A New Hotspot of Cave Leptodirini (Coleoptera: Leiodidae) from the Romanian Carpathians †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
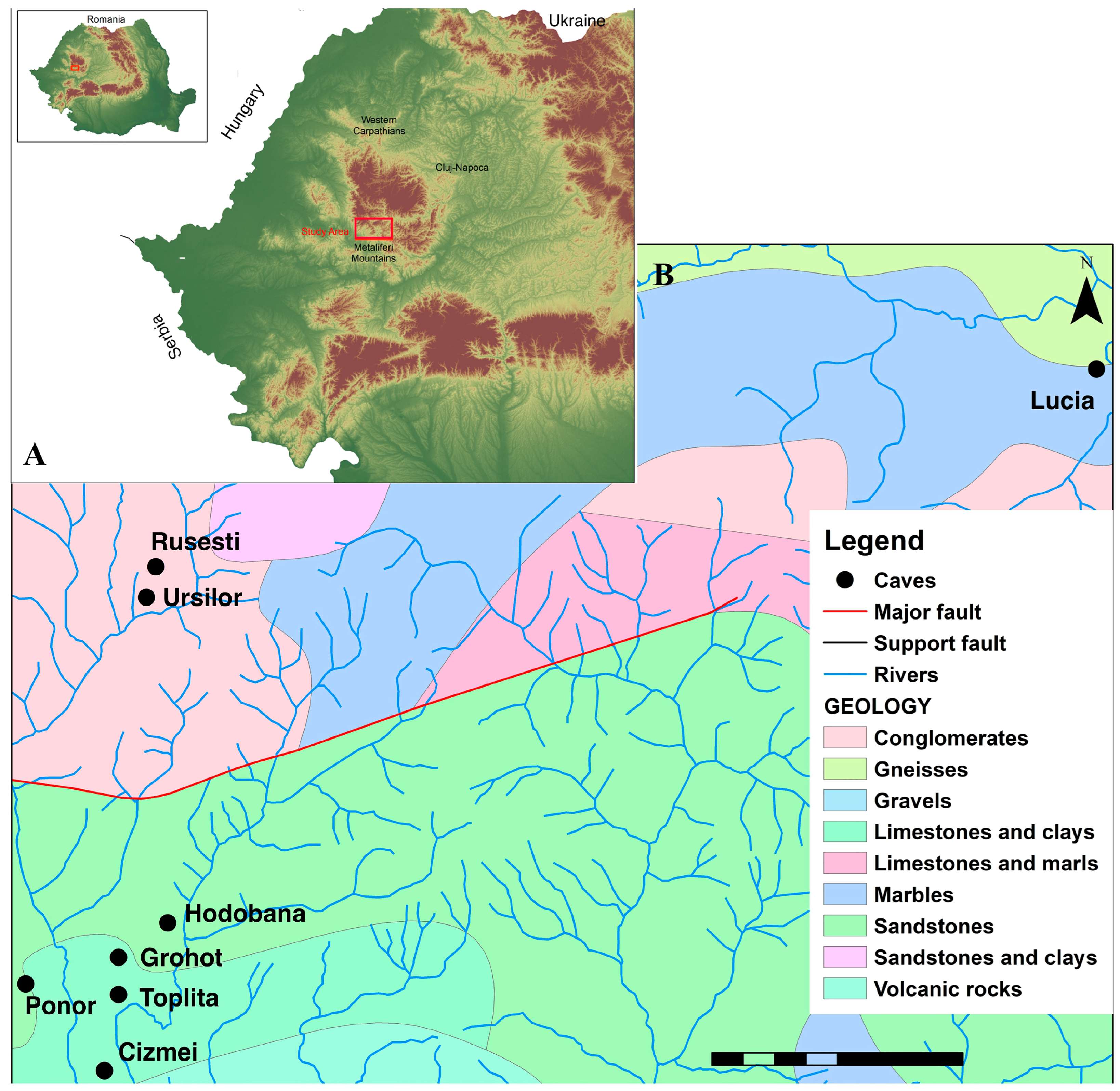
2.1. Study Area
2.2. Sampling
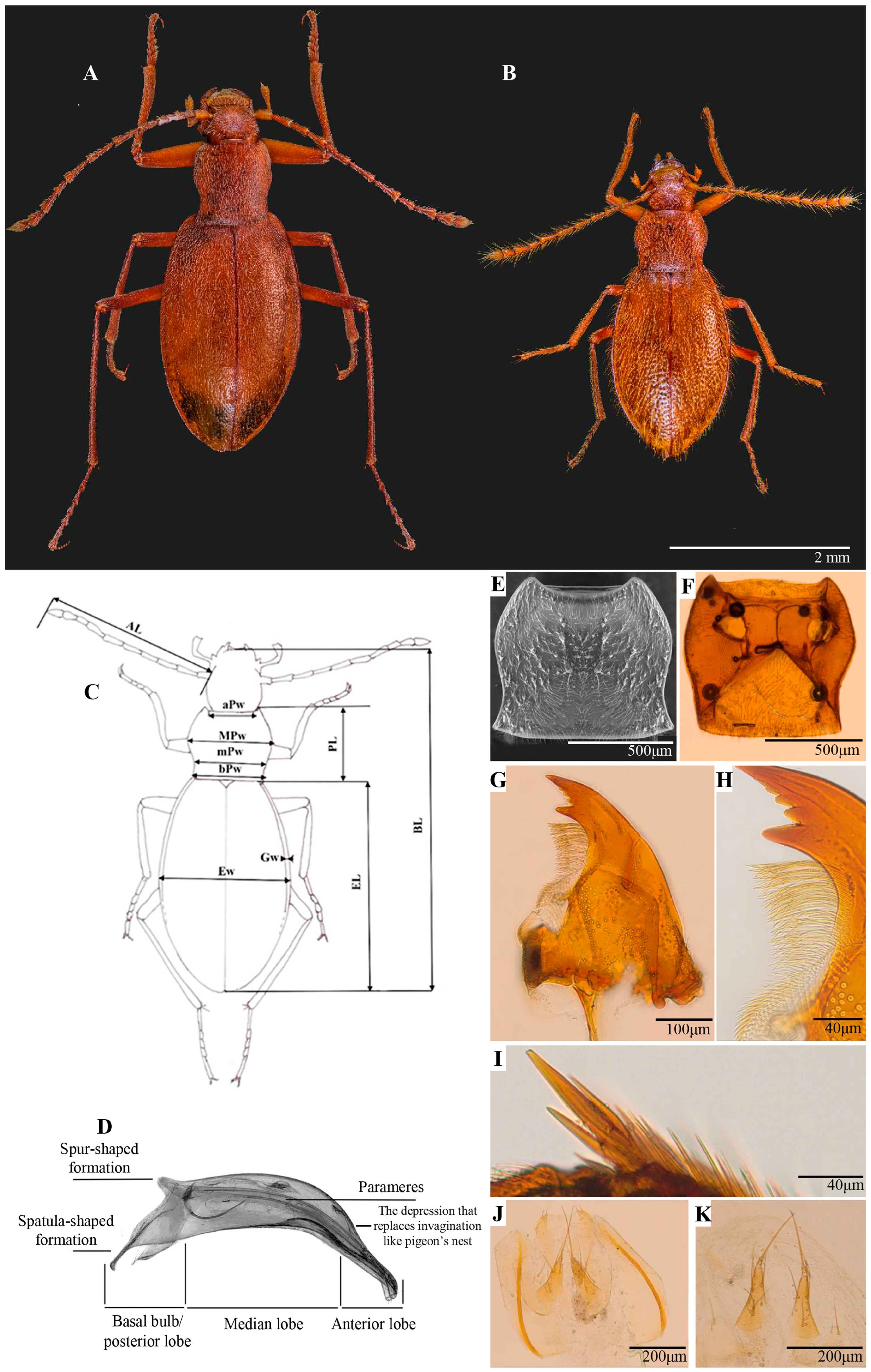
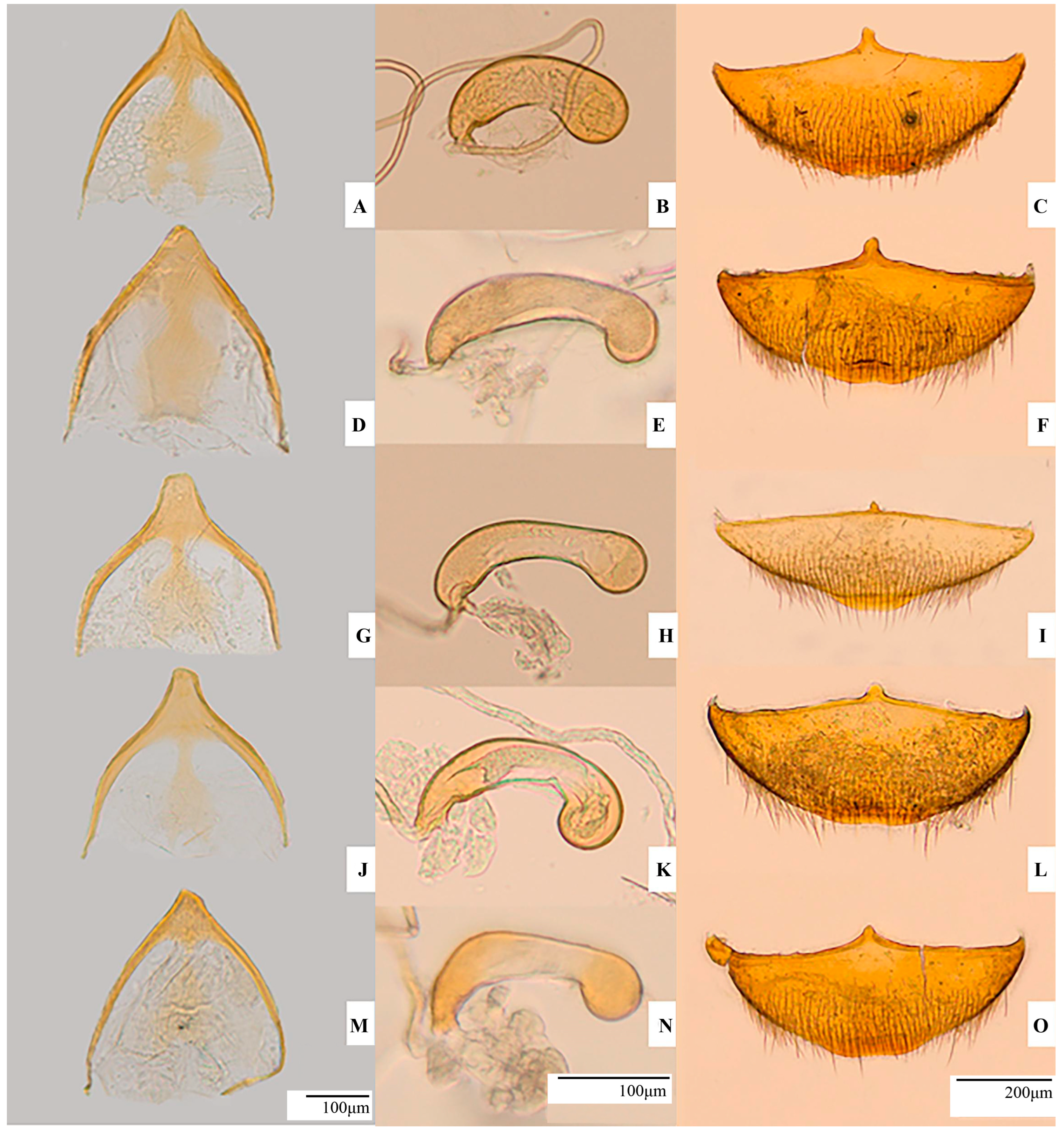
2.3. Morphological Analyses and Measurements
2.4. Scanning Electron Microscopy (SEM)
3. Results
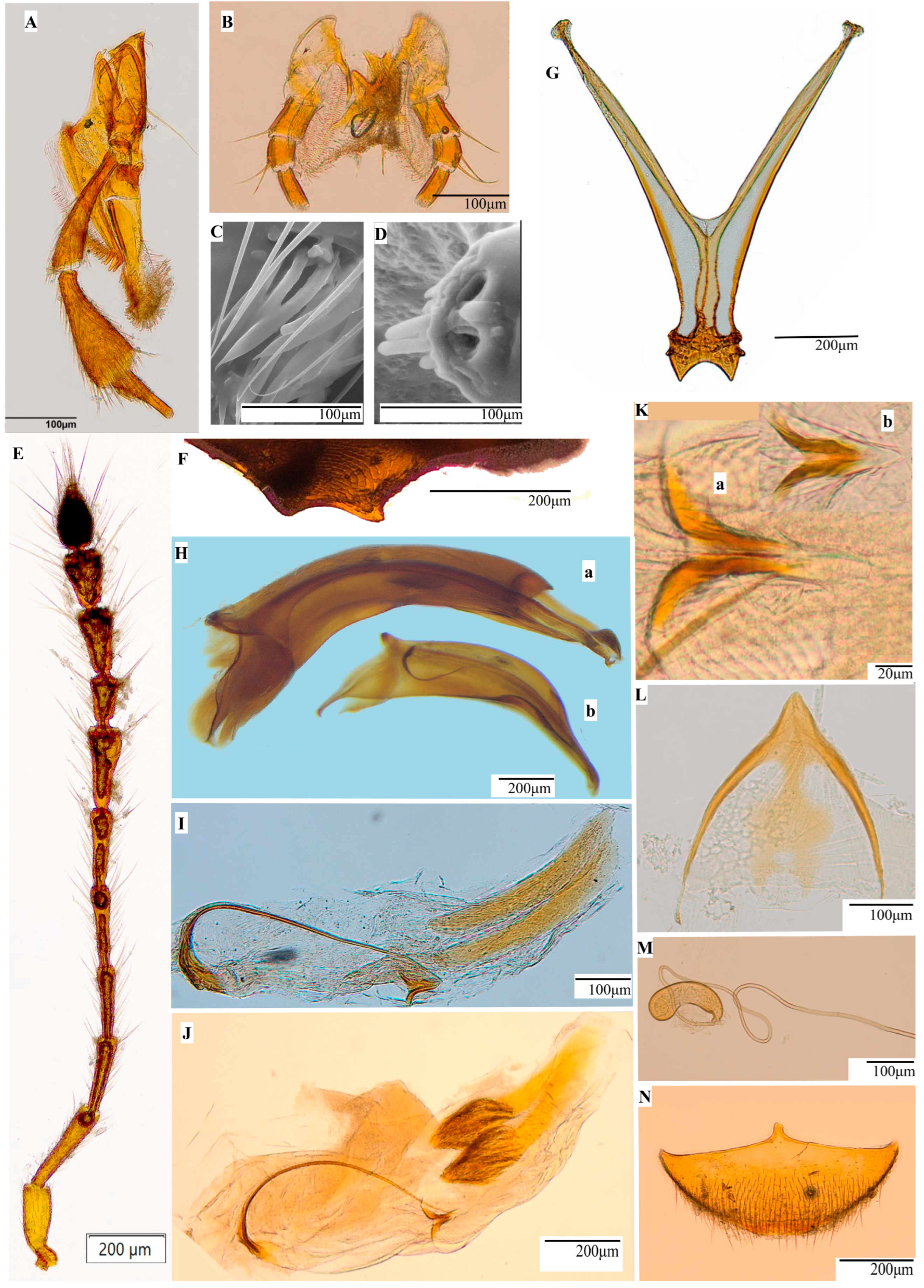
3.1. Redescription of the Subgenus Protopholeuon with the Type Species P. hungaricum
3.2. Protopholeuon (s. str.) rusescuae Sitar & Moldovan sp. nov.
- urn:lsid:zoobank.org:act:9456EC63-FB81-440D-892D-3A51D69AD083
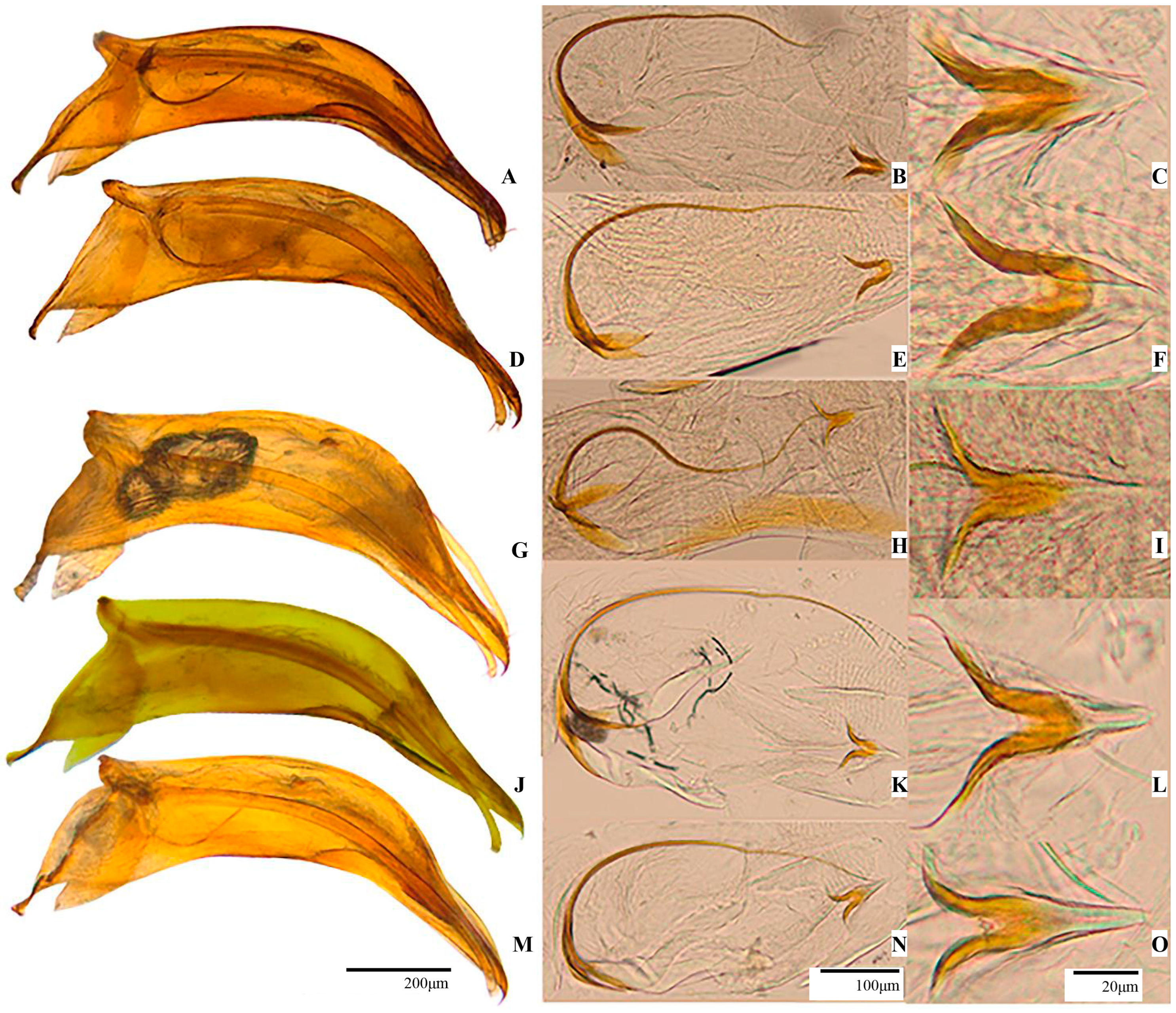
3.3. Description of the Subgenus Protopholeuon (Pachyphallus) Sitar & Moldovan subgen. nov.
- urn:lsid:zoobank.org:act:1264462C-7151-4FBC-A1A5-F8B92254ABC6
3.4. Protopholeuon (Pachyphallus) ponoricum Sitar & Moldovan sp. nov.
- urn:lsid:zoobank.org:act:D672507D-83CB-488C-BC38-24CE5C203F6E
3.5. Protopholeuon (Pachyphallus) grohotae Sitar & Moldovan sp. nov.
- urn:lsid:zoobank.org:act:F5A8F2DF-692B-47E5-A336-4813C137965F
