Suppression of Spotted Wing Drosophila, Drosophila suzukii (Matsumura), in Raspberry Using the Sterile Insect Technique
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Quality of Irradiated Sterile Male D. suzukii
2.1.1. Flight Performance
2.1.2. Mating Competitiveness
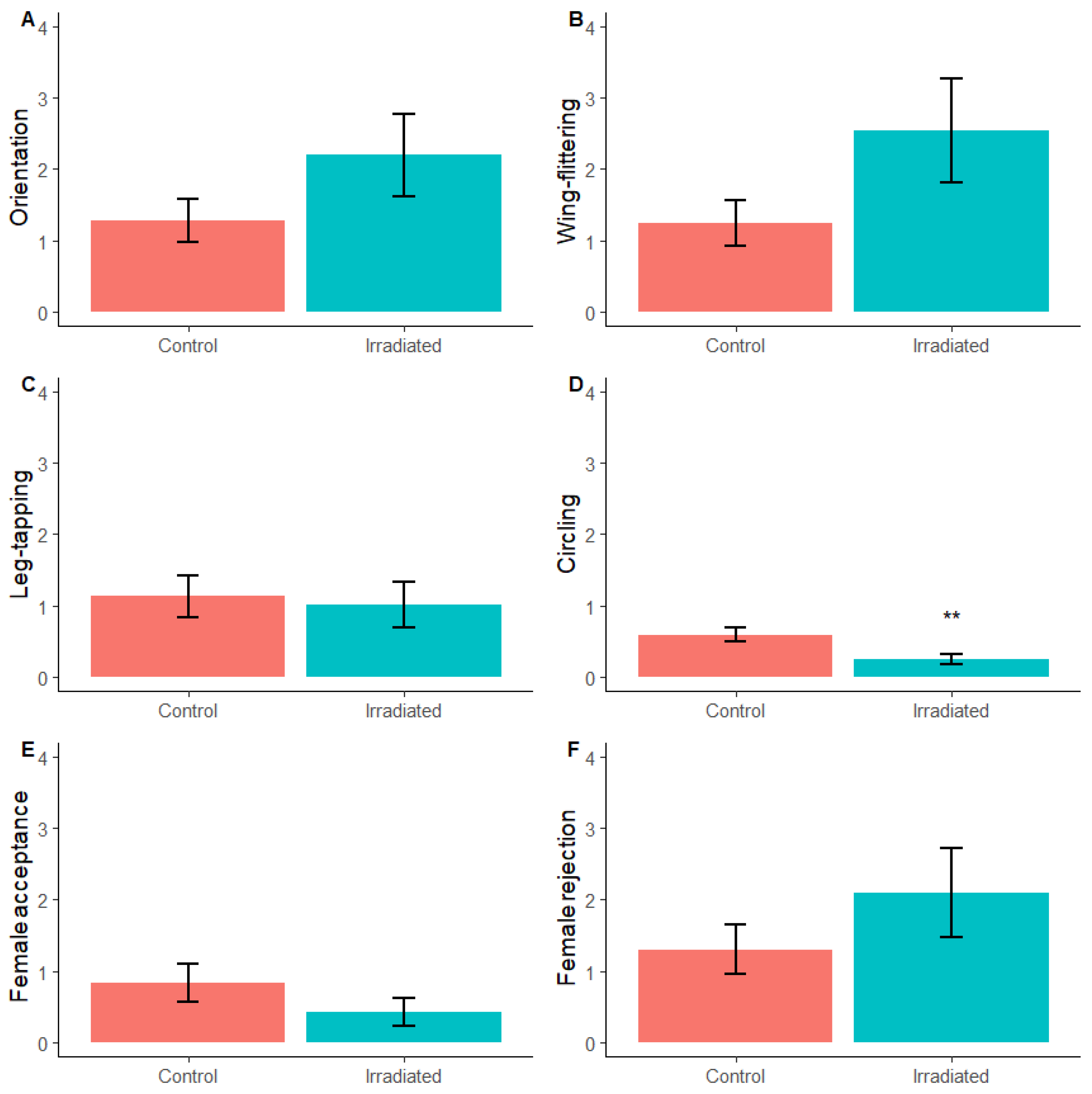
2.1.3. Courtship
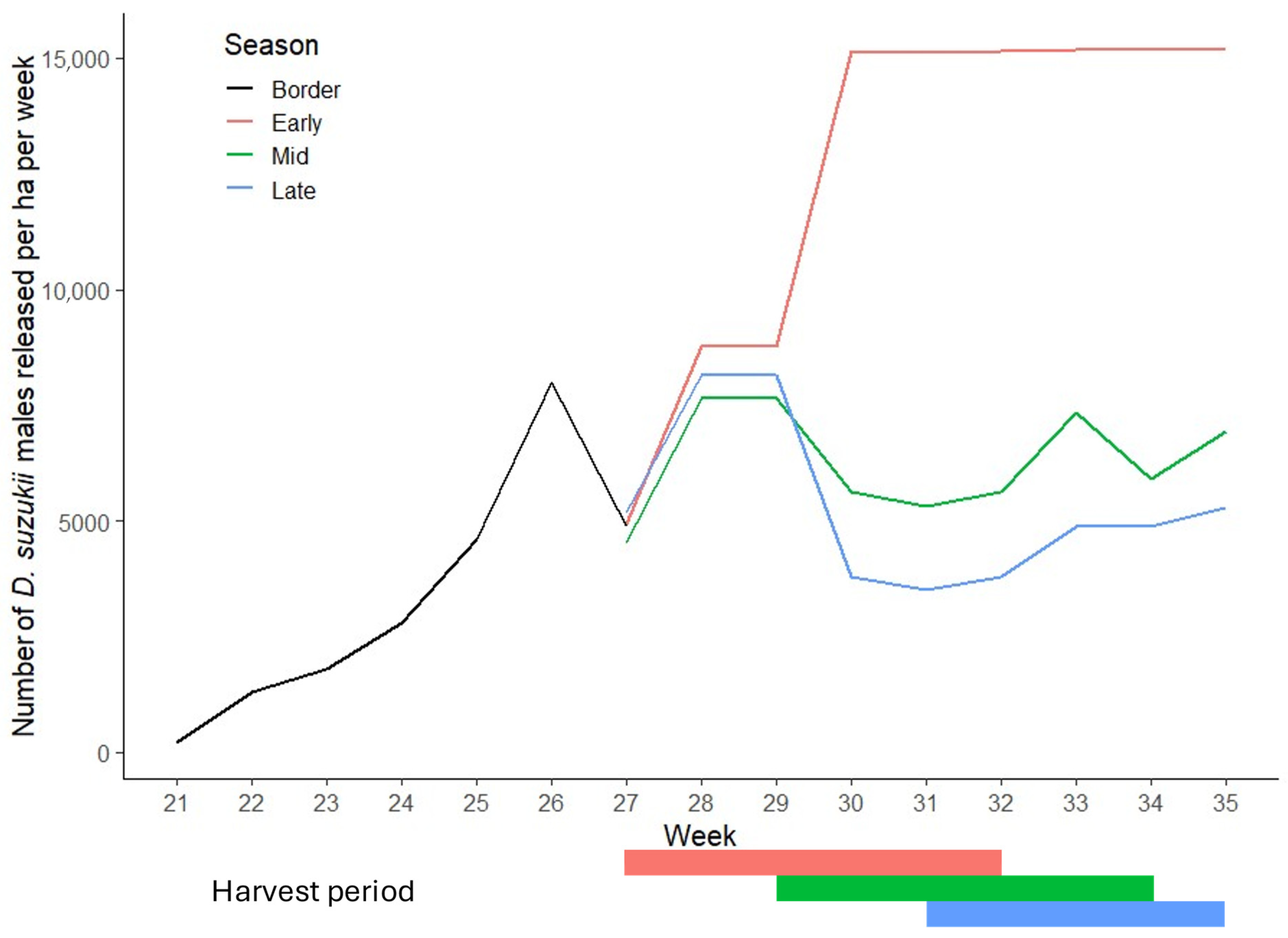
2.2. Efficacy of SIT in Commercial Raspberry Crops
2.3. Statistical Analysis
2.3.1. Quality of Irradiated Sterile Male D. suzukii
2.3.2. Efficacy of SIT in Commercial Raspberry Crops
3. Results
3.1. Quality of Irradiated Sterile Male D. suzukii
3.1.1. Flight Performance
3.1.2. Mating Competitiveness
3.1.3. Courtship
3.2. Efficacy of SIT in Commercial Raspberry Crops
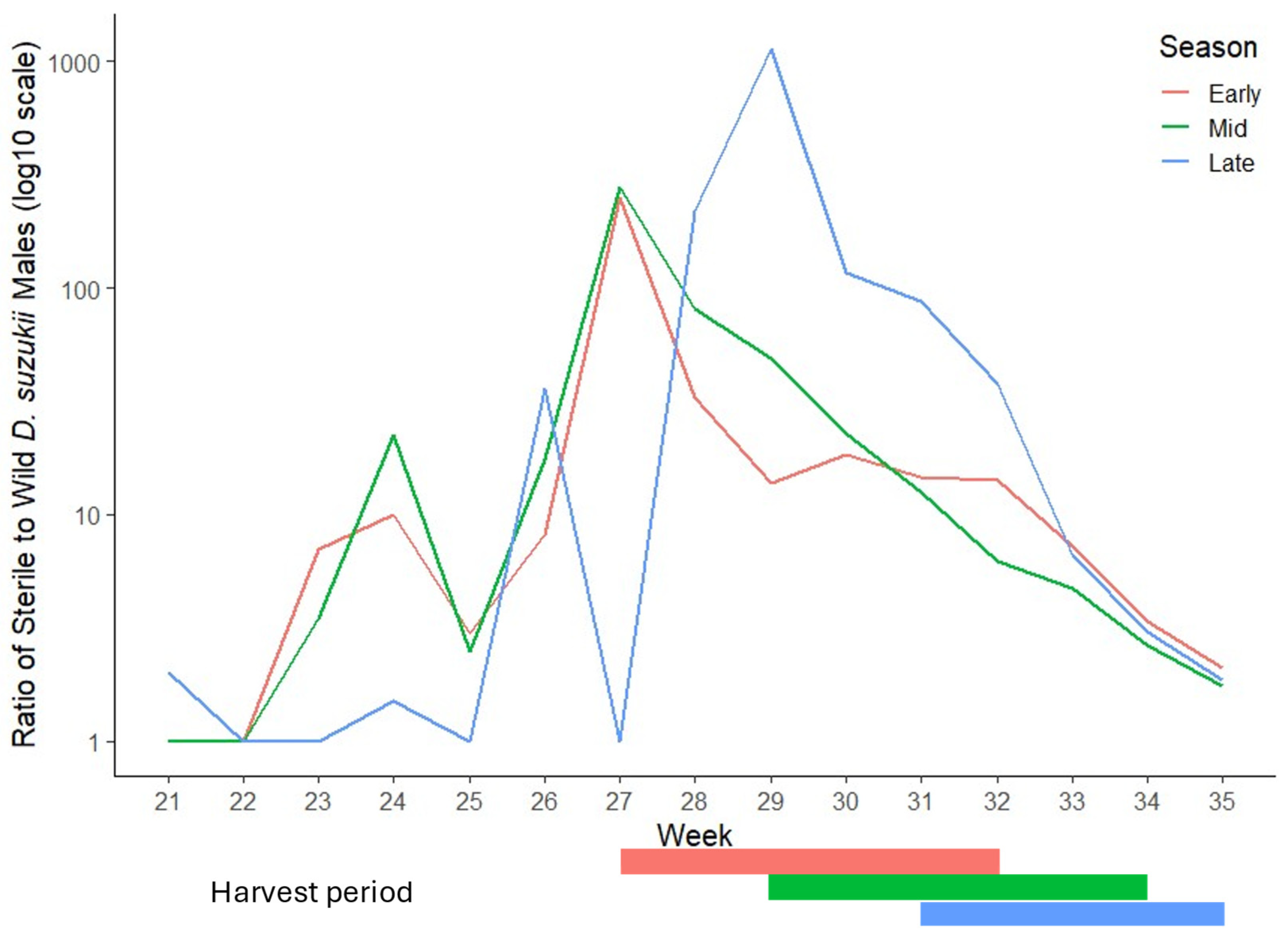
3.2.1. Ratio of Sterile to Wild Male D. suzukii
3.2.2. Suppression of Wild Adult Female D. suzukii
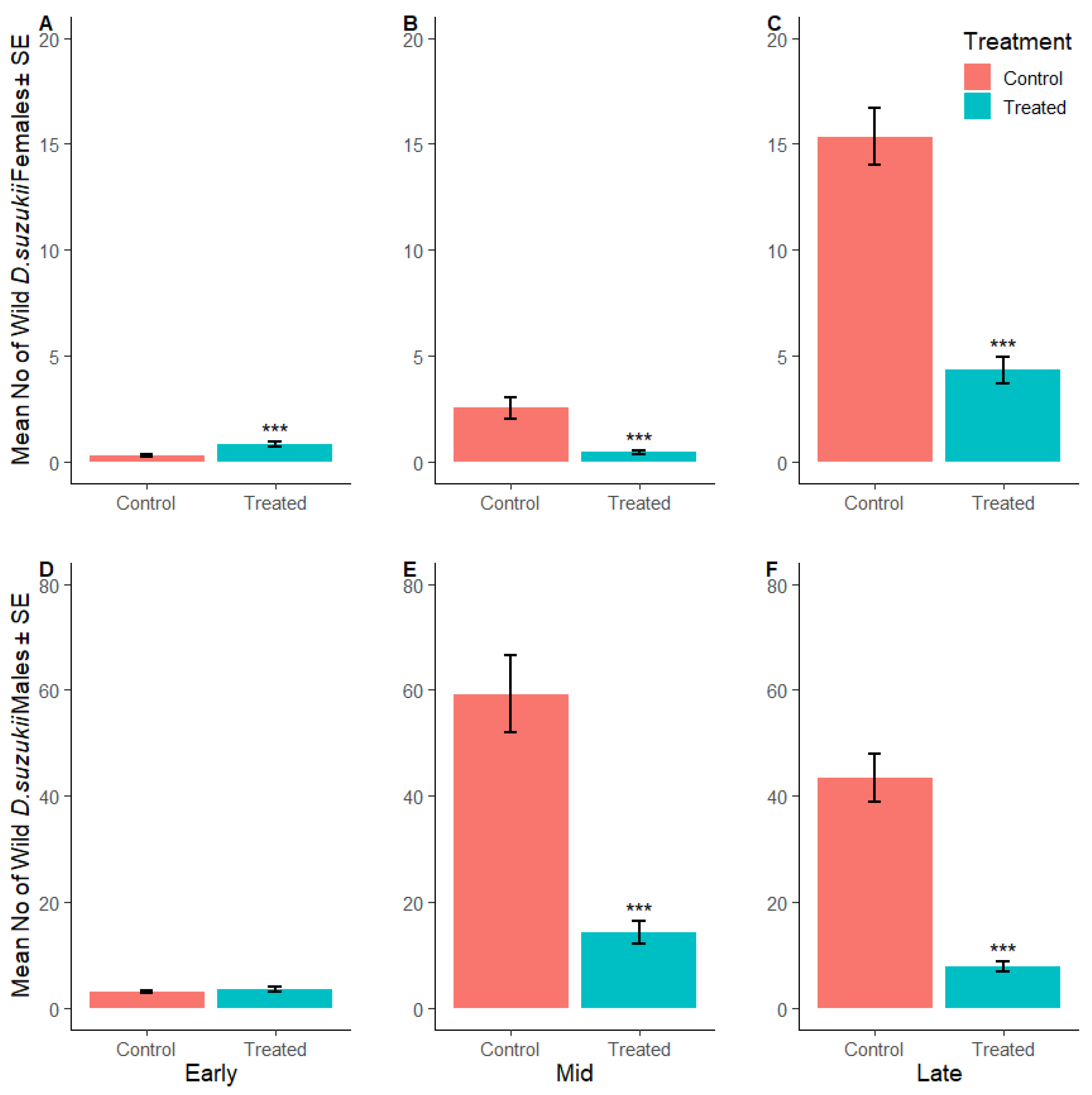
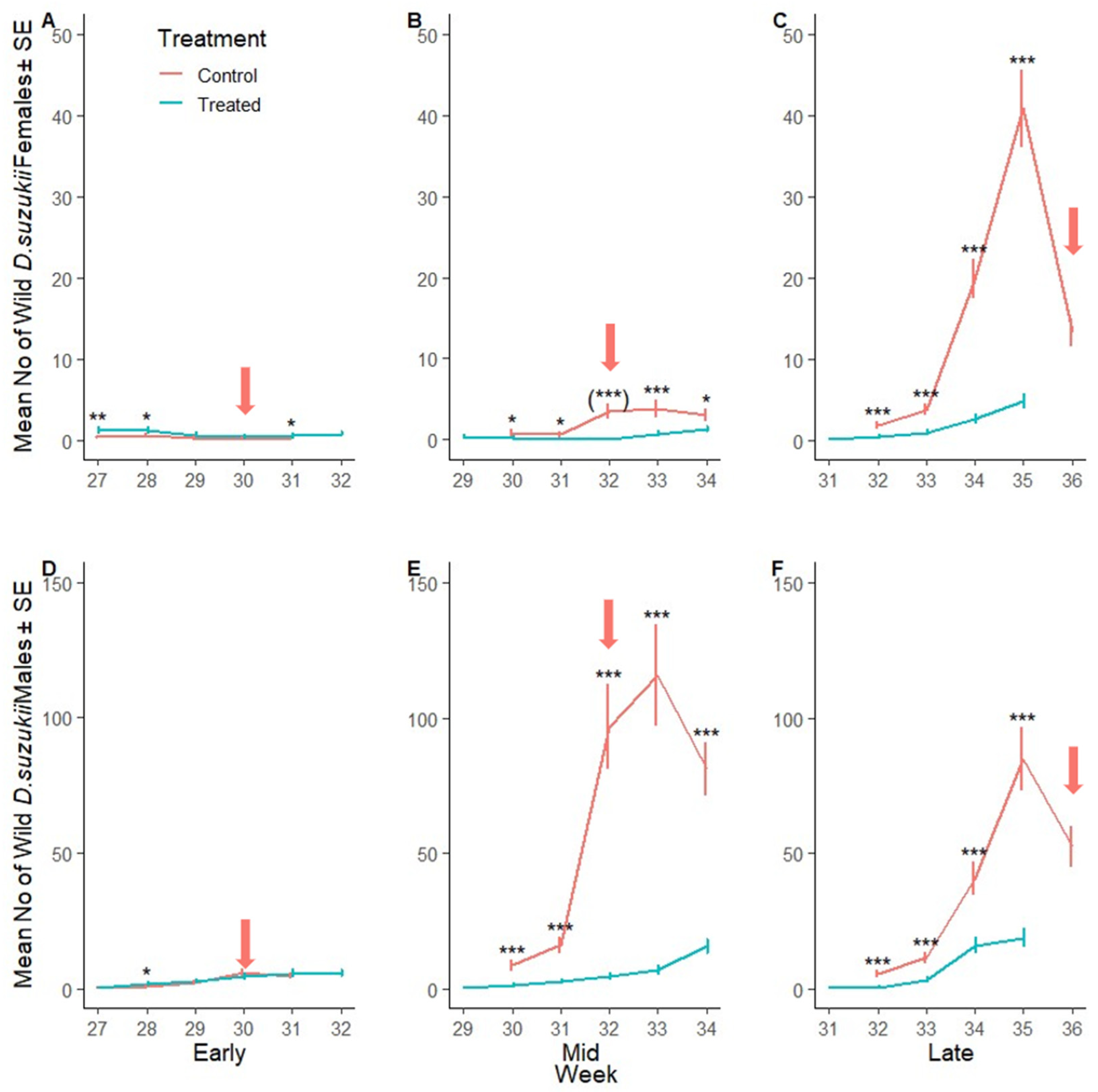
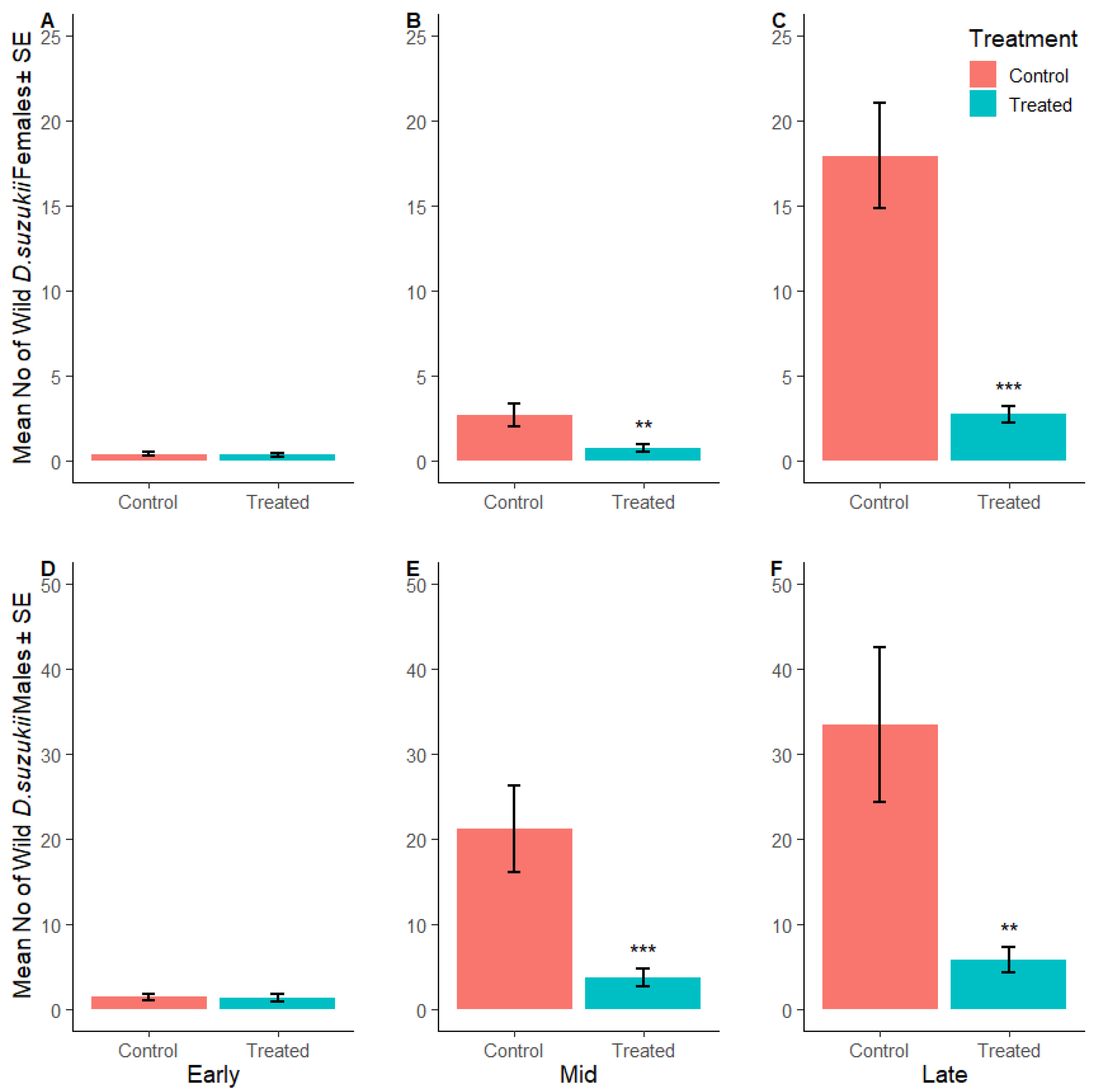
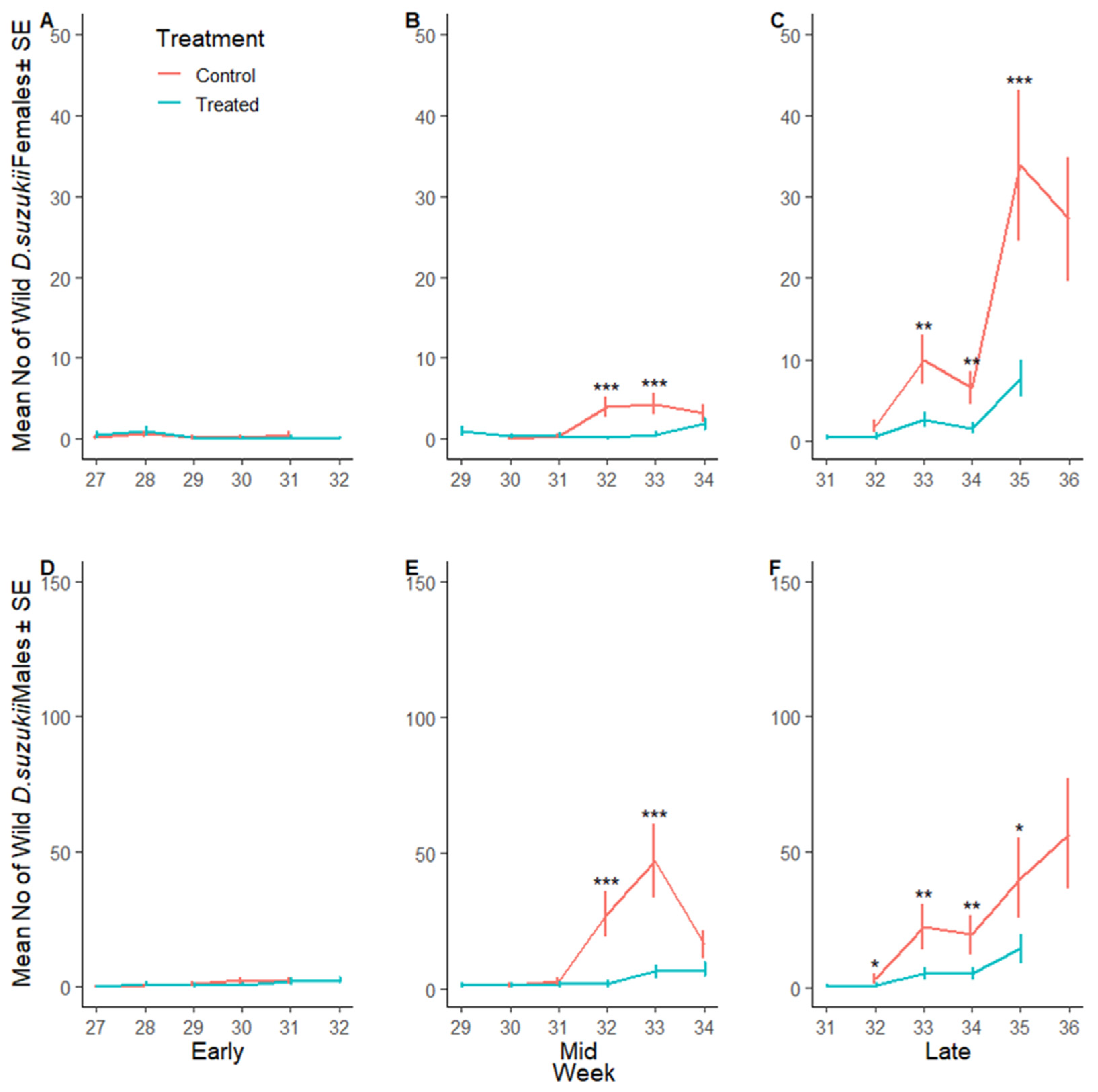
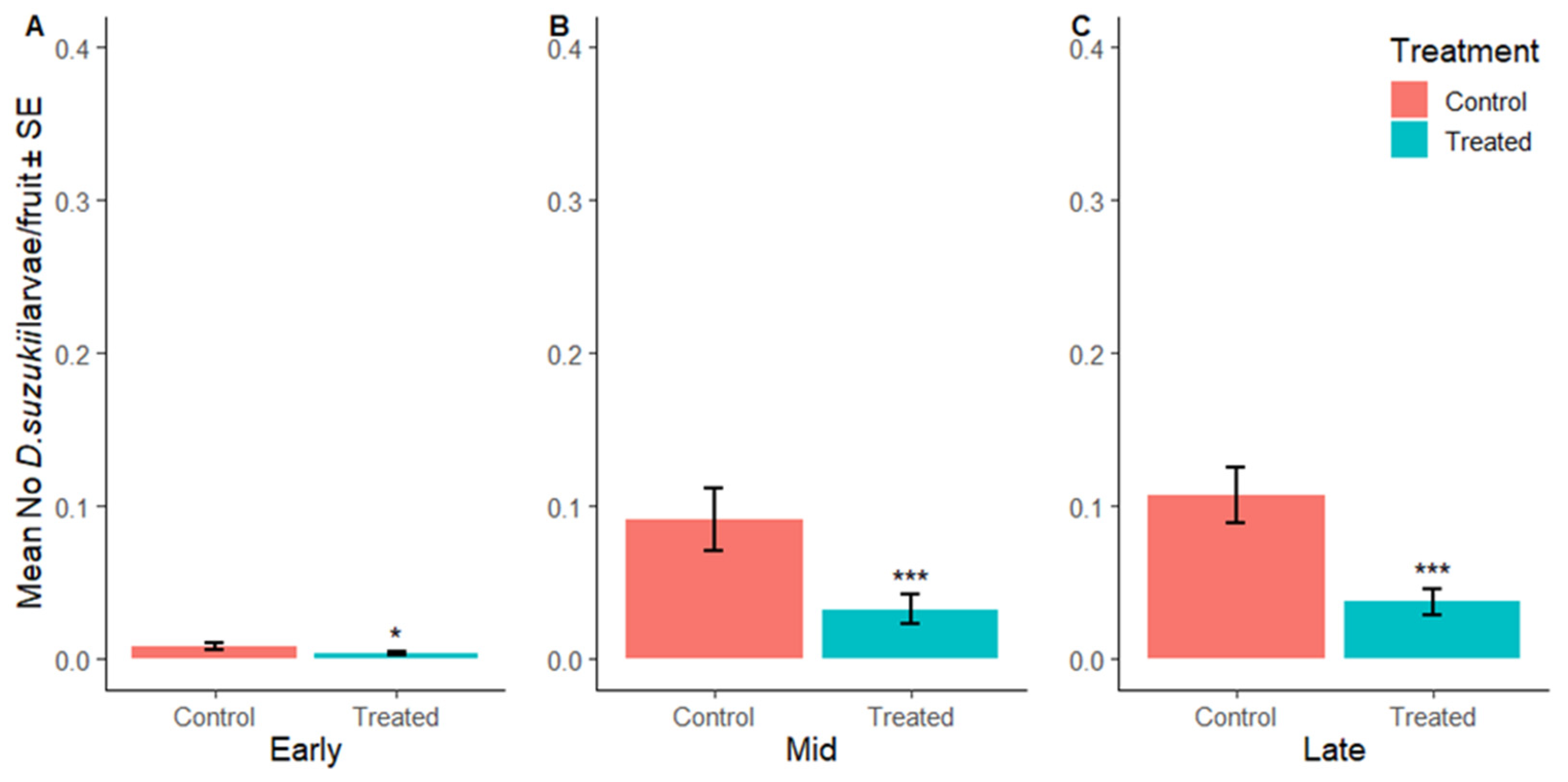
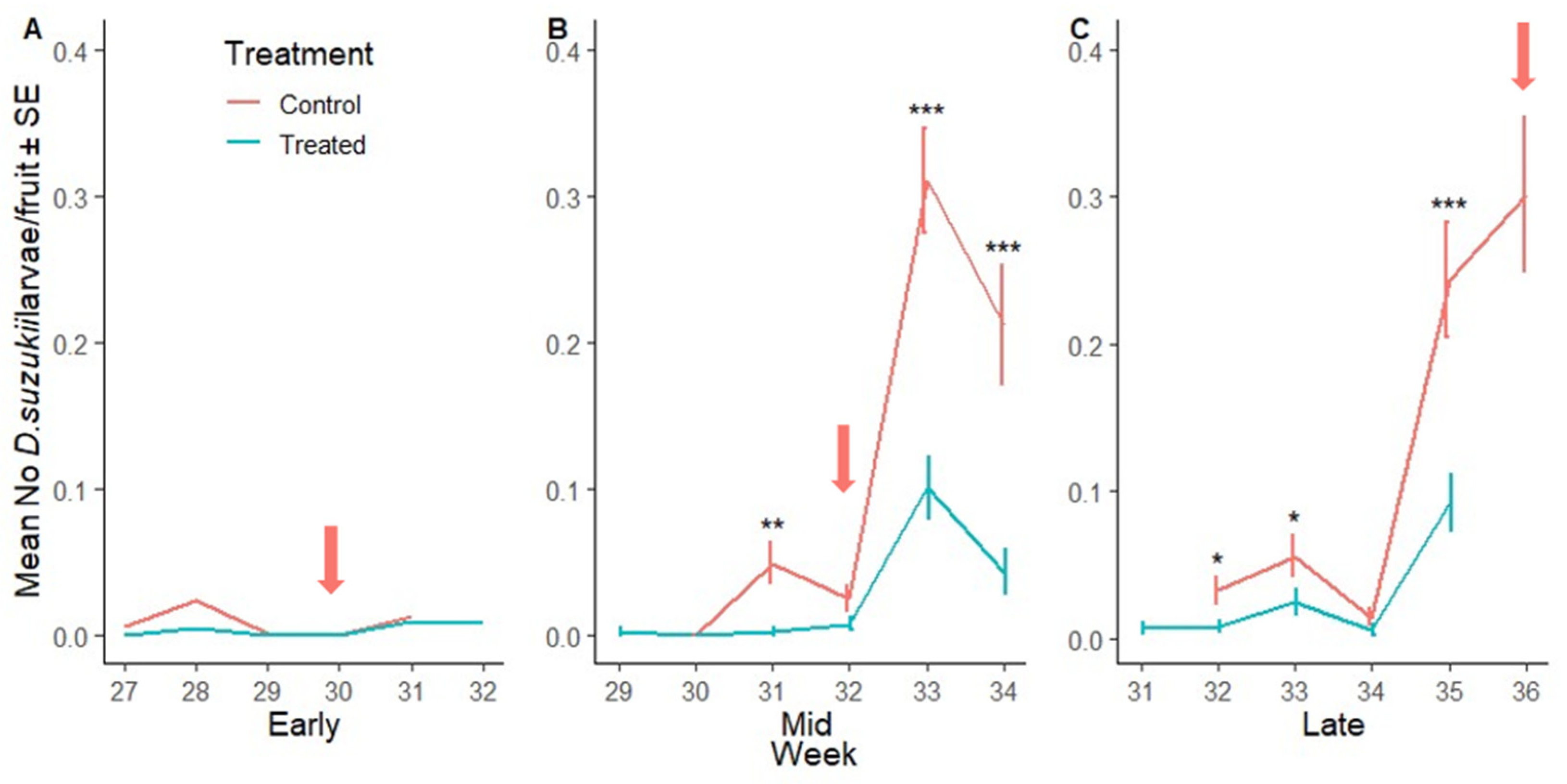
3.2.3. Suppression of D. suzukii Larvae per Fruit
3.2.4. Reduction in Fruit Waste
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SIT | Sterile Insect Technique |
| AI | Artificial Intelligence |
| GLMM | Generalised Linear Mixed Model |
| GLM | Generalised Linear Model |
| IIT | Incompatible Insect Technique |
References
- Vargas-Terán, M.; Spradbery, J.P.; Hofmann, H.C.; Tweddle, N.E. Impact of Screwworm Eradication Programmes Using the Sterile Insect Technique. In Sterile Insect Technique; CRC Press: Boca Raton, FL, USA, 2021; pp. 949–978. [Google Scholar]
