The Journey of the Bacterial Symbiont Through the Olive Fruit Fly: Lessons Learned and Open Questions
Simple Summary
Abstract
1. Introduction
2. Biology of Bactrocera oleae–Ca. Erwinia dacicola Interaction
2.1. The Symbiont Ca. Erwinia dacicola
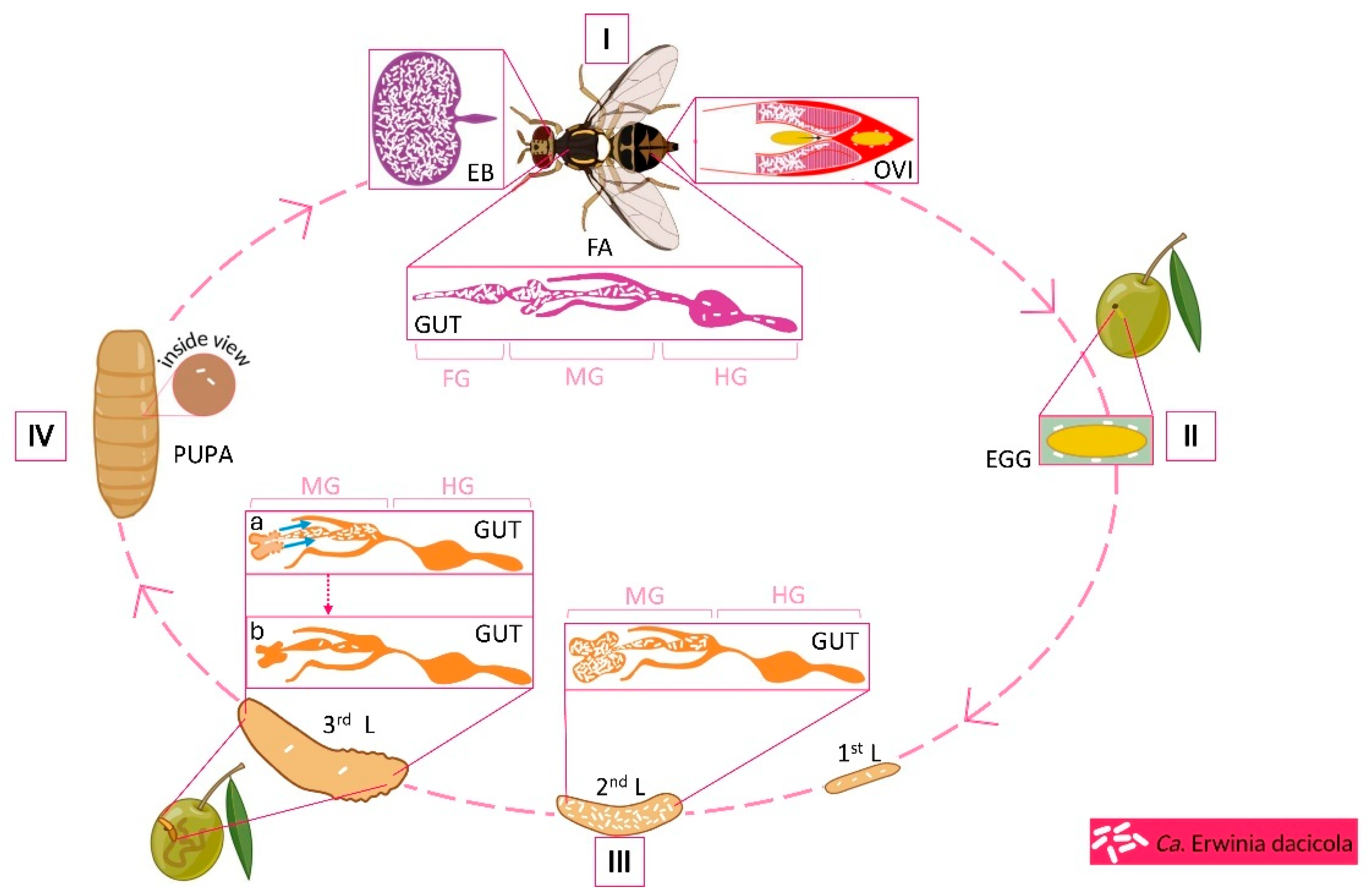
2.2. Abundance of Bacteria During the Life Cycle
2.3. The Role of Ca. E. Dacicola in the Adult and Larval Stages of the Olive Fly
2.4. Association of the Symbiont with the Host
2.4.1. Larval Stage
2.4.2. Pupal and Adult Stages
2.5. Vertical Transmission of the Bacteria
2.6. Genomic and Transcriptomic Analysis of Host–Symbiont Interaction
2.6.1. Genomic and Transcriptomic Analyses of B. oleae
2.6.2. Genomic and Transcriptomic Analyses of Ca. E. Dacicola
Genome Sequencing of Ca. E. Dacicola Reveals Metabolic Function Complementing Host Needs
Transcriptomic Comparison of Ca. E. Dacicola Developing in Unripe and Ripe Olives
3. Targeting the Symbiont for Olive Fly Control
3.1. Antisymbiotic Approaches–Laboratory Experiments
| Compounds and Treatment | Stage and Tissue Treated | Stage and Tissue Tested | Method for Determining Bacterial Abundance | Results | Comments | References |
|---|---|---|---|---|---|---|
| Antibiotic (100–200 µg mL−1 Piperacillin) Addition to food | ♀ Adults | Adult: dissected esophageal bulbs | Epifluorescence microscope Statistical analysis | LD | [29] | |
| Antibiotic (200 µg mL−1 Piperacillin) Addition to food | ♀ Adults | 3rd instar larvae: gut | HTS Bacterial counts under microscope | LD | Total bacterial community in the gut counted | [8] |
| Propolis 20% Copper 5% Copper Addition to food | Adults Eggs | Adult: dissected esophageal bulbs Eggs | DNA extraction PCR and DGGE analyses RT-qPCR Bioinformatics and statistical analysis | Bulbs: SD (copper compounds) SI (propolis) Eggs: NC | [48] | |
| Propionic acid solution (0.3% PA) Mixture of sodium hypochlorite and Triton X (1:1 SHTX) Soaking | Eggs | Eggs: surface and rinse solution | DNA extraction and DGGE Stereomicroscope RT-q-PCR Scanning electron microscopy | SD (All treatments) | Bacteria are lost even when eggs are washed with water | [43] |
| Antibiotic (0.08% Streptomycin) Addition to food | Adults | Adult: dissected guts | DNA extraction and 16S rRNA gene amplicon library Sequencing Bioinformatics and statistical analysis | NC | [9] | |
| Copper oxychloride (0.5%, 0.1%, and 0.02% w/w) Fungal metabolite Viridiol (0.5% and 0.1% w/w) Negative control: antibiotic (Piperacillin) Addition to food | Adults | Adult: head and abdomen | DNA extraction and qPCR Statistical analysis | Heads: All treatments (≠CO 0.02%): SD CO 0.02%: NC Abdomens: All treatments (≠CO 0.02% and Vi 0.1%): SD CO 0.02% and Vi 0.1%: NC | All treatments (≠ antibiotic and CO 0.02%): LD (total bacterial community) | [46] |