3.6. Protopholeuon (Pachyphallus) hodobanae Sitar & Moldovan sp. nov.
- urn:lsid:zoobank.org:act:1D4349AF-6CC9-4DD8-88ED-F226EE8CE84A
3.7. Dichotomous key of Protopholeuon species
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decou, V. Le catalogue des Coléoptèra cavernicoles de Roumanie (Coleoptera). Acta Zool. Cracov. 1964, 9, 441–467. [Google Scholar]
- Moldovan, O.T.; Iepure, S.; Brad, T.; Kenesz, M.; Mirea, I.C.; Năstase-Bucur, R. Database of Romanian cave invertebrates with a Red List of cave species and a list of hotspot/coldspot caves. Biodivers. Data J. 2020, 8, e53571. [Google Scholar] [CrossRef]
- Moldovan, O.T.; Racoviţă, G.; Dunay, G. Reconsidering Pholeuon C. Hampe (Coleoptera: Leiodidae: Cholevinae), with the description of a new subgenus. Zootaxa 2007, 1449, 31–43. [Google Scholar] [CrossRef]
- Fresneda, J.; Grebennikov, V.; Ribera, I. The phylogenetic and geographic limits of Leptodirini (Insecta: Coleoptera: Leiodidae: Cholevinae), with a description of Sciaphyes shestakovi sp. n. from the Russian Far East. Arthropod. Syst. Phylogeny 2011, 69, 99–123. [Google Scholar] [CrossRef]