- Plá, I.; García de Oteyza, J.; Tur, C.; Martínez, M.Á.; Laurín, M.C.; Alonso, E.; Martínez, M.; Martín, Á.; Sanchis, R.; Navarro, M.C.; et al. Sterile Insect Technique Programme against Mediterranean Fruit Fly in the Valencian Community (Spain). Insects 2021, 12, 415. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Staples, D.; Díaz-Fleischer, F.; Montoya, P. The Sterile Insect Technique: Success and Perspectives in the Neotropics. Neotrop. Entomol. 2021, 50, 172–185. [Google Scholar] [CrossRef]
- Vreysen, M.J.B.; Carpenter, J.E.; Marec, F. Improvement of the Sterile Insect Technique for Codling Moth Cydia pomonella (Linnaeus) (Lepidoptera Tortricidae) to Facilitate Expansion of Field Application. J. Appl. Entomol. 2010, 134, 165–181. [Google Scholar] [CrossRef]
- Bourtzis, K.; Vreysen, M.J.B. Sterile Insect Technique (SIT) and Its Applications. Insects 2021, 12, 638. [Google Scholar] [CrossRef]
- Klassen, W.; Curtis, C.F.; Hendrichs, J. History of the Sterile Insect Technique. In Sterile Insect Technique; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–44. [Google Scholar]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In Focus: Spotted Wing Drosophila, Drosophila suzukii, across Perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef]
- Cini, A.; Ioriatti, C.; Anfora, G. A Review of the Invasion of Drosophila suzukii in Europe and a Draft Research Agenda for Integrated Pest Management. Bull. Insectology 2012, 65, 149–160. [Google Scholar]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion Biology of Spotted Wing Drosophila (Drosophila suzukii): A Global Perspective and Future Priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Klick, J.; Yang, W.Q.; Walton, V.M.; Dalton, D.T.; Hagler, J.R.; Dreves, A.J.; Lee, J.C.; Bruck, D.J. Distribution and Activity of Drosophila suzukii in Cultivated Raspberry and Surrounding Vegetation. J. Appl. Entomol. 