| Copper oxychloride (3.75%) Dodine (52.9%) Tannins (0.13%) Flavonoids (2% propolis) Micronutrient EC fertilizer [Cu (2%) + Zn (4%) + citric acid (23.8%)] Sodium hypochlorite (NaClO 1%) Soaking | Eggs Infested olives | Larvae | DNA extraction and qPCR Statistical analysis | Egg treatment: LD: NaClO SD: All other treatments Olive treatment: SD/NC: All treatments | Eggs exposed directly and indirectly | [47] |
| Copper nanoparticles (Cu-NPs) Copper oxide nanoparticles (CuO-NPs) Ethylenedia-minetetraacetic acid (EDTA) Copper hydroxide (Cu (OH)2) Deltamethrin (Decis 2.5 EC) Addition to food | Adults | Adult: thorax–head and abdomen | Scanning electron microscopy DNA extraction and qPCR Statistical analysis | SD/NC: All treatments | [49] | |
| 6-pentyl-α- pyrone 0.5%, 0.1% and 0.02% p/p (6PP) Harzianic acid 0.5%, 0.1% and 0.02% p/p (HA) Positive controls: A) Piperacillin (100 µg mL−1) B) Copper oxychloride (0.5%, 0.1% and 0.02% p/p) Addition to food | Adults | Adult: dissected esophageal bulbs | Cloning (E. coli vectors) qPCR Statistical analysis | LD: Piperacillin, SD:0.5% HA and 0.5% Cu SI: 0.1% Cu NC: 0.02% HA and 0.02% 6PP | [28] |
3.2. Disruption of Symbiosis in Other Insects
3.3. Biotechnology Approaches for Targeting the Symbiont
4. Discussion
- What is the critical function of the symbiont in the larval stage? The essential role of Ca. E. dacicola in the larvae developing in unripe olives is not yet clarified. One possibility is the synthesis of amino acids by the bacteria, which are made available to the host by the digestion of the bacteria, thus overcoming the nutritional restriction by oleuropein. This notion is supported by reports that lysis of the bacteria takes place in the gastric caeca and that genes encoding digestive proteases are differentially expressed in larvae harboring the bacterium compared to aposymbiotic samples [26]. Alternatively, the bacterium may directly contribute to the detoxification of oleuropein in larvae, thus increasing the availability of the fruit’s protein. Other possibilities include providing precursors for molecules secreted by the larvae, which ultimately deactivate oleuropein. Symbiotic bacteria of many insects play a direct role in the defense against plant toxins, utilizing a multitude of pathways (for a review, see [58]), and this aspect could be investigated for Ca. E. dacicola using, as a starting point, the genome sequences. Metabolites in the caeca could also be directly identified by metabolomic analyses.