- Perreau, M. Catalogue des Coléoptères Leiodidae Cholevinae et Platypsyllinae. Mem. Soc. Ent. Fr. 2000, 4, 1–460. [Google Scholar]
- Perreau, M. Catalogue of Palaearctic Coleoptera, Volume 2: Hydrophiloidea–Histeroidea–Staphylinoidea; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2004; pp. 133–203. [Google Scholar]
- Bouchard, P.; Bousquet, Y.; Davies, A.; Alonso-Zarazaga, M.; Lawrence, J.; Lyal, C.; Newton, A.; Reid, C.; Schmitt, M.; Slipiński, A.; et al. Family-Group Names in Coleoptera (Insecta). ZooKeys 2011, 88, 1–972. [Google Scholar] [CrossRef]
- Fresneda, J.; Giachino, P.M.; Salgado, J.M.; Faille, A.; Bourdeau, C.; Cieslak, A.; Ribera, I. A phylogenetic classification of Leptodirini (Coleoptera, Leiodidae, Cholevinae). Mem. Soc. Entomol. Ital. 2024, 101, 3–936. [Google Scholar] [CrossRef]
- Schmitt, T. Biogeographical and evolutionary importance of the European high mountain systems. Front. Zool. 2009, 6, 9. [Google Scholar] [CrossRef]
- Mráz, P.; Ronikier, M. Biogeography of the Carpathians: Evolutionary and spatial facets of biodiversity. Biol. J. Linn. Soc. 2016, 119, 528–559. [Google Scholar] [CrossRef]