2016, 140, 37–46. [Google Scholar] [CrossRef]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic Impact of the Introduction and Establishment of Drosophila suzukii on Sweet Cherry Production in Switzerland. Insects 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, D.S.; Funes, C.F.; Buonocore-Biancheri, M.J.; Suárez, L.; Ovruski, S.M. The Biology and Ecology of Drosophila suzukii (Diptera: Drosophilidae). In Drosophila suzukii Management; Springer International Publishing: Cham, Switzerland, 2020; pp. 41–91. [Google Scholar]
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The Making of a Pest: The Evolution of a Fruit-Penetrating Ovipositor in Drosophila suzukii and Related Species. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132840. [Google Scholar] [CrossRef] [PubMed]
- De Ros, G.; Grassi, A.; Pantezzi, T. Recent Trends in the Economic Impact of Drosophila suzukii. In Drosophila suzukii Management; Springer International Publishing: Cham, Switzerland, 2020; pp. 11–27. [Google Scholar]
- De Ros, G. The Economic Analyses of the Drosophila suzukii’s Invasions: A Mini-Review. Neotrop. Entomol. 2024, 53, 244–253. [Google Scholar] [CrossRef]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research Towards a Sustainable Integrated Pest Management Program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef]
- Buck, N.; Fountain, M.T.; Potts, S.G.; Bishop, J.; Garratt, M.P.D. The Effects of Non-crop Habitat on Spotted Wing Drosophila (Drosophila suzukii) Abundance in Fruit Systems: A Meta-analysis. Agric. For. Entomol. 2023, 25, 66–76. [Google Scholar] [CrossRef]
- Homem, R.; Mateos-Fierro, Z.; Jones, R.; Gilbert, D.; Mckemey, A.; Slade, G.; Fountain, M. Field Suppression of Spotted Wing Drosophila (SWD) (Drosophila suzukii Matsumura) Using the Sterile Insect Technique (SIT). Insects 2022, 13, 328. [Google Scholar] [CrossRef]