- What is the role of the symbiont in the adult? The current evidence points to the function of the symbiont in supplementing the poor nutrition of the fly, especially the lack of a nitrogen source [29,30]. This is supported by the fact that the bacteria eventually undergo lysis as they transit to the hind section of the midgut [10]. Other Tephritids share similar gut morphologies with B. oleae and also cultivate a large and metabolically active mass of bacteria, which are digested [59]. Adult olive flies and other Tephritids may be regarded as specialized to gain nutrition by digesting bacteria propagating internally; thus, they do not rely on external microbes for gaining nutrition. Similar mechanisms can be seen in the bacteria-directed digestive physiology of dipterans, such as Drosophila and other flies, which are evolutionary specialized for obtaining nutrition by digesting microbes; although, in this case, the microbes are obtained from the environment [60,61].The specific function of the esophageal bulb, which is also found in other Tephritids, is unknown. At least in Ceratititis capitata and Rhagoletis pomonella, this organ harbors bacteria [62,63]. In the olive fly, it maintains the association with the symbiont by enclosing the symbiont, and in addition, it may keep harmful microbes outnumbered. Crucial knowledge that is lacking is the specific characteristics of the esophageal bulb that allow bacterial reproduction. Proteomic or transcriptomic analyses of this organ may suggest specific proteins that are crucial for the symbiont and that may constitute targets for interventions.
- What is the mechanism for the vertical transmission of the symbiont? In the laboratory, transmission to the next generation is strictly dependent upon maternal transfer of the symbiont [41], although trophallaxis may take place [39,40]. This should be investigated under simulated field conditions to establish whether the symbiont is stably transmitted for several generations if acquired by trophallaxis. The detailed mechanism for vertical transmission of the symbiont, for example, whether special interactions are necessary for the bacterium to adhere to the egg during oviposition, remains to be clarified. Vertical transmission has been suggested as a target for intervention [51], although complicating this issue is the fact that the process largely takes place inside the ovipositor as it penetrates the olive mesocarp. However, promising results have been reported from the treatment of the olive fruit, and new formulations may lead to improvement of penetration of the fruit [47].The exact mechanism of the uptake of the bacteria in the young larva is not completely resolved, as well as how they reach the gastric caeca. Imaging carefully staged eggs could provide more information. Fluorescently labeled bacteria would be ideal for identifying their position and proliferation, and this method was successfully used for the establishment of a synthetic insect–bacteria mutualism between the grain weevil and a free-living relative of the symbiont Sodalis pierantonius [64]. However, this would require the generation of transgenic bacteria, which would be challenging, given that the symbiont cannot be cultivated. Alternatively, an antibody recognizing the bacterial surface could be useful for this purpose.
- How can Ca. E. dacicola be cultivated? It will be clear from this review that one important hindrance in studying symbiosis in B. oleae is the fact that the symbiont cannot be cultivated using standard media, as well as more specialized media for bacterial culture [7,12]. To be able to test growth in a systematic fashion, analysis of the genome may provide clues for critical requirements of the bacterium. Comparisons with genomes of relatives, for which complete genomes exist, within the genus could be one starting point. One drawback is that the genome is not yet complete for Ca. E. dacicola, and the lack of specific genes may be because the specific genome sequence is missing. Thus, completion of the sequence would be worthwhile. Alternatively, if a limited list of candidate genes is put together, based on the present genome sequences, the presence of the specific genes could be determined directly via PCR.
