Demographic Parameters and Life History Traits of Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) Influenced by Different Temperatures and Two Types of Food
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Rearing of Tetranychus urticae
2.2. Laboratory Rearing of Predator Mites
2.3. Life Table Study
2.4. Data Analysis
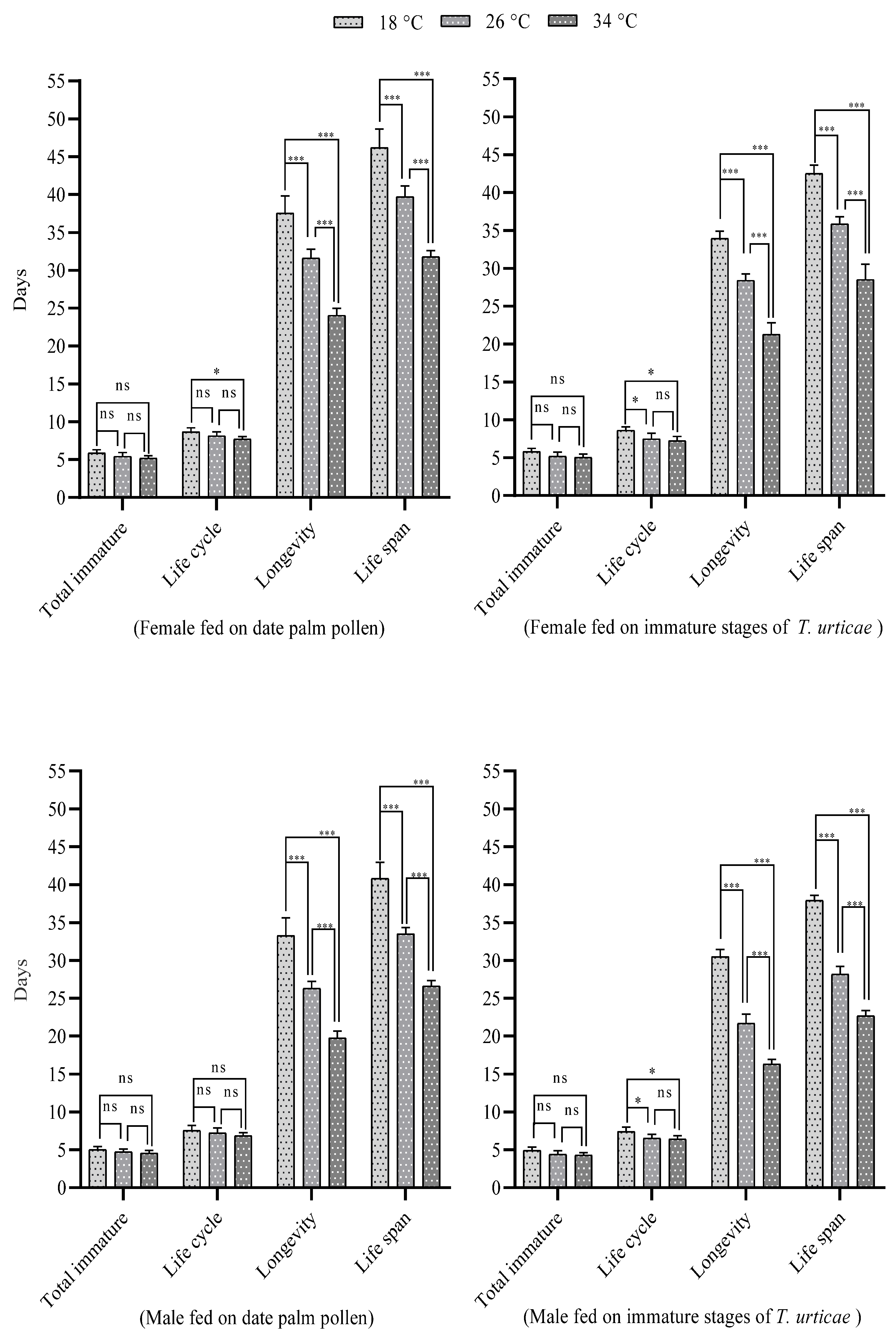
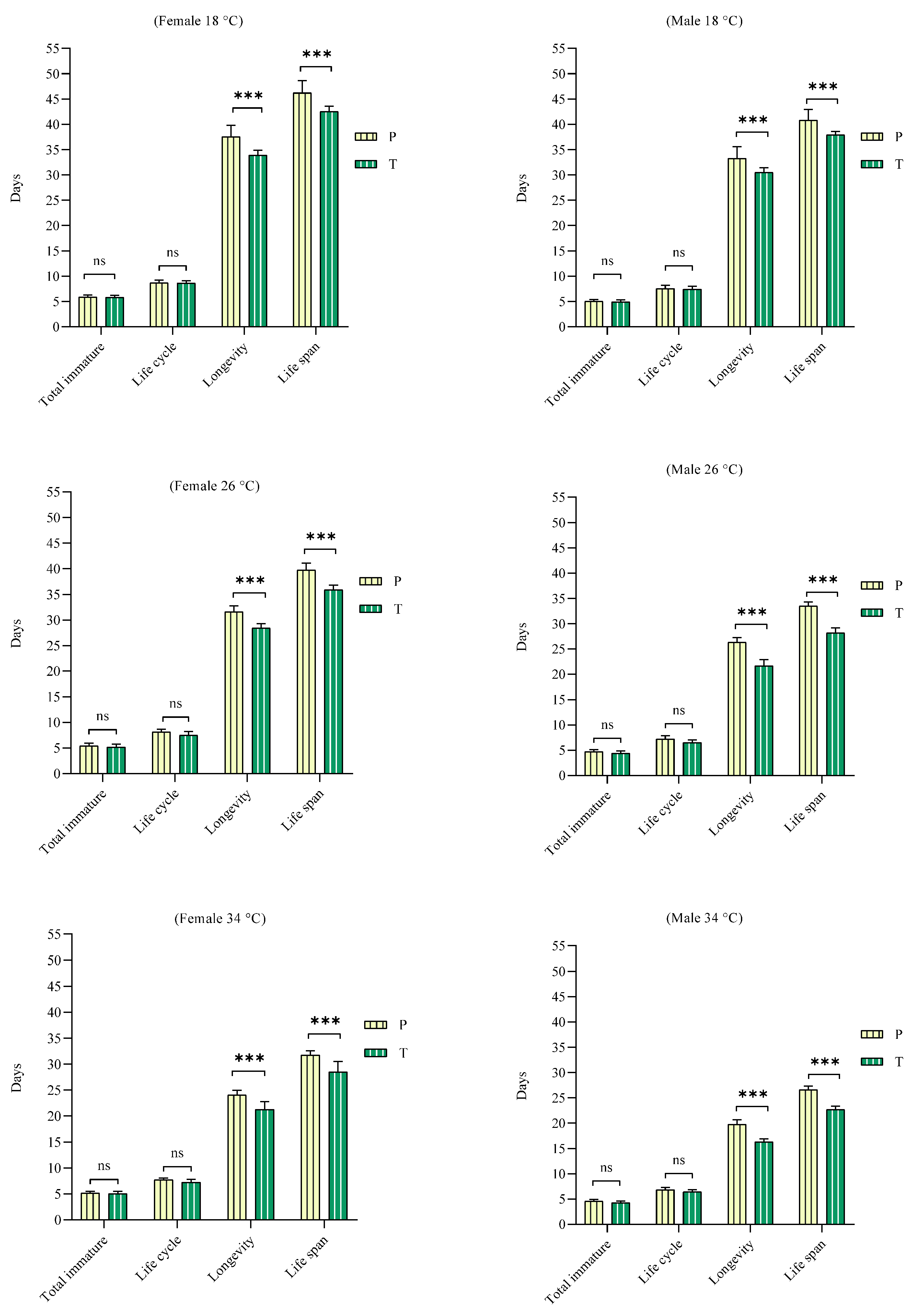
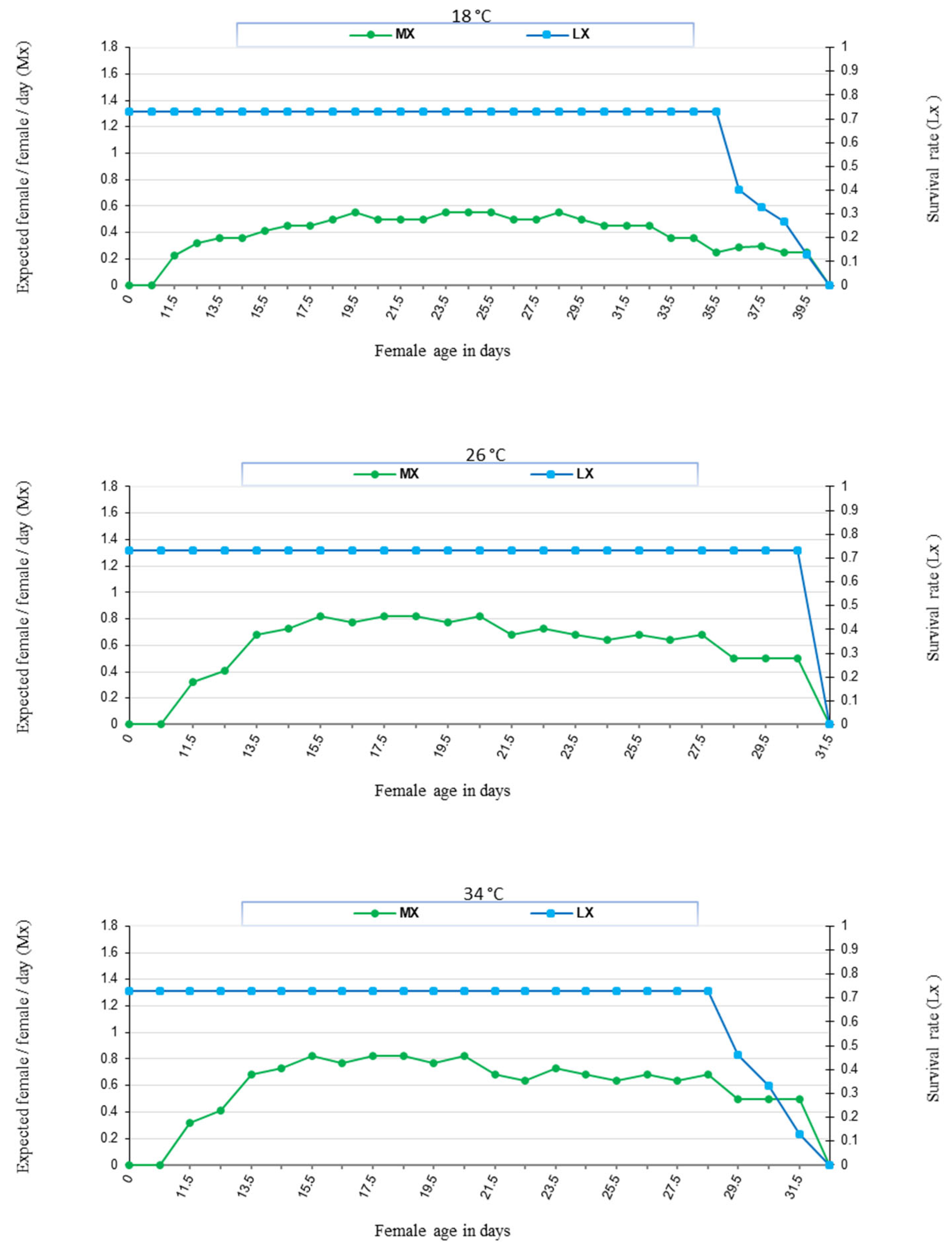
3. Results
3.1. Influence of Temperature
3.2. Effect of Feeding
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghazy, N.A.; Osakabe, M.; Negm, M.W.; Schausberger, P.; Gotoh, T.; Amano, H. Phytoseiid mites under environmental stress. Biol. Control 2016, 96, 120–134. [Google Scholar] [CrossRef]
- McMurtry, J.A.; De Moraes, G.J.; Sourassou, N.F. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 2013, 18, 297–320. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Momen, F.; Nasr, A.-E.K.; Metwally, A.-E.M.; Mahmoud, Y.; Saleh, K. Performance of five species of phytoseiid mites (Acari: Phytoseiidae) on Bactrocera zonata eggs (Diptera: Tephritidae) as a factitious food. Acta Phytopathol. Entomol. Hung. 2016, 51, 123–132. [Google Scholar] [CrossRef]
- Demite, P.R.; Cavalcante, A.C.; Dias, M.A.; Lofego, A.C. A new species and redescription of two species of Euseius wainstein (Acari: Phytoseiidae) from Cerrado biome areas in Brazil. Int. J. Acarol. 2016, 42, 334–340. [Google Scholar] [CrossRef]
