Flight Phenology of Spodoptera eridania (Stoll, 1781) (Lepidoptera: Noctuidae) in Its Native Range: A Baseline for Managing an Emerging Invasive Pest
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pheromone Trapping
2.2. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, I.B.D.; Paula-Moraes, S.V.; Beuzelin, J.M.; Hahn, D.A.; Perera, O.P.; Fraisse, C. Factors Affecting Population Dynamics of Helicoverpa zea (Lepidoptera: Noctuidae) in a Mixed Landscape with Bt Cotton and Peanut. Insects 2023, 14, 395. [Google Scholar] [CrossRef] [PubMed]
- Paula-Moraes, S.V.; Calixto, E.S.; Santos, A.A.; Reay-Jones, F.P.F.; Reisig, D.D.; Farhan, Y.; Smith, J.L.; Hutchison, W.D. Continental-Scale Migration Patterns and Origin of Helicoverpa zea (Lepidoptera: Noctuidae) Based on a Biogeochemical Marker. Environ. Entomol. 2024, 53, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Montezano, D.G.; Specht, A.; Sosa–Gómez, D.R.; Roque–Specht, V.F.; De Barros, N.M. Immature Stages of Spodoptera eridania (Lepidoptera: Noctuidae): Developmental Parameters and Host Plants. J. Insect Sci. 2014, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Montezano, D.; Specht, A. Spodoptera Eridania (Southern Armyworm). 2019. Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.44518 (accessed on 11 July 2025).
- Capinera, J.L. Handbook of Vegetable Pests; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-814488-6. [Google Scholar]
- Huseth, A.S.; Koch, R.L.; Reisig, D.; Davis, J.A.; Paula-Moraes, S.V.; Hodgson, E.W. Current Distribution and Population Persistence of Five Lepidopteran Pests in U.S. Soybean. J. Integr. Pest Manag. 2021, 12, 11. [Google Scholar] [CrossRef]