- Is dysbiosis-based control of B. oleae possible in the field? Ca. E. dacicola is present through the life cycle of the host, and possibly any of the stages could be targeted to eliminate or reduce the symbiont. The egg is deposited inside the olive mesocarp, where larval development takes place. As mentioned above, the application of antibacterial compounds on the olive has shown a minor reduction in bacterial abundance. Thus, such a strategy is theoretically feasible, although it is currently not a realistic option due to the difficulty in implementing this in the field. The pupae reside in the Earth, and the olive groves could possibly be sprayed with agents that have the ability to penetrate the pupal case. A possible approach could be a combination of entomopathogenic fungi targeting the host with copper compounds to kill the bacteria. This would have the advantage that the bacterial number in this stage is very low, and it may be easier to achieve complete elimination, but the method would need careful consideration for its practical implementation. The adult stage is perhaps the easiest to target with baits containing sugar to which antimicrobials could be added. After uptake by the fly, the agents should reach the EB and gut, where the bacteria reside. This is a theoretically simple method, but there are several unknown factors that may confound such a solution. First, one would need to verify that indeed antimicrobials will reach the organs of interest and that their activity is not diminished by the environment in the digestive system. It would also be preferable for the agents to remain in the esophagus, bulb, and midgut and not be transferred throughout the fly. Detoxification mechanisms should also be taken into consideration. For all approaches, the choice of agent would also be critical, and at present, it is limited to conventional antibiotics used in humans and animals. While, as discussed above, copper has been suggested, it has not yet been shown to be able to eliminate the bacterial symbiont. However, it is possible that improved formulations could circumvent this obstacle, and a combination with other agents could improve efficacy. Although challenging, the development of new and safe agents targeting Ca. E. dacicola should be a priority for research on dysbiosis.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roots, S. The Olive Fruit Fly: A Persistent Pest in a Changing Climate. Available online: https://www.oliveoiltimes.com/basics/the-olive-fruit-fly-a-persistent-pest-in-a-changing-climate/125055 (accessed on 18 July 2025).
- European and Mediterranean Plant Protection Organization (EPPO). Available online: https://gd.eppo.int/taxon/DACUOL/ (accessed on 18 July 2025).
- Martinez-Sañudo, I.; Perotti, M.A.; Carofano, I.; Santoiemma, G.; Marri, L.; Mazzon, L. The biogeographic patterns of the olive fly and its primary symbiont Erwinia dacicola across the distribution area of the olive tree. Sci. Rep. 2024, 14, 22483. [Google Scholar] [CrossRef]
- Daane, K.M.; Johnson, M.W. Olive Fruit Fly: Managing an Ancient Pest in Modern Times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef]
- Kampouraki, A.; Stavrakaki, M.; Karataraki, A.; Katsikogiannis, G.; Pitika, E.; Varikou, K.; Vlachaki, A.; Chrysargyris, A.; Malandraki, E.; Sidiropoulos, N.; et al. Recent evolution and operational impact of insecticide resistance in olive fruit fly Bactrocera oleae populations from Greece. J. Pest. Sci. 2018, 91, 1429–1439. [Google Scholar] [CrossRef]
- Genç, H.; Nation, J.L. Maintaining Bactrocera oleae (Gmelin.) (Diptera: Tephritidae) colony on its natural host in the laboratory. J. Pest. Sci. 2008, 81, 167–174. [Google Scholar] [CrossRef]