- Mamos, T.; Jażdżewski, K.; Čiamporová-Zaťovičová, Z.; Fedor, Č., Jr.; Grabowski, M. Fuzzy species borders of glacial survivalists in the Carpathian biodiversity hotspot revealed using a multimarker approach. Sci. Rep. 2021, 11, 21629. [Google Scholar] [CrossRef]
- Bálint, M.; Ujvárosi, L.; Theissinger, K.; Lehrian, S.; Mészáros, N.; Pauls, S.U. The Carpathians as a Major Diversity Hotspot in Europe. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, E.F., Habel, J.C., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; pp. 189–205. [Google Scholar]
- Năstase-Bucur, R.; Allegrucci, G.; Ketmaier, V.; Mirea, I.C.; Moldovan, O.T. Comparative phylogeography of two troglobitic Coleoptera (Leiodidae, Leptodirini) species from Romania based on mitochondrial DNA. Subterr. Biol. 2022, 42, 61–78. [Google Scholar] [CrossRef]
- Racoviţă, G. Révision systématique des Bathysciinae souterrains des Monts Apuseni. I. Variabilité individuelle et valeur taxonomique des caractères morphologiques dans la série phylétique de Drimeotus (Coleoptera, Bathysciinae). Trav. Inst. Spéol. E. Racovitza 1995, 34, 103–129. [Google Scholar]