- EPPO. 2025 EPPO Global Database. Available online: https://gd.eppo.int/taxon/PRODER (accessed on 27 May 2025).
- Pogue, M.G. A World Revision of the Genus Spodoptera Guenée (Lepidoptera: Noctuidae); American Entomological Society: Philadelphia, PA, USA, 2002; Volume 43. [Google Scholar]
- Weinberg, J.; Ota, N.; Goergen, G.; Fagbohoun, J.R.; Tepa-Yotto, G.T.; Kriticos, D.J. Spodoptera eridania: Current and Emerging Crop Threats from Another Invasive, Pesticide-Resistant Moth. Entomol. Gen. 2022, 42, 701–712. [Google Scholar] [CrossRef]
- Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.; Jaques Miret, J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; Navas-Cortes, J.A.; et al. Pest Categorisation of Spodoptera eridania. EFSA J. 2020, 18, e05932. [Google Scholar] [CrossRef]
- Goergen, G.E. Southern Armyworm, a New Alien Invasive Pest Identified in West and Central Africa. Crop Prot. 2018, 112, 371–373. [Google Scholar]
- Gaikwad, S.M. First Report of Spodoptera eridania (Stoll) (Lepidoptera: Noctuidae) on Soybean [Glycine max (L.) Merrill] from Kolhapur, Maharashtra, India. J. Entomol. Zool. Stud. 2021, 9, 1419–1422. [Google Scholar] [CrossRef]
- Tepa-Yotto, G.T.; Gouwakinnou, G.N.; Fagbohoun, J.R.; Tamò, M.; Sæthre, M. Horizon Scanning to Assess the Bioclimatic Potential for the Alien Species Spodoptera eridania and Its Parasitoids after Pest Detection in West and Central Africa. Pest Manag. Sci. 2021, 77, 4437–4446. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Qi, Y.; Li, M.; Yang, N.; Guo, J.; Xian, X.; Liu, W. Global Potential Geographical Distribution of the Southern Armyworm (Spodoptera eridania) under Climate Change. Biology 2023, 12, 1040. [Google Scholar] [CrossRef]