- Capuzzo, C.; Firrao, G.; Mazzon, L.; Squartini, A.; Girolami, V. ‘Candidatus Erwinia dacicola’, a coevolved symbiotic bacterium of the olive fly Bactrocera oleae (Gmelin). Int. J. Syst. Evol. Microbiol. 2005, 55, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria enable olive fly larvae to overcome host defences. R. Soc. Open Sci. 2015, 2, 150170. [Google Scholar] [CrossRef]
- Koskinioti, P.; Ras, E.; Augustinos, A.A.; Tsiamis, G.; Beukeboom, L.W.; Caceres, C.; Bourtzis, K. The effects of geographic origin and antibiotic treatment on the gut symbiotic communities of Bactrocera oleae populations. Entomol. Exp. Appl. 2019, 167, 197–208. [Google Scholar] [CrossRef]
- Petri, L. Ricerche Sopra i Batteri Intestinali Della Mosca Olearia; Tipografia Nazionale Giovanni Bertero Ec: Rome, Italy, 1909; pp. 1–129. [Google Scholar]
- Girolami, V.; Cavalloro, R. Aspects of Bacterial Symbiosis of Dacus-Oleae-Gmelin in Nature and Rearing of Laboratory. Ann. Soc. Entomol. Fr. 1972, 8, 561. [Google Scholar] [CrossRef]
- Estes, A.M.; Hearn, D.J.; Bronstein, J.L.; Pierson, E.A. The olive fly endosymbiont, “Candidatus Erwinia dacicola,” switches from an intracellular existence to an extracellular existence during host insect development. Appl. Environ. Microbiol. 2009, 75, 7097–7106. [Google Scholar] [CrossRef] [PubMed]
- da Costa, L.T.; Powell, C.; van Noort, S.; Costa, C.; Sinno, M.; Caleca, V.; Rhode, C.; Kennedy, R.J.; van Staden, M.; van Asch, B. The complete mitochondrial genome of Bactrocera biguttula (Bezzi) (Diptera: Tephritidae) and phylogenetic relationships with other Dacini. Int. J. Biol. Macromol. 2019, 126, 130–140. [Google Scholar] [CrossRef]
- Mazzon, L.; Martinez-Sañudo, I.; Simonato, M.; Girolami, V. Conziderazione filogenetiche e biogeografiche su “Candidatus Erwinia dacidol” e pospettive per l’allevamento di Bactrocera oleae (Rossi). Atti Della Accad. Naz. Ital. Entomol. Rend. 2016, Anno LXIV, 85–91. [Google Scholar]
- Savio, C.; Mazzon, L.; Martinez-Sanudo, I.; Simonato, M.; Squartini, A.; Girolami, V. Evidence of two lineages of the symbiont ‘Candidatus Erwinia dacicola’ in Italian populations of Bactrocera oleae (Rossi) based on 16S rRNA gene sequences. Int. J. Syst. Evol. Microbiol. 2012, 62, 179–187. [Google Scholar] [CrossRef]
- Nobre, T. Olive fruit fly and its obligate symbiont Erwinia dacicola: Two new symbiont haplotypes in the Mediterranean basin. PLoS ONE 2021, 16, e0256284. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.M.; Hearn, D.J.; Agrawal, S.; Pierson, E.A.; Dunning Hotopp, J.C. Comparative genomics of the Erwinia and Enterobacter olive fly endosymbionts. Sci. Rep. 2018, 8, 15936. [Google Scholar] [CrossRef]
- Estes, A.M.; Hearn, D.J.; Nadendla, S.; Pierson, E.A.; Dunning Hotopp, J.C. Draft Genome Sequence of Erwinia dacicola, a Dominant Endosymbiont of Olive Flies. Microbiol. Resour. Announc. 2018, 7, e01067-18. [Google Scholar] [CrossRef] [PubMed]
- Blow, F.; Gioti, A.; Starns, D.; Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Vontas, J.; Darby, A.C. Draft Genome Sequence of the Bactrocera oleae Symbiont “Candidatus Erwinia dacicola”. Genome Announc. 2016, 4, e00896-16. [Google Scholar] [CrossRef]
- Estes, A.M.; Nestel, D.; Belcari, A.; Jessup, A.; Rempoulakis, P.; Economopoulos, A.P. A basis for the renewal of sterile insect technique for the olive fly, Bactrocera oleae (Rossi). J. Appl. Entomol. 2012, 136, 1–16. [Google Scholar] [CrossRef]
- Bigiotti, G.; Sacchetti, P.; Pastorelli, R.; Lauzon, C.R.; Belcari, A. Bacterial symbiosis in Bactrocera oleae, an Achilles’ heel for its pest control. Insect Sci. 2021, 28, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Nobre, T. Symbiosis in Sustainable Agriculture: Can Olive Fruit Fly Bacterial Microbiome Be Useful in Pest Management? Microorganisms 2019, 7, 238. [Google Scholar] [CrossRef] [PubMed]