- Knapp, M.; van Houten, Y.; van Baal, E.; Groot, T. Use of predatory mites in commercial biocontrol: Current status and future prospects. Acarologia 2018, 58, 72–82. [Google Scholar] [CrossRef]
- Sousa, V.C.; Zélé, F.; Rodrigues, L.R.; Godinho, D.P.; de la Masselière, M.C.; Magalhães, S. Rapid host-plant adaptation in the herbivorous spider mite Tetranychus urticae occurs at low cost. Curr. Opin. Insect Sci. 2019, 36, 82–89. [Google Scholar] [CrossRef]
- Pavela, R. Extract from the roots of Saponaria officinalis as a potential acaricide against Tetranychus urticae. J. Pest Sci. 2017, 90, 683–692. [Google Scholar] [CrossRef]
- Metwally, A.; Abdallah, A.; Abd El-Hady, M. Effect of temperature degrees on the duration of the phytophagous mite, Eutetranychus orientalis complex (klein)(acari: Tetranychidae) when fed on green bean (Phaseolus vulgaris L.). Al-Azhar J. Agric. Res. 2019, 44, 172–179. [Google Scholar] [CrossRef]
- Elmoghazy, M.M.E.; Abd Elgalil, Y.M.A. Effect of dusty conditions on abundance of phytophagous, predaceous mites and population density of Tetranychus urticae koch on citrus trees in Dakahlia governorate. Al-Azhar J. Agric. Res. 2011, 11, 169–179. [Google Scholar]
- Abou Shosha, M.; Taha, T.M.; Fawzy, M.A. Toxicity of some Microalgae to the citrus Brown Mite Eutetranychus orientalis (Klein). Al-Azhar Bull. Sci. 2013, 24, 81–92. [Google Scholar] [CrossRef]