- Bernardi, O.; Sorgatto, R.J.; Barbosa, A.D.; Domingues, F.A.; Dourado, P.M.; Carvalho, R.A.; Martinelli, S.; Head, G.P.; Omoto, C. Low Susceptibility of Spodoptera cosmioides, Spodoptera eridania and Spodoptera Frugiperda (Lepidoptera: Noctuidae) to Genetically-Modified Soybean Expressing Cry1Ac Protein. Crop Prot. 2014, 58, 33–40. [Google Scholar] [CrossRef]
- Machado, E.P.; Dos S Rodrigues Junior, G.L.; Somavilla, J.C.; Führ, F.M.; Zago, S.L.; Marques, L.H.; Santos, A.C.; Nowatzki, T.; Dahmer, M.L.; Omoto, C.; et al. Survival and Development of Spodoptera eridania, Spodoptera cosmioides and Spodoptera albula (Lepidoptera: Noctuidae) on Genetically-modified Soybean Expressing Cry1Ac and Cry1F Proteins. Pest Manag. Sci. 2020, 76, 4029–4035. [Google Scholar] [CrossRef]
- Sampaio, F.; Krechemer, F.S.; Marchioro, C.A. The Hotter the Better? Climate Change and Voltinism of Spodoptera eridania Estimated with Different Methods. J. Therm. Biol. 2021, 98, 102946. [Google Scholar] [CrossRef]
- Sampaio, F.; Batista, M.M.; Marchioro, C.A. Temperature-dependent Reproduction of Spodoptera eridania: Developing an Oviposition Model for a Novel Invasive Species. Pest Manag. Sci. 2024, 80, 1118–1125. [Google Scholar] [CrossRef]
- Trumble, J.T.; Baker, T.C. Flight Phenology and Pheromone Trapping of Spodoptera Exigua (Hübner) (Lepidoptera: Noctuidae) in Southern Coastal California. Environ. Entomol. 1984, 13, 1278–1282. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 11 July 2025).