- Blow, F.; Gioti, A.; Goodhead, I.B.; Kalyva, M.; Kampouraki, A.; Vontas, J.; Darby, A.C. Functional Genomics of a Symbiotic Community: Shared Traits in the Olive Fruit Fly Gut Microbiota. Genome Biol. Evol. 2020, 12, 3778–3791. [Google Scholar] [CrossRef] [PubMed]
- De Cock, M.; Virgilio, M.; Vandamme, P.; Bourtzis, K.; De Meyer, M.; Willems, A. Comparative Microbiomics of Tephritid Frugivorous Pests (Diptera: Tephritidae) From the Field: A Tale of High Variability Across and Within Species. Front. Microbiol. 2020, 11, 1890. [Google Scholar] [CrossRef]
- Campos, C.; Gomes, L.; Rei, F.T.; Nobre, T. Olive Fruit Fly Symbiont Population: Impact of Metamorphosis. Front. Microbiol. 2022, 13, 868458. [Google Scholar] [CrossRef]
- Siden-Kiamos, I.; Koidou, V.; Livadaras, I.; Skoufa, E.; Papadogiorgaki, S.; Papadakis, S.; Chalepakis, G.; Ioannidis, P.; Vontas, J. Dynamic interactions between the symbiont Candidatus Erwinia dacicola and its olive fruit fly host Bactrocera oleae. Insect Biochem. Mol. Biol. 2022, 146, 103793. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.M.; Hearn, D.J.; Burrack, H.J.; Rempoulakis, P.; Pierson, E.A. Prevalence of Candidatus Erwinia dacicola in wild and laboratory olive fruit fly populations and across developmental stages. Environ. Entomol. 2012, 41, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Jesu, G.; Vinale, F.; Lorito, M.; Laudonia, S. Trichoderma metabolites 6-pentyl-α-pyrone and harzianic acid affect the reproduction and microbiome of Bactrocera oleae. J. Pest. Sci. 2024, 98, 389–398. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Aharon, Y.; Jurkevitch, E.; Yuval, B. Give us the tools and we will do the job: Symbiotic bacteria affect olive fly fitness in a diet-dependent fashion. Proc. Biol. Sci. 2010, 277, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria enable olive flies (Bactrocera oleae) to exploit intractable sources of nitrogen. J. Evol. Biol. 2014, 27, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- Hagen, K.S. Dependence of Olive Fly Dacus Oleae Larvae on Symbiosis with Pseudomonas Savastanoi for Utilization of Olive. Nature 1966, 209, 423. [Google Scholar] [CrossRef]
- Fytizas, E.; Tzanakakis, M.E. Some Effects of Streptomycin, When Added to the Adult Food, on the Adults of Dacus oleae (Diptera: Tephritidae) and Their Progeny. Ann. Entomol. Soc. Am. 1966, 59, 269–273. [Google Scholar] [CrossRef]
- Lambrou, P.D.; Tzanakakis, M.E. Inhibition of Larval Growth of Dacus-Oleae (Diptera-Tephritidae) by Streptomycin 2. Effect of Treating Parents. Entomol. Exp. Appl. 1978, 23, 163–170. [Google Scholar] [CrossRef]
- Tzanakakis, M.E.; Stavrinides, A.S. Inhibition of Development of Larvae of Olive Fruit-Fly, Dacus-Oleae (Diptera—Tephritidae), in Olives Treated with Streptomycin. Entomol. Exp. Appl. 1973, 16, 39–47. [Google Scholar] [CrossRef]
- Tzanakakis, Μ.Ε.; Prophetou, D.A.; Vassiliou, G.N.; Papadopoulos, J.J. Inhibition of larval growth of Dαcus oleαe by topical application of streptomycin to olives. Entomol. Hell. 1983, 1, 65–70. [Google Scholar] [CrossRef][Green Version]
- Spadafora, A.; Mazzuca, S.; Chiappetta, F.F.; Parise, A.; Perri, E.; Innocenti, A.M. Oleuropein-Specific-β-Glucosidase Activity Marks the Early Response of Olive Fruits (Olea europaea) to Mimed Insect Attack. Agric. Sci. China 2008, 7, 703–712. [Google Scholar] [CrossRef]
- Mazzini, M.; Vita, G. Identificazione submicroscopica del meccanismo di trasmissione del batterio simbionte in Dacus oleae (Gmelin) (Diptera, Trypetidae). Redia 1981, 64, 277–301. [Google Scholar]
- Salem, H.; Florez, L.; Gerardo, N.; Kaltenpoth, M. An out-of-body experience: The extracellular dimension for the transmission of mutualistic bacteria in insects. Proc. R. Soc. B—Biol. Sci. 2015, 282, 20142957. [Google Scholar] [CrossRef]