- Racoviţă, G. Révision systématique des Leptodirinae souterrains des Monts Apuseni. II. Le sous-genre Parapholeuon Ganglb. du bassin de Crişul Repede (Monts Pădurea Craiului). Trav. Inst. Spéol. E. Racovitza 1996, 35, 71–108. [Google Scholar]
- Racoviţă, G. Révision systématique des Leptodirinae souterrains des Monts Apuseni. III. Le sous-genre Parapholeuon Ganglb. du bassin de Crişul Negru (Monts Pădurea Craiului). Trav. Inst. Spéol. E. Racovitza 1999, 38, 175–216. [Google Scholar]
- Racoviţă, G. Révision systématique des Leptodirinae souterrains des Monts Apuseni. IV. Le sous-genre Pholeuon (s. str.) du bassin de l’Arieş (Monts du Bihor). Trav. Inst. Spéol. E. Racovitza 2005, 44, 165–192. [Google Scholar]
- Racoviţă, G. Révision systématique des Leptodirinae souterrains des Monts Apuseni. VII. Aperçu synthétique sur le genre Pholeuon. Trav. Inst. Spéol. E. Racovitza 2010, 50, 37–60. [Google Scholar]
- Moldovan, O. Révision systématique de l’espèce Pholeuon (s. str.) angusticolle (Coleoptera, Catopidae, Bathysciinae). Trav. Inst. Spéol. E. Racovitza 1989, 28, 29–41. [Google Scholar]