- Lanouette, G.; Brodeur, J.; Fournier, F.; Martel, V.; Vreysen, M.; Cáceres, C.; Firlej, A. The Sterile Insect Technique for the Management of the Spotted Wing Drosophila, Drosophila suzukii: Establishing the Optimum Irradiation Dose. PLoS ONE 2017, 12, e0180821. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Yadav, A.K.; Scott, M.J. Evaluation of Additional Drosophila suzukii Male-Only Strains Generated Through Remobilization of an FL19 Transgene. Front. Bioeng. Biotechnol. 2022, 10, 829620. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yamamoto, A.; Belikoff, E.J.; Berger, A.; Griffith, E.H.; Scott, M.J. A Conditional Female Lethal System for Genetic Suppression of the Global Fruit Crop Pest Drosophila suzukii. Pest Manag. Sci. 2021, 77, 4915–4922. [Google Scholar] [CrossRef]
- Krüger, A.P.; Schlesener, D.C.H.; Martins, L.N.; Wollmann, J.; Deprá, M.; Garcia, F.R.M. Effects of Irradiation Dose on Sterility Induction and Quality Parameters of Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2018, 111, 741–746. [Google Scholar] [CrossRef]
- Abdelhafiz, I.; Gerth, S.; Claussen, J.; Weule, M.; Hufnagel, E.; Vilcinskas, A.; Lee, K. Radioactivity and GMO-Free Sterile Insect Technology for the Sustainable Control of the Invasive Pest Drosophila suzukii. Adv. Biol. 2024, 8, 2400100. [Google Scholar] [CrossRef] [PubMed]
- Gard, B.; Panel, A.; Labbetoul, A.; Bosshard, N.; Xuereb, A.; Cariou, B.; Debelle, A.; Oliva, C.; Fellous, S. The Sterile Insect Technique Can Efficiently Reduce the Reproduction of the Spotted Wing Drosophila (Drosophila suzukii) in Strawberry. Acta Hortic. 2023, 1378, 237–244. [Google Scholar] [CrossRef]
- Bellamy, D.E.; Sisterson, M.S.; Walse, S.S. Quantifying Host Potentials: Indexing Postharvest Fresh Fruits for Spotted Wing Drosophila, Drosophila suzukii. PLoS ONE 2013, 8, e61227. [Google Scholar] [CrossRef]
- Jones, R.; Fountain, M.T.; Andreani, N.A.; Günther, C.S.; Goddard, M.R. The Relative Abundances of Yeasts Attractive to Drosophila suzukii Differ between Fruit Types and Are Greatest on Raspberries. Sci. Rep. 2022, 12, 10382. [Google Scholar] [CrossRef]