- Abdallah, A.; El-Saiedy, E.; Maklad, A.M. Biological and chemical control of the spider mite species, Tetranychus urticae koch. On two faba bean cultivars. Egypt. J. Biol. Pest Control 2014, 24, 7–10. [Google Scholar]
- Marinosci, C.; Magalhaes, S.; Macke, E.; Navajas, M.; Carbonell, D.; Devaux, C.; Olivieri, I. Effects of host plant on life-history traits in the polyphagous spider mite Tetranychus urticae. Ecol. Evol. 2015, 5, 3151–3158. [Google Scholar] [CrossRef]
- Kwon, D.H.; Kang, T.-J.; Kim, Y.H.; Lee, S.H. Phenotypic-and genotypic-resistance detection for adaptive resistance management in Tetranychus urticae Koch. PLoS ONE 2015, 10, e0139934. [Google Scholar] [CrossRef]
- Dermauw, W.; Wybouw, N.; Rombauts, S.; Menten, B.; Vontas, J.; Grbić, M.; Clark, R.M.; Feyereisen, R.; Van Leeuwen, T. A link between host plant adaptation and pesticide resistance in the polyphagous spider mite Tetranychus urticae. Proc. Natl. Acad. Sci. USA 2013, 110, E113–E122. [Google Scholar] [CrossRef]
- Jacobson, R.; Croft, P.; Fenlon, J. Suppressing establishment of Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) in cucumber crops by prophylactic release of Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae). Biocontrol Sci. Technol. 2001, 11, 27–34. [Google Scholar] [CrossRef]