- Moldovan, O. Drimeotus octaviani (Coleoptera: Cholevidae: Bathysciinae) nouvelle espèce des monts Padurea Craiului (Transylvanie, Roumanie). Mém. Biospéol. 1997, 24, 169–173. [Google Scholar]
- Moldovan, O. Drimeotus plesai (Coleoptera, Cholevidae) a new species of Leptodirinae from Transylvania (Romania). Mém. Biospéol. 2000, 27, 89–92. [Google Scholar]
- Moldovan, O.T. Révision de Drimeotus str. Miller, 1856 (Coleoptera, Cholevidae, Leptodirinae) de Transylvanie (Roumanie) avec description de deux nouvelles espèces et clé de détermination des taxa. Zoosystema 2000, 22, 139–152. [Google Scholar]
- Bucur, R. Filogenia și Biogeografia Coleopterelor Subterane din Munții Apuseni; Editura Napoca Star: Cluj-Napoca, Romania, 2007. [Google Scholar]
- Csiki, E. Pholeuon hungaricum, barlanglakó új vak bogár Magyarországból. Ann. Musei Natl. Hung. 1904, 2, 565–566. [Google Scholar]
- Jeannel, R. Monographie des Bathysciinae. Arch. Zool. Exp. Gén. 1924, 63, 1–436. [Google Scholar]
- European Space Agency (ESA). Generated Using E.U. Copernicus DEM. Available online: https://dataspace.copernicus.eu/explore-data/data-collections/copernicus-contributing-missions/collections-description/COP-DEM (accessed on 10 August 2024).