- Clymans, R.; Van Kerckvoorde, V.; Beliën, T.; Bylemans, D.; De Clercq, P. Marking Drosophila suzukii (Diptera: Drosophilidae) with Fluorescent Dusts. Insects 2020, 11, 152. [Google Scholar] [CrossRef]
- Sassù, F.; Bakhoum, T.; Bouyer, J.; Cáceres, C. Mating Competitiveness of Sterile Male Drosophila suzukii Under Different Atmosphere Conditions. In Proceedings of the 1st International Electronic Conference on Entomology, Virtual, 1–15 July 2021; p. 10494. [Google Scholar]
- Huang, J.; Gut, L.J. Impact of Background Fruit Odors on Attraction of Drosophila suzukii (Diptera: Drosophilidae) to Its Symbiotic Yeast. J. Insect Sci. 2021, 21, 4. [Google Scholar] [CrossRef]
- Revadi, S.; Lebreton, S.; Witzgall, P.; Anfora, G.; Dekker, T.; Becher, P. Sexual Behavior of Drosophila suzukii. Insects 2015, 6, 183–196. [Google Scholar] [CrossRef]
- Dreves, A.J.; Cave, A.; Lee, J.C.-T.; Oregon State University. A Detailed Guide for Testing Fruit for the Presence of Spotted Wing Drosophila (SWD) Larvae; Extension Service: Corvallis, OR, USA, 2014. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R package version 1.10.7. In CRAN: Contributed Packages 2025; Vienna University of Technology: Vienna, Austria, 2025. [Google Scholar] [CrossRef]
- Kosmidis, I.; Firth, D. Jeffreys-Prior Penalty, Finiteness and Shrinkage in Binomial-Response Generalized Linear Models. Biometrika 2021, 108, 71–82. [Google Scholar] [CrossRef]
- Rix, R.R.; Cutler, G.C. Review of Molecular and Biochemical Responses during Stress Induced Stimulation and Hormesis in Insects. Sci. Total Environ. 2022, 827, 154085. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, H.; Li, J.; Pan, D.; Liu, P.; Hu, H. Effects of Irradiated Sterile Male and Mating Sequence on the Fertility of Drosophila suzukii (Diptera: Drosophilidae). J. Insect Sci. 2022, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Lanouette, G.; Brodeur, J.; Fournier, F.; Martel, V.; Firlej, A. Effect of Irradiation on the Mating Capacity and Competitiveness of Drosophila suzukii (Diptera: Drosophilidae) for the Development of the Sterile Insect Technique. Can. Entomol. 2020, 152, 563–574. [Google Scholar] [CrossRef]