- Agostinelli, C.; Lund, U. R Package “Circular”: Circular Statistics (Version 0.5-0). 2023. Available online: https://CRAN.R-project.org/package=circular (accessed on 11 July 2025).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publishing: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.3.0. 2020. Available online: https://cran.r-project.org/package=emmeans (accessed on 11 July 2025).
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2022. Available online: https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html (accessed on 11 July 2025).
- Morellato, L.P.C.; Alberti, L.F.; Hudson, I.L. Applications of Circular Statistics in Plant Phenology: A Case Studies Approach. In Phenological Research; Hudson, I.L., Keatley, M.R., Eds.; Springer: Heidelberg, Germany, 2010; pp. 339–359. ISBN 978-90-481-3335-2. [Google Scholar]
- Novaes, L.R.; Calixto, E.S.; de Oliveira, M.L.; Alves-de-Lima, L.; de Almeida, O.; Torezan-Silingardi, H.M. Environmental Variables Drive Phenological Events of Anemocoric Plants and Enhance Diaspore Dispersal Potential: A New Wind-Based Approach. Sci. Total. Environ. 2020, 730, 139039. [Google Scholar] [CrossRef] [PubMed]
- Calixto, E.S.; Novaes, L.R.; dos Santos, D.F.B.; Lange, D.; Moreira, X.; Del-Claro, K. Climate Seasonality Drives Ant–Plant–Herbivore Interactions via Plant Phenology in an Extrafloral Nectary-Bearing Plant Community. J. Ecol. 2021, 109, 639–651. [Google Scholar] [CrossRef]
- Montezano, D.G.; Sosa-Gómez, D.; Specht, A.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T. Host Plants of Spodoptera Frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Luginbill, P. The Fall Army Worm; US Department of Agriculture: Washington, DC, USA, 1928. [Google Scholar]
- Nagoshi, R.N.; Meagher, R.L. Review of Fall Armyworm (Lepidoptera: Noctuidae) Genetic Complexity and Migration. Fla. Entomol. 2008, 91, 546–554. [Google Scholar] [CrossRef]