- Estes, A.M.; Segura, D.F.; Jessup, A.; Wornoayporn, V.; Pierson, E.A. Effect of the symbiont Candidatus Erwinia dacicola on mating success of the olive fly Bactrocera oleae (Diptera: Tephritidae). Int. J. Trop. Insect Sc. 2014, 34, S123–S131. [Google Scholar] [CrossRef]
- Bigiotti, G.; Pastorelli, R.; Guidi, R.; Belcari, A.; Sacchetti, P. Horizontal transfer and finalization of a reliable detection method for the olive fruit fly endosymbiont, Candidatus Erwinia dacicola. BMC Biotechnol. 2019, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Livadaras, I.; Koidou, V.; Pitsili, E.; Moustaka, J.; Vontas, J.; Siden-Kiamos, I. Stably inherited transfer of the bacterial symbiont Candidatus Erwinia dacicola from wild olive fruit flies Bactrocera oleae to a laboratory strain. Bull. Entomol. Res. 2021, 111, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, P.; Granchietti, A.; Landini, S.; Viti, C.; Giovannetti, L.; Belcari, A. Relationships between the olive fly and bacteria. J. Appl. Entomol. 2008, 132, 682–689. [Google Scholar] [CrossRef]
- Sacchetti, P.; Pastorelli, R.; Bigiotti, G.; Guidi, R.; Ruschioni, S.; Viti, C.; Belcari, A. Olive fruit fly rearing procedures affect the vertical transmission of the bacterial symbiont Candidatus Erwinia dacicola. BMC Biotechnol. 2019, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Bayega, A.; Djambazian, H.; Tsoumani, K.T.; Gregoriou, M.E.; Sagri, E.; Drosopoulou, E.; Mavragani-Tsipidou, P.; Giorda, K.; Tsiamis, G.; Bourtzis, K.; et al. De novo assembly of the olive fruit fly (Bactrocera oleae) genome with linked-reads and long-read technologies minimizes gaps and provides exceptional Y chromosome assembly. BMC Genom. 2020, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Pavlidi, N.; Gioti, A.; Wybouw, N.; Dermauw, W.; Ben-Yosef, M.; Yuval, B.; Jurkevich, E.; Kampouraki, A.; Van Leeuwen, T.; Vontas, J. Transcriptomic responses of the olive fruit fly Bactrocera oleae and its symbiont Candidatus Erwinia dacicola to olive feeding. Sci. Rep. 2017, 7, 42633. [Google Scholar] [CrossRef] [PubMed]
- Sinno, M.; Bézier, A.; Vinale, F.; Giron, D.; Laudonia, S.; Garonna, A.P.; Pennacchio, F. Symbiosis disruption in the olive fruit fly, Bactrocera oleae (Rossi), as a potential tool for sustainable control. Pest Manag. Sci. 2020, 76, 3199–3207. [Google Scholar] [CrossRef]
- Perin, C.; Martinez-Sañudo, I.; Carofano, I.; Mori, N.; Santoiemma, G.; Squartini, A.; Tondello, A.; Mazzon, L. Impairing the development of an olive fly pest by targeting its symbiotic bacteria in egg-infested fruits. Entomol. Gen. 2023, 43, 831–838. [Google Scholar] [CrossRef]
- Bigiotti, G.; Pastorelli, R.; Belcari, A.; Sacchetti, P. Symbiosis interruption in the olive fly: Effect of copper and propolis on Erwinia dacicola. J. Appl. Entomol. 2019, 143, 357–364. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Varikou, K.; Kavroulakis, N.; Nikolakakis, A.; Dervisi, I.; Reppa, C.; Papadakis, S.; Holeva, M.C.; Chrysikopoulos, C. Copper nanoparticles interfere with insecticide sensitivity, fecundity and endosymbiont abundance in olive fruit fly Bactrocera oleae (Diptera: Tephritidae). Pest Manag. Sci. 2024, 80, 3640–3649. [Google Scholar] [CrossRef]
- Kaltenpoth, M.; Flórez, L.V.; Vigneron, A.; Dirksen, P.; Engl, T. Origin and function of beneficial bacterial symbioses in insects. Nat. Rev. Microbiol. 2025. [Google Scholar] [CrossRef]
- Gonella, E.; Alma, A. The Role of Symbiont-Targeted Strategies in the Management of Pentatomidae and Tephritidae Pests under an Integrated Vision. Agron. 2023, 13, 868. [Google Scholar] [CrossRef]