- Shaw, B.; Hemer, S.; Cannon, M.F.L.; Rogai, F.; Fountain, M.T. Insecticide Control of Drosophila suzukii in Commercial Sweet Cherry Crops under Cladding. Insects 2019, 10, 196. [Google Scholar] [CrossRef]
- Noble, R.; Walker, A.; Whitfield, C.; Harris, A.; Dobrovin-Pennington, A.; Fountain, M.T. Minimizing Insecticides for Control of Spotted Wing Drosophila (Drosophila suzukii) in Soft Fruit Using Bait Sprays. J. Appl. Entomol. 2021, 145, 977–985. [Google Scholar] [CrossRef]
- Noble, R.; Shaw, B.; Walker, A.; Whitfield, E.C.; Deakin, G.; Harris, A.; Dobrovin-Pennington, A.; Fountain, M.T. Control of Spotted Wing Drosophila (Drosophila suzukii) in Sweet Cherry and Raspberry Using Bait Sprays. J. Pest Sci. 2023, 96, 623–633. [Google Scholar] [CrossRef]
- Buonocore Biancheri, M.J.; Núñez-Campero, S.R.; Suárez, L.; Ponssa, M.D.; Kirschbaum, D.S.; Garcia, F.R.M.; Ovruski, S.M. Implications of the Niche Partitioning and Coexistence of Two Resident Parasitoids for Drosophila suzukii Management in Non-Crop Areas. Insects 2023, 14, 222. [Google Scholar] [CrossRef]
- Deans, C.; Hutchison, W.D. Propensity for Resistance Development in the Invasive Berry Pest, Spotted-wing Drosophila (Drosophila suzukii), under Laboratory Selection. Pest Manag. Sci. 2022, 78, 5203–5212. [Google Scholar] [CrossRef]
- Disi, J.O.; Sial, A.A. Laboratory Selection and Assessment of Resistance Risk in Drosophila suzukii (Diptera: Drosophilidae) to Spinosad and Malathion. Insects 2021, 12, 794. [Google Scholar] [CrossRef]
- Ganjisaffar, F.; Gress, B.E.; Demkovich, M.R.; Nicola, N.L.; Chiu, J.C.; Zalom, F.G. Spatio-Temporal Variation of Spinosad Susceptibility in Drosophila suzukii (Diptera: Drosophilidae), a Three-Year Study in California’s Monterey Bay Region. J. Econ. Entomol. 2022, 115, 972–980. [Google Scholar] [CrossRef]