- Ianovici, V.; Giuşcă, D. Evoluţia Geologică a Munţilor Metaliferi; Editura Academiei Republicii Socialiste România: București, Romania, 1969. [Google Scholar]
- Enciu, V. Carstul. III-4 (1). Atlasul R.S.R.; Editura Academiei R.S.R.: București, Romania, 1973. [Google Scholar]
- Perreau, M. Description of a new genus and two new species of Leiodidae (Coleoptera) from Baltic amber using phase contrast synchrotron X-ray microtomography. Zootaxa 2012, 3455, 81–88. [Google Scholar] [CrossRef]
- Ćurčić, S.; Radja, T.; Mulaomerović, J.; Vrbica, M.; Antić, D.; Tomić, V.; Ćurčić, B.; Apostolska, B.; Vesović, N. Three new cave-dwelling leiodid beetles (Coleoptera: Leiodidae: Cholevinae: Leptodirini) from Bosnia and Herzegovina. Arch. Biol. Sci. 2014, 66, 919–933. [Google Scholar] [CrossRef]
- Ćurčić, S.; Pavićević, D.; Vesović, N.; Mulaomerović, J.; Radja, T.; Antić, D.; Bosco, F.; Marković, Đ.; Petković, M. Seven new taxa of Leptodirini (Coleoptera: Leiodidae: Cholevinae) from the Balkan Peninsula. Zootaxa 2018, 4483, 523–548. [Google Scholar] [CrossRef] [PubMed]
- Ćurčić, S.; Pavićević, D.; Vesović, N.; Vrbica, M.; Kuraica, M.; Marković, Đ.; Petković, M.; Lazović, V.; Pantelić, D.; Bosco, F. On the diversity of subterranean beetles of the Dinarides: New leiodid taxa (Coleoptera: Leiodidae) from Serbia. Eur. J. Taxon. 2021, 782, 55–81. [Google Scholar] [CrossRef]
- Moldovan, O.T.; Jalzic, B.; Erichsen, E. Diversity of mouthparts in some European representatives of subterranean Cholevidae (Coleoptera). Nat. Croat. 2004, 13, 1–18. [Google Scholar]
- Antunes-Carvalho, C.A.I.O.; Gnaspini, P. Pretarsus and distal margin of the terminal tarsomere as an unexplored character system for higher-level classification in Cholevinae (Coleoptera, Leiodidae). Syst. Entomol. 2016, 41, 392–415. [Google Scholar] [CrossRef]
- Antunes-Carvalho, C.; Ribera, I.; Beutel, R.G.; Gnaspini, P. Morphology-based phylogenetic reconstruction of Cholevinae (Coleoptera: Leiodidae): A new view on higher-level relationships. Cladistics 2019, 35, 1–41. [Google Scholar] [CrossRef]
- Sitar, C.; Barbu-Tudoran, L.; Moldovan, O.T. Morphological and micromorphological description of the larvae of two endemic species of Duvalius (Coleoptera, Carabidae, Trechini). Biology 2021, 10, 627. [Google Scholar] [CrossRef]
- Habitat Directive. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. 1992. Available online: https://eur-lex.europa.eu/eli/dir/1992/43/oj/eng (accessed on 14 July 2025).
- Trochet, A.; Schmeller, D. Effectiveness of the Natura 2000 network to cover threatened species. Nat. Conserv. 2013, 4, 35–53. [Google Scholar] [CrossRef]
- Manes, S.; Costello, M.J.; Beckett, H.; Debnath, A.; Devenish-Nelson, E.; Grey, K.A.; Jenkins, R.; Ming Khan, T.; Kiessling, W.; Krause, C.S.; et al. Endemism increases species’ climate change risk in areas of global biodiversity importance. Biol. Conserv. 2021, 257, 109070. [Google Scholar] [CrossRef]

| Cave | Air Temperature (°C) | Karstic Basin | Absolute Altitude (m) | Rock Type | Coordinates |
|---|---|---|---|---|---|
| Ponor | 11.0 | Rîșculiței | 480 | Limestone | 46.236979° N, 22.733602° E |
| Hodobana | 11.0 | Grohot | 470 | Limestone | 46.257245° N, 22.785063° E |
| Grohot | 11.4 | Grohot | 600 | Limestone | 46.255324° N. 22.772105° E |
| Topliței | 11.4 | Grohot | 530 | Limestone | 46.235599° N, 22.780594° E |
| Cizmei | 11.5 | Grohot | 430 | Limestone | 46.221721° N, 22.769745° E |
| Urșilor | 9.9 | Bulzești–Rusești | 620 | Metamorphosed limestone | 46.306961° N, 22.759897° E |