- Shigenobu, S.; Wilson, A.C.C. Genomic revelations of a mutualism: The pea aphid and its obligate bacterial symbiont. Cell Mol. Life Sci. 2011, 68, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Jing, X.F.; Luo, Y.; Douglas, A.E. Targeting symbiosis-related insect genes by RNAi in the pea aphid-symbiosis. Insect Biochem. Mol. Biol. 2018, 95, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.X.Y.; Shigenobu, S. In vivo interference of pea aphid endosymbiont gene by synthetic peptide nucleic acids. Sci. Rep. 2024, 14, 5378. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Oliver, S.V. Gene drives: An alternative approach to malaria control? Gene Ther. 2025, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Carballar-Lejarazú, R.; Dong, Y.; Pham, T.B.; Tushar, T.; Corder, R.M.; Mondal, A.; Sánchez, C.H.M.; Lee, H.-F.; Marshall, J.M.; Dimopoulos, G.; et al. Dual effector population modification gene-drive strains of the African malaria mosquitoes, Anopheles gambiae and Anopheles coluzzii. Proc. Natl. Acad. Sci. USA 2023, 120, e2221118120. [Google Scholar] [CrossRef] [PubMed]
- Hoermann, A.; Habtewold, T.; Selvaraj, P.; Del Corsano, G.; Capriotti, P.; Inghilterra, M.G.; Kebede, T.M.; Christophides, G.K.; Windbichler, N. Gene drive mosquitoes can aid malaria elimination by retarding Plasmodium sporogonic development. Sci. Adv. 2022, 8, eabo1733. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Tago, K.; Hayatsu, M.; Kikuchi, Y. Detoxifying symbiosis: Microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat. Prod. Rep. 2018, 35, 434–454. [Google Scholar] [CrossRef]
- Girolami, V. Mediterranean Fruit Fly Associated Bacteria: Transmission and Larval Survival; Springer: Berlin/Heidelberg, Germany, 1986; pp. 135–146. [Google Scholar]
- Lemos, F.J.A.; Terra, W.R. Digestion of Bacteria and the Role of Midgut Lysozyme in Some Insect Larvae. Comp. Biochem. Phys. B 1991, 100, 265–268. [Google Scholar] [CrossRef]
- Terra, W.; Ferreira, C. Biochemistry and Molecular Biology of Digestion. In Insect Molecular Biology and Biochemistry; Academic Press: Cambridge, MA, USA, 2012; pp. 365–418. [Google Scholar]
- Marchini, D.; Rosetto, M.; Dallai, R.; Marri, L. Bacteria associated with the oesophageal bulb of the medfly Ceratitis capitata (Diptera:Tephritidae). Curr. Microbiol. 2002, 44, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Ratner, S.S. Structure and Function of the Esophageal Bulb of the Apple Maggot Fly, Rhagoletis pomonella Walsh. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 1981. [Google Scholar]
- Su, Y.H.; Lin, H.C.; Teh, L.; Chevance, F.; James, I.; Mayfield, C.; Golic, K.G.; Gagnon, J.A.; Rog, O.; Dale, C. Rational engineering of a synthetic insect-bacterial mutualism. Curr. Biol. 2022, 32, 3925. [Google Scholar] [CrossRef]
- Rupawate, P.S.; Roylawar, P.; Khandagale, K.; Gawande, S.; Ade, A.B.; Jaiswal, D.K.; Borgave, S. Role of gut symbionts of insect pests: A novel target for insect-pest control. Front. Microbiol. 2023, 14, 1146390. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siden-Kiamos, I.; Pantidi, G.; Vontas, J. The Journey of the Bacterial Symbiont Through the Olive Fruit Fly: Lessons Learned and Open Questions. Insects 2025, 16, 789. https://doi.org/10.3390/insects16080789
Siden-Kiamos I, Pantidi G, Vontas J. The Journey of the Bacterial Symbiont Through the Olive Fruit Fly: Lessons Learned and Open Questions. Insects. 2025; 16(8):789. https://doi.org/10.3390/insects16080789
Chicago/Turabian StyleSiden-Kiamos, Inga, Georgia Pantidi, and John Vontas. 2025. "The Journey of the Bacterial Symbiont Through the Olive Fruit Fly: Lessons Learned and Open Questions" Insects 16, no. 8: 789. https://doi.org/10.3390/insects16080789
APA StyleSiden-Kiamos, I., Pantidi, G., & Vontas, J. (2025). The Journey of the Bacterial Symbiont Through the Olive Fruit Fly: Lessons Learned and Open Questions. Insects, 16(8), 789. https://doi.org/10.3390/insects16080789