- Gress, B.E.; Zalom, F.G. Identification and Risk Assessment of Spinosad Resistance in a California Population of Drosophila suzukii. Pest Manag. Sci. 2019, 75, 1270–1276. [Google Scholar] [CrossRef]
- Civolani, S.; Vaccari, G.; Caruso, S.; Finetti, L.; Bernacchia, G.; Chicca, M.; Cassanelli, S. Evaluation of Insecticide Efficacy and Insecticide Adaptive Response in Italian Populations of Drosophila suzukii. Bull. Insectology 2021, 74, 103–114. [Google Scholar]
- Schöneberg, T.; Lewis, M.T.; Burrack, H.J.; Grieshop, M.; Isaacs, R.; Rendon, D.; Rogers, M.; Rothwell, N.; Sial, A.A.; Walton, V.M.; et al. Cultural Control of Drosophila suzukii in Small Fruit—Current and Pending Tactics in the U.S. Insects 2021, 12, 172. [Google Scholar] [CrossRef]
- Saridaki, A.; Bourtzis, K. Wolbachia: More than Just a Bug in Insects Genitals. Curr. Opin. Microbiol. 2010, 13, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master Manipulators of Invertebrate Biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Nikolouli, K.; Colinet, H.; Renault, D.; Enriquez, T.; Mouton, L.; Gibert, P.; Sassu, F.; Cáceres, C.; Stauffer, C.; Pereira, R.; et al. Sterile Insect Technique and Wolbachia Symbiosis as Potential Tools for the Control of the Invasive Species Drosophila suzukii. J. Pest Sci. 2018, 91, 489–503. [Google Scholar] [CrossRef]
- Nikolouli, K.; Sassù, F.; Mouton, L.; Stauffer, C.; Bourtzis, K. Combining Sterile and Incompatible Insect Techniques for the Population Suppression of Drosophila suzukii. J. Pest Sci. 2020, 93, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Sassù, F.; Nikolouli, K.; Stauffer, C.; Bourtzis, K.; Cáceres, C. Sterile Insect Technique and Incompatible Insect Technique for the Integrated Drosophila suzukii Management. In Drosophila suzukii Management; Springer International Publishing: Cham, Switzerland, 2020; pp. 169–194. [Google Scholar]