- Santos, I.B.; Paula-Moraes, S.V.; Beuzelin, J.; Hahn, D.; Fraisse, C. Phenology of Flight and Number of Generations of Helicoverpa zea Boddie (Lepidoptera: Noctuidae) in a Migratory Region. In Proceedings of the Entomological Society of America (ESA 2022), Vancouver, BC, Canada, 13–16 November 2022. [Google Scholar]
- Lawton, D.; Huseth, A.S.; Kennedy, G.G.; Morey, A.C.; Hutchison, W.D.; Reisig, D.D.; Dorman, S.J.; Dillard, D.; Venette, R.C.; Groves, R.L.; et al. Pest Population Dynamics Are Related to a Continental Overwintering Gradient. Proc. Natl. Acad. Sci. USA 2022, 119, e2203230119. [Google Scholar] [CrossRef]
- Tessnow, A.E.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J. Revisiting Fall Armyworm Population Movement in the United States and Canada. Front. Insect Sci. 2023, 3, 1104793. [Google Scholar] [CrossRef]
- Gould, F.; Blair, N.; Reid, M.; Rennie, T.; Lopez, J.; Micinski, S. Bacillus thuringiensis—Toxin Resistance Management: Stable Isotope Assessment of Alternate Host Use by Helicoverpa zea. Proc. Natl. Acad. Sci. USA 2002, 99, 16581–16586. [Google Scholar] [CrossRef]
- Westbrook, J.K. Noctuid Migration in Texas within the Nocturnal Aeroecological Boundary Layer. Integr. Comp. Biol. 2008, 48, 99–106. [Google Scholar] [CrossRef]
- Westbrook, J.K.; López, J.D. Long-Distance Migration in Helicoverpa zea: What We Know and Need to Know. Southwest. Entomol. 2010, 35, 355–360. [Google Scholar] [CrossRef]