| Rusești | 10.0 | Bulzești–Rusești | 650 | Metamorphosed limestone | 46.323134° N, 22.776788° E |
| Lucia | 9.6 | Poieni | 570 | Metamorphosed limestone | 46.361234° N, 23.006931° E |
| Laptelui de Piatră | 9.3 | Arieșul Mare | 960 | Limestone | 46.468274° N, 22.926859° E |
| Species | Pholeuon (s. str.) knirschi intermittens | Protopholeuon (s. str.) hungaricum | Protopholeuon (s. str.) rusescuae sp. nov. | Protopholeuon (Pachyphallus) ponoricum sp. nov | Protopholeuon (Pachyphallus) grohotae sp. nov. | Protopholeuon (Pachyphallus) hodobanae sp. nov. | |
|---|---|---|---|---|---|---|---|
| Females | n | 42 | 100 | 6 | 10 | 18 | 6 |
| BL | 5.64 ± 0.10 | 3.82 ± 0.03 | 4.16 ± 0.16 | 4.00 ± 0.10 | 3.93 ± 0.14 | 4.12 ± 0.25 | |
| PL | 1.31 ± 0.03 | 0.89 ± 0.01 | 0.86 ± 0.03 | 0.81 ± 0.03 | 0.79 ± 0.03 | 0.82 ± 0.02 | |
| aPw | 0.88 ± 0.03 | 0.66 ± 0.01 | 0.64 ± 0.02 | 0.64 ± 0.01 | 0.62 ± 0.01 | 0.66 ± 0.03 | |
| MPw | 1.43 ± 0.04 | 0.98 ± 0.01 | 1.01 ± 0.03 | 9.43 ± 0.01 | 9.44 ± 0.03 | 9.78 ± 0.04 | |
| mPw | 1.26 ± 0.05 | 0.83 ± 0.01 | 0.85 ± 0.03 | 0.81 ± 0.02 | 0.81 ± 0.02 | 0.82 ± 0.04 | |
| bPw | 1.26 ± 0.04 | 0.85 ± 0.01 | 0.84 ± 0.04 | 0.82 ± 0.02 | 0.80 ± 0.02 | 0.84 ± 0.04 | |
| EL | 3.83 ± 0.10 | 2.73 ± 0.02 | 2.53 ± 0.09 | 2.49 ± 0.05 | 2.46 ± 0.07 | 2.61 ± 0.16 | |
| Ew | 2.30 ± 0.06 | 1.56 ± 0.01 | 1.60 ± 0.04 | 1.50 ± 0.04 | 1.52 ± 0.05 | 1.54 ± 0.06 | |
| jw | - | - | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | |
| AL | 3.48 ± 0.12 | 2.42 ± 0.03 | 2.40 ± 0.10 | 2.31 ± 0.08 | 2.26 ± 0.07 | 2.27 ± 0.10 | |
| BL/AL | 0.61 ± 0.02 | 0.63 | 0.57 | 0.57 | 0.57 | 0.55 | |
| Males | n | 34 | 100 | 14 | 8 | 14 | 7 |
| BL | 5.20 ± 0.14 | 3.65 ± 0.04 | 3.89 ± 0.18 | 3.98 ± 0.04 | 4.07 ± 0.13 | 3.74 ± 0.12 | |
| PL | 1.28 ± 0.04 | 0.87 ± 0.01 | 0.83 ± 0.04 | 0.83 ± 0.04 | 0.83 ± 0.03 | 0.80 ± 0.02 | |
| aPw | 0.84 ± 0.02 | 0.63 ± 0.01 | 0.61 ± 0.02 | 0.64 ± 0.06 | 0.64 ± 0.02 | 0.62 ± 0.01 | |
| MPw | 1.24 ± 0.04 | 0.93 ± 0.01 | 0.94 ± 0.03 | 9.21 ± 0.07 | 1.00 ± 0.04 | 0.92 ± 0.02 | |
| mPw | 1.17 ± 0.05 | 0.78 ± 0.01 | 0.78 ± 0.03 | 0.78 ± 0.07 | 0.85 ± 0.03 | 0.77 ± 0.02 | |
| bPw | 1.18 ± 0.04 | 0.80 ± 0.01 | 0.78 ± 0.04 | 8.14 ± 0.07 | 0.84 ± 0.03 | 0.79 ± 0.01 | |
| EL | 3.61 ± 0.10 | 2.58 ± 0.03 | 2.39 ± 0.12 | 2.48 ± 0.04 | 2.56 ± 0.04 | 2.37 ± 0.06 | |
| Ew | 2.17 ± 0.06 | 1.44 ± 0.01 | 1.45 ± 0.06 | 1.44 ± 0.01 | 1.56 ± 0.06 | 1.44 ± 0.04 | |
| jw | - | - | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | |
| AL | 3.78 ± 0.14 | 2.57 ± 0.04 | 2.45 ± 0.10 | 2.60 ± 0.05 | 2.38 ± 0.06 | 2.39 ± 0.05 | |
| BL/AL | 0.72 ± 0.03 | 0.7 | 0.63 | 0.65 | 0.58 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitar, C.; Kenesz, M.; Barbu-Tudoran, L.; Moldovan, O.T. A New Hotspot of Cave Leptodirini (Coleoptera: Leiodidae) from the Romanian Carpathians. Insects 2025, 16, 806. https://doi.org/10.3390/insects16080806
Sitar C, Kenesz M, Barbu-Tudoran L, Moldovan OT. A New Hotspot of Cave Leptodirini (Coleoptera: Leiodidae) from the Romanian Carpathians. Insects. 2025; 16(8):806. https://doi.org/10.3390/insects16080806
Chicago/Turabian StyleSitar, Cristian, Marius Kenesz, Lucian Barbu-Tudoran, and Oana Teodora Moldovan. 2025. "A New Hotspot of Cave Leptodirini (Coleoptera: Leiodidae) from the Romanian Carpathians" Insects 16, no. 8: 806. https://doi.org/10.3390/insects16080806
APA StyleSitar, C., Kenesz, M., Barbu-Tudoran, L., & Moldovan, O. T. (2025). A New Hotspot of Cave Leptodirini (Coleoptera: Leiodidae) from the Romanian Carpathians. Insects, 16(8), 806. https://doi.org/10.3390/insects16080806