| Early Season | Mid Season | Late Season | ||||
|---|---|---|---|---|---|---|
| Calendar Week | SIT-Treated | Control | SIT-Treated | Control | SIT-Treated | Control |
| 27 | X | X | ||||
| 28 | X | X | ||||
| 29 | X | X | X | |||
| 30 | X | X | X | X | ||
| 31 | X | X | X | X | X | |
| 32 | X | X | X | X | X | |
| 33 | X | X | X | X | ||
| 34 | X | X | X | X | ||
| 35 | X | X | ||||
| 36 | X | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemer, S.; Mateos-Fierro, Z.; Brough, B.; Deakin, G.; Moar, R.; Carvalho, J.P.; Randall, S.; Harris, A.; Klick, J.; Seagraves, M.P.; et al. Suppression of Spotted Wing Drosophila, Drosophila suzukii (Matsumura), in Raspberry Using the Sterile Insect Technique. Insects 2025, 16, 791. https://doi.org/10.3390/insects16080791
Hemer S, Mateos-Fierro Z, Brough B, Deakin G, Moar R, Carvalho JP, Randall S, Harris A, Klick J, Seagraves MP, et al. Suppression of Spotted Wing Drosophila, Drosophila suzukii (Matsumura), in Raspberry Using the Sterile Insect Technique. Insects. 2025; 16(8):791. https://doi.org/10.3390/insects16080791
Chicago/Turabian StyleHemer, Sebastian, Zeus Mateos-Fierro, Benjamin Brough, Greg Deakin, Robert Moar, Jessica P. Carvalho, Sophie Randall, Adrian Harris, Jimmy Klick, Michael P. Seagraves, and et al. 2025. "Suppression of Spotted Wing Drosophila, Drosophila suzukii (Matsumura), in Raspberry Using the Sterile Insect Technique" Insects 16, no. 8: 791. https://doi.org/10.3390/insects16080791
APA StyleHemer, S., Mateos-Fierro, Z., Brough, B., Deakin, G., Moar, R., Carvalho, J. P., Randall, S., Harris, A., Klick, J., Seagraves, M. P., Slade, G., Fountain, M. T., & Homem, R. A. (2025). Suppression of Spotted Wing Drosophila, Drosophila suzukii (Matsumura), in Raspberry Using the Sterile Insect Technique. Insects, 16(8), 791. https://doi.org/10.3390/insects16080791