- Calixto, E.S.; Paula-Moraes, S.V. Hydrogen Stable Isotopes Indicate Reverse Migration of Fall Armyworm in North America. Insects 2025, 16, 471. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.C.; Hilburn, D.J.; Wright, B. The Lepidoptera of Bermuda: Their Food Plants, Biogeography, and Means of Dispersal. Mem. Entomol. Soc. Can. 1991, 123, 3–105. [Google Scholar] [CrossRef]
- Johnson, S.J. Migration and the Life History Strategy of the Fall Armyworm, Spodoptera frugiperda in the Western Hemisphere. Int. J. Trop. Insect Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Goergen, G.; Tounou, K.A.; Agboka, K.; Koffi, D.; Meagher, R.L. Analysis of Strain Distribution, Migratory Potential, and Invasion History of Fall Armyworm Populations in Northern Sub-Saharan Africa. Sci. Rep. 2018, 8, 3710. [Google Scholar] [CrossRef]
- Silva, D.M.D.; Bueno, A.D.F.; Stecca, C.D.S.; Andrade, K.; Neves, P.M.O.J.; Oliveira, M.C.N.D. Biology of Spodoptera eridania and Spodoptera cosmioides (Lepidoptera: Noctuidae) on Different Host Plants. Fla. Entomol. 2017, 100, 752–760. [Google Scholar] [CrossRef]
- Sampaio, F.; Krechemer, F.; Marchioro, C.A. Temperature-dependent Development Models Describing the Effects of Temperature on the Development of Spodoptera eridania. Pest Manag. Sci. 2021, 77, 919–929. [Google Scholar] [CrossRef] [PubMed]
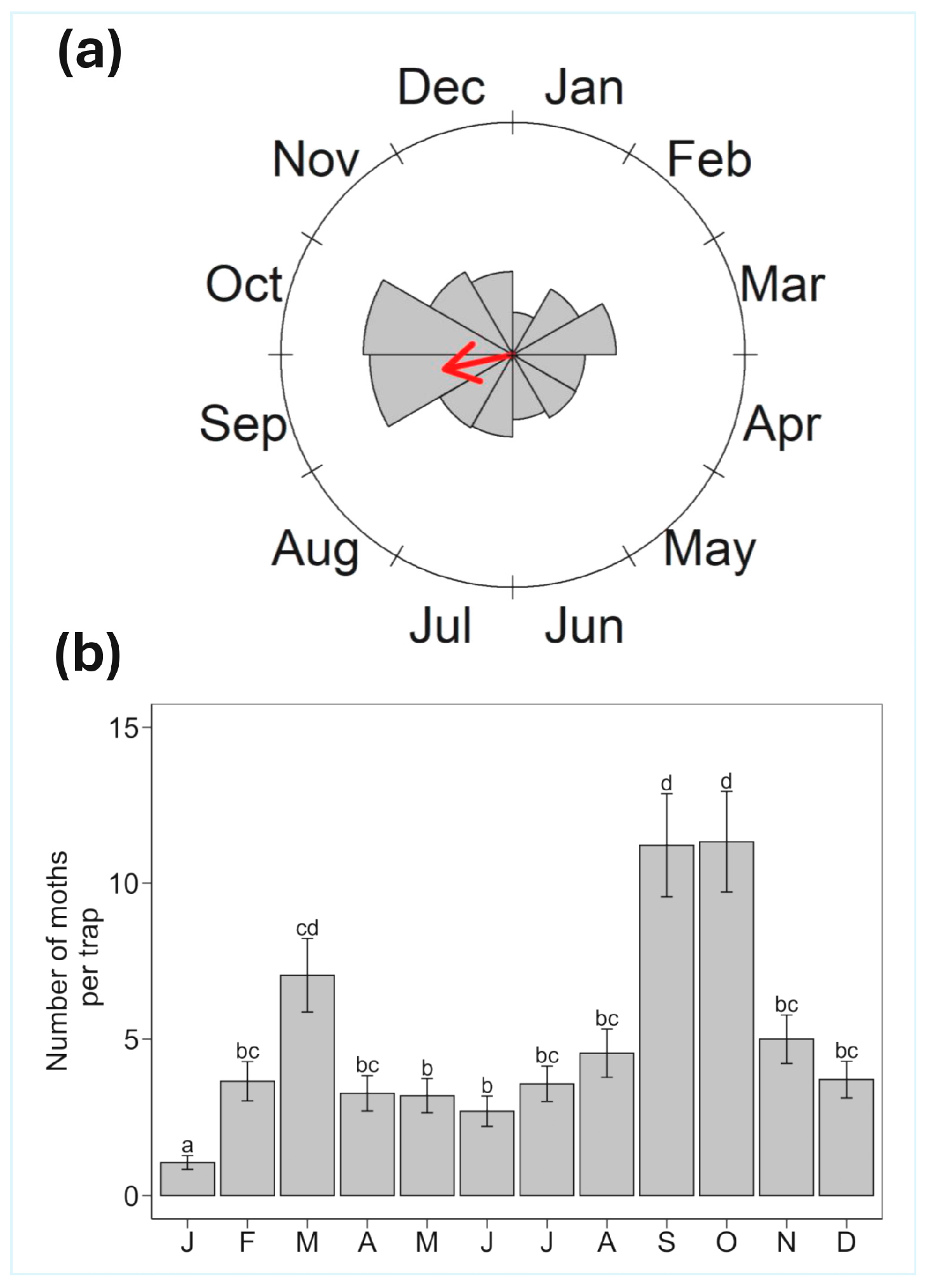
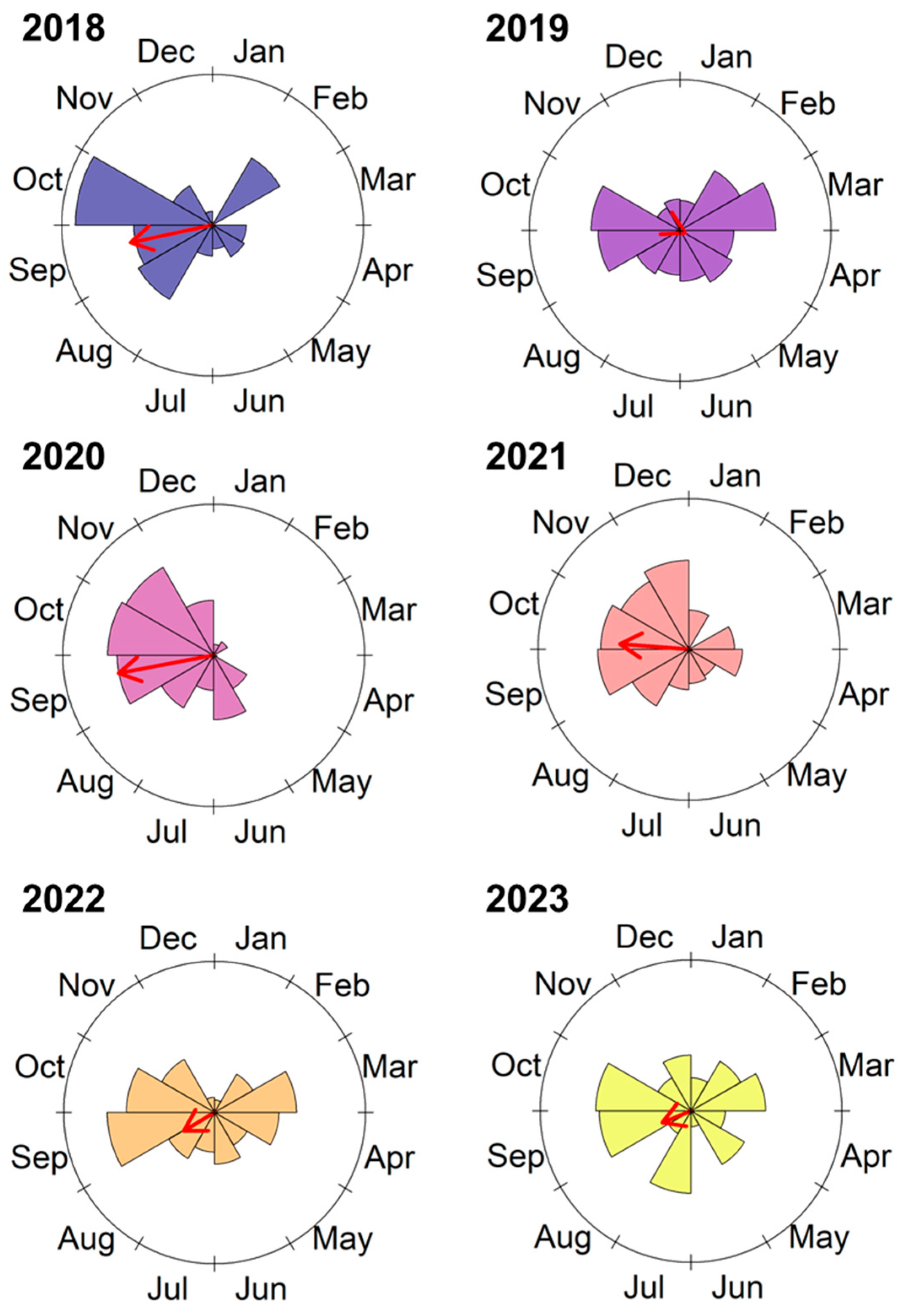
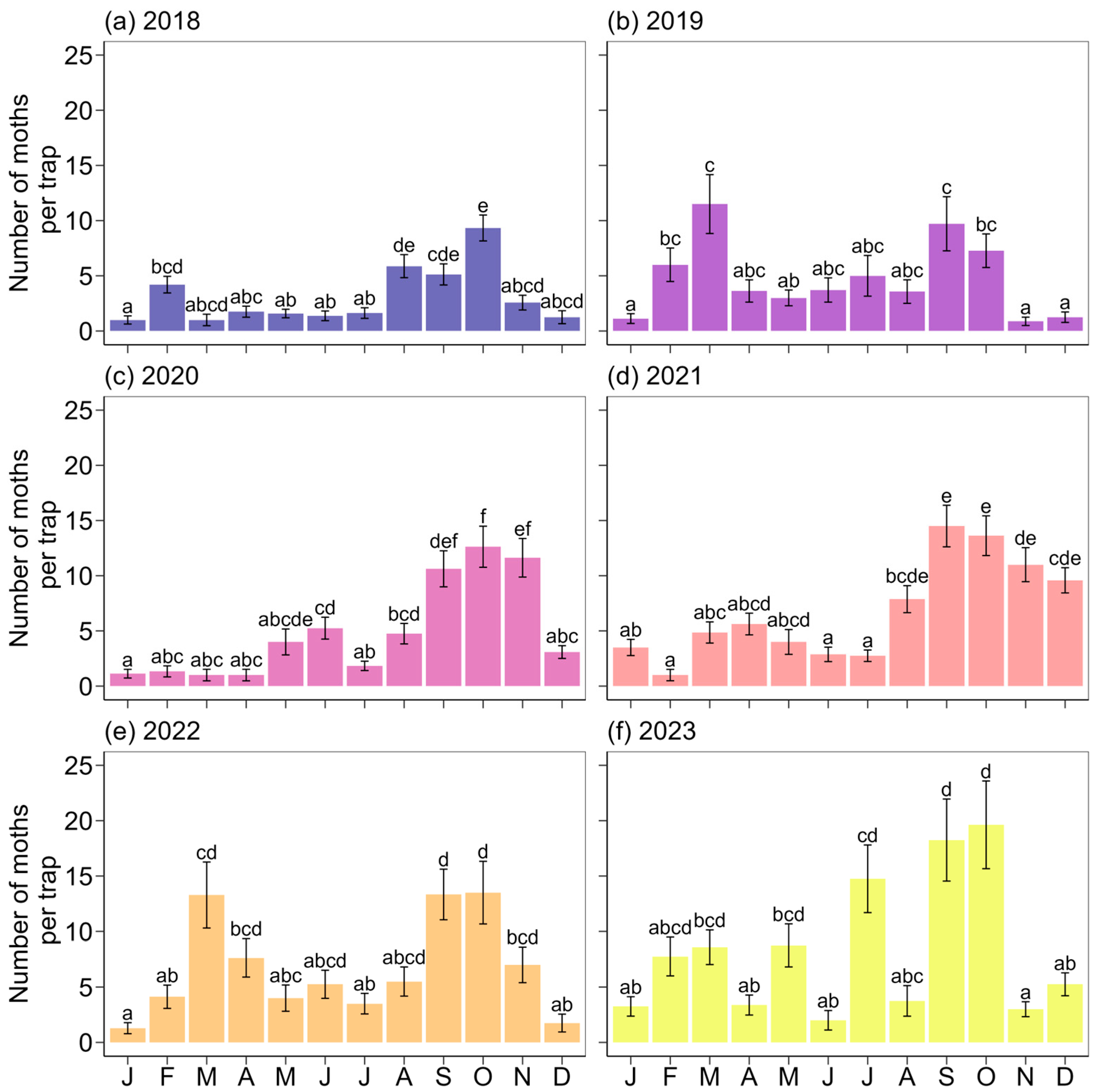
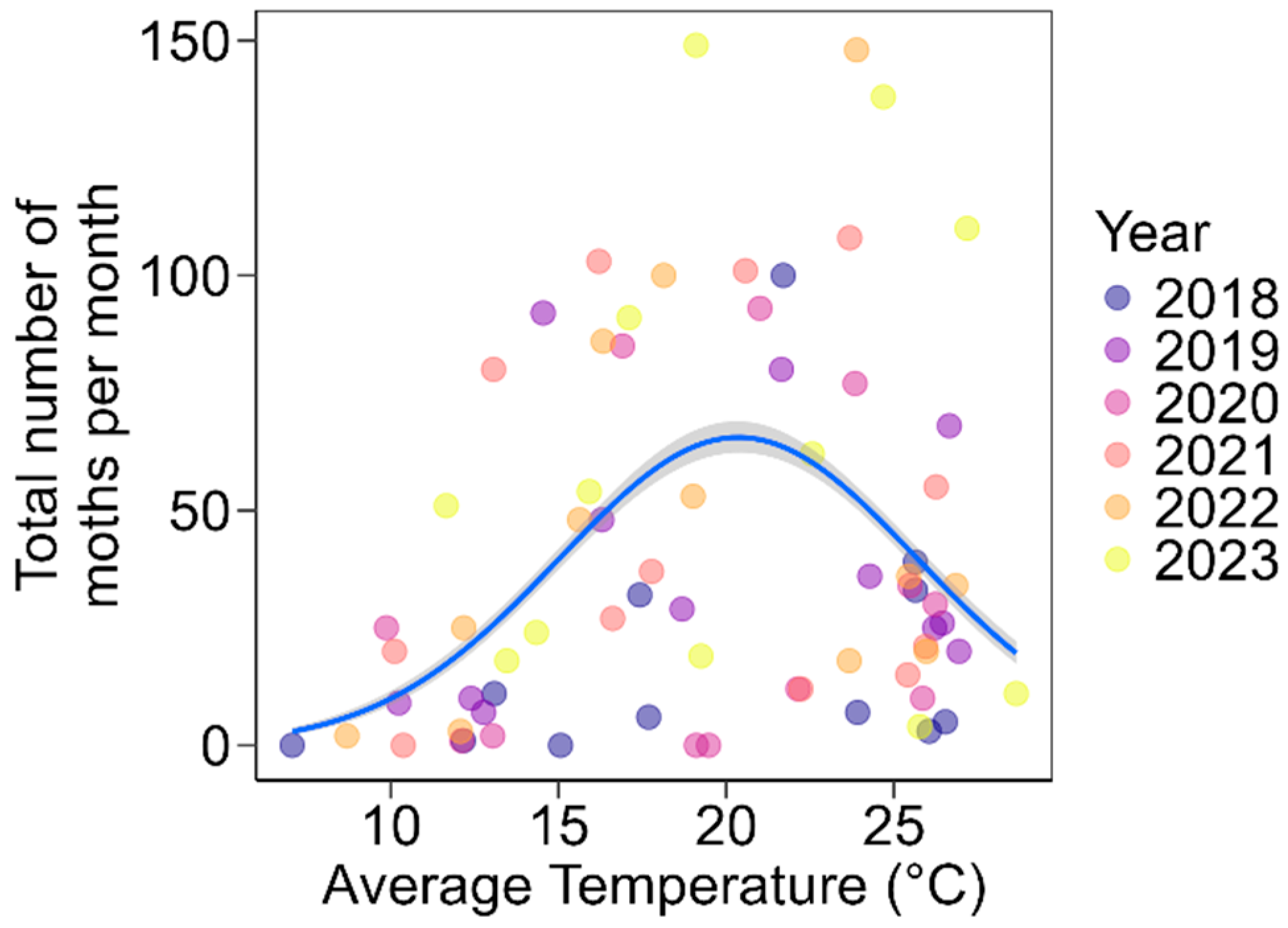
| Year | Mean Vector (Degrees) | Mean Month | Vector Length (rho) | Watson U2 Test | p-Value |
|---|---|---|---|---|---|
| All years | 258.05 | Sep | 0.30 | 7.47 | <0.01 |
| 2018 | 258.01 | Sep | 0.55 | 5.21 | <0.01 |
| 2019 | 117.32 | Apr | 0.04 | 1.57 | <0.01 |
| 2020 | 259.51 | Sep | 0.64 | 8.84 | <0.01 |
| 2021 | 274.56 | Oct | 0.46 | 7.19 | <0.01 |
