 | Artemisia alcohol | 0.4 | 1580 | 1578 | Spathulenol | 1.1 |
| 1102 | 1098 | trans-Sabinene hydrate | 0.3 | 1586 | 1583 | Caryophyllene oxide | 1.0 |
| 1138 | 1139 | trans-Sabinol | 1.5 | 1595 | 1594 | Salvial-4(14)-en-1-one | 0.2 |
| 1150 | 1146 | Camphor | 18.7 | 1602 | 1601 | Helifolen-12-al B | 0.5 |
| 1170 | 1164 | Pinocarvone | 2.4 | 1625 | 1630 | Caryophylla-4(12),8(13)-dien-5α-ol | 0.9 |
| 1172 | 1169 | Borneol | 0.9 | 1634 | 1636 | cis- Cadin-4en-7-ol | 0.9 |
| 1177 | 1177 | Terpinen-4-ol | 0.5 | 1638 | 1642 | Caryophylla-4(12),8(13)-dien-5β-ol | 1.2 |
| 1192 | 1188 | α-Terpineol | 0.2 | 1666 | 1669 | ar-Turmerone | 2.9 |
| 1199 | 1195 | Myrtenol | 1.0 | 1675 | 1669 | 14-Hydroxy-9-epi-(E)-caryophyllene | 0.3 |
| 1218 | 1216 | trans-Carveol | 0.3 | 1690 | 1686 | Germacra-4(15),5,10(14)-trien-1α-ol | 1.6 |
| 1244 | 1243 | Carvone | 0.4 | 1701 | 1699 | β-Turmerone | 1.0 |
| 1287 | 1290 | Thymol | 0.7 | 1841 | 1841 | Phytone | 0.3 |
| 1298 | 1299 | Carvacrol | 1.5 | 2108 | 2109 | Phytol | 0.6 |
| 1336 | 1338 | δ-Elemene | 0.2 | Monoterpene hydrocarbons | 13.5 | ||
| 1376 | 1376 | α-Copaene | 1.4 | Oxygenated monoterpenoids | 40.8 | ||
| 1396 | 1395 | Benzyl pentanoate | 0.3 | Sesquiterpene hydrocarbons | 21.6 | ||
| 1390 | 1388 | β-Cubebene | 0.1 | Oxygenated sesquiterpenoids | 11.6 | ||
| 1398 | 1392 | (Z)-Jasmone | 0.1 | Diterpenoids | 0.6 | ||
| 1416 | 1419 | (E)-β-Caryophyllene | 6.4 | Others | 3.7 | ||
| Total identified | 91.7 | ||||||
| Tested Agent | Time (h) | Lethal Concentrations with 95% Confidence Limits (µL/L Air) | Slope ± SE | χ2 (df = 3) | p Value | RP | R2 | ||
|---|---|---|---|---|---|---|---|---|---|
| LC30 | LC50 | LC90 | |||||||
| A. annua essential oil | 24 | 0.107 (0.032–0.186) | 0.289 (0.158–0.433) | 3.266 (1.640–16.087) | 1.217 ± 0.268 | 1.741 | 0.628 | 2.215 | 0.926 |
| 48 | 0.066 (0.019–0.118) | 0.147 (0.069–0.221) | 1.027 (0.660–2.479) | 1.518 ± 0.319 | 2.438 | 0.487 | 4.354 | 0.987 | |
| 1,8-Cineole | 24 | 0.199 (0.087–0.310) | 0.533 (0.349–0.836) | 5.890 (2.642–37.458) | 1.228 ± 0.262 | 2.170 | 0.538 | 1.201 | 0.913 |
| 48 | 0.138 (0.054–0.223) | 0.351 (0.216–0.514) | 3.427 (1.763–14.679) | 1.296 ± 0.268 | 2.090 | 0.554 | 1.823 | 0.919 | |
| Camphor | 24 | 0.241 (0.115–0.367) | 0.640 (0.428–1.041) | 6.939 (3.023–47.308) | 1.238 ± 0.263 | 0.748 | 0.862 | 1.000 | 0.971 |
| 48 | 0.158 (0.056–0.260) | 0.446 (0.274–0.695) | 5.665 (2.482–41.601) | 1.161 ± 0.260 | 0.283 | 0.936 | 1.435 | 0.987 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr Azadani, F.; Jalali Sendi, J.; Ebadollahi, A.; Azizi, R.; Setzer, W.N. Toxicity and Detoxification Enzyme Inhibition in the Two-Spotted Spider Mite (Tetranychus urticae Koch) by Artemisia annua L. Essential Oil and Its Major Monoterpenoids. Insects 2025, 16, 811. https://doi.org/10.3390/insects16080811
Nasr Azadani F, Jalali Sendi J, Ebadollahi A, Azizi R, Setzer WN. Toxicity and Detoxification Enzyme Inhibition in the Two-Spotted Spider Mite (Tetranychus urticae Koch) by Artemisia annua L. Essential Oil and Its Major Monoterpenoids. Insects. 2025; 16(8):811. https://doi.org/10.3390/insects16080811
Chicago/Turabian StyleNasr Azadani, Fatemeh, Jalal Jalali Sendi, Asgar Ebadollahi, Roya Azizi, and William N. Setzer. 2025. "Toxicity and Detoxification Enzyme Inhibition in the Two-Spotted Spider Mite (Tetranychus urticae Koch) by Artemisia annua L. Essential Oil and Its Major Monoterpenoids" Insects 16, no. 8: 811. https://doi.org/10.3390/insects16080811
APA StyleNasr Azadani, F., Jalali Sendi, J., Ebadollahi, A., Azizi, R., & Setzer, W. N. (2025). Toxicity and Detoxification Enzyme Inhibition in the Two-Spotted Spider Mite (Tetranychus urticae Koch) by Artemisia annua L. Essential Oil and Its Major Monoterpenoids. Insects, 16(8), 811. https://doi.org/10.3390/insects16080811