| 2022 | 238.9 | Aug | 0.24 | 4.21 | <0.01 |
| 2023 | 247.97 | Sep | 0.21 | 3.37 | <0.01 |
| Predictor | Estimate | SE | Degrees of Freedom | χ2 | p-Value |
|---|---|---|---|---|---|
| Temperature | 0.764 | 0.171 | 1 | 19.8 | <0.001 |
| Temperature2 | −0.018 | 0.004 | 1 | 16.9 | <0.001 |
| Precipitation | −0.004 | 0.003 | 1 | 2.1 | 0.144 |
| Precipitation2 | 0.000 | 0.000 | 1 | 0.5 | 0.457 |
| Max wind speed | −0.082 | 0.086 | 1 | 0.9 | 0.338 |
| Max wind speed2 | 0.001 | 0.001 | 1 | 0.9 | 0.320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzate, C.; Calixto, E.S.; Paula-Moraes, S.V. Flight Phenology of Spodoptera eridania (Stoll, 1781) (Lepidoptera: Noctuidae) in Its Native Range: A Baseline for Managing an Emerging Invasive Pest. Insects 2025, 16, 779. https://doi.org/10.3390/insects16080779
Alzate C, Calixto ES, Paula-Moraes SV. Flight Phenology of Spodoptera eridania (Stoll, 1781) (Lepidoptera: Noctuidae) in Its Native Range: A Baseline for Managing an Emerging Invasive Pest. Insects. 2025; 16(8):779. https://doi.org/10.3390/insects16080779
Chicago/Turabian StyleAlzate, Claudia, Eduardo Soares Calixto, and Silvana V. Paula-Moraes. 2025. "Flight Phenology of Spodoptera eridania (Stoll, 1781) (Lepidoptera: Noctuidae) in Its Native Range: A Baseline for Managing an Emerging Invasive Pest" Insects 16, no. 8: 779. https://doi.org/10.3390/insects16080779
APA StyleAlzate, C., Calixto, E. S., & Paula-Moraes, S. V. (2025). Flight Phenology of Spodoptera eridania (Stoll, 1781) (Lepidoptera: Noctuidae) in Its Native Range: A Baseline for Managing an Emerging Invasive Pest. Insects, 16(8), 779. https://doi.org/10.3390/insects16080779