- Zhang, Z.-Q. Mites of Greenhouses: Identification, Biology and Control; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- Houten, Y.V.; Østlie, M.L.; Hoogerbrugge, H.; Bolckmans, K. Biological control of western flower thrips on sweet pepper using the predatory mites Amblyseius cucumeris, Iphiseius degenerans, A. andersoni and A. swirskii. IOBC/WPRS Bull. 2005, 28, 283–286. [Google Scholar]
- Li, G.-Y.; Zhang, Z.-Q. Some factors affecting the development, survival and prey consumption of Neoseiulus cucumeris (Acari: Phytoseiidae) feeding on Tetranychus urticae eggs (Acari: Tetranychidae). Syst. Appl. Acarol. 2016, 21, 555–566. [Google Scholar] [CrossRef]
- Kakkar, G.; Kumar, V.; Seal, D.R.; Liburd, O.E.; Stansly, P.A. Predation by Neoseiulus cucumeris and Amblyseius swirskii on Thrips palmi and Frankliniella schultzei on cucumber. Biol. Control. 2016, 92, 85–91. [Google Scholar] [CrossRef]
- Li, M.; Yang, N.; Wan, F.; Liu, L.; Chen, Y.; Li, J.; Fu, J. Functional response of Neoseiulus cucumeris (Oudemans)(Acari: Phytoseiidae) to Bemisia tabaci (Gennadius) on tomato leaves. Biocontrol Sci. Technol. 2017, 27, 677–685. [Google Scholar] [CrossRef]
- Patel, K.; Zhang, Z.-Q. Functional and numerical responses of Amblydromalus limonicus and Neoseiulus cucumeris to eggs and first instar nymph of tomato/potato psyllid (Bactericera cockerelli). Syst. Appl. Acarol. 2017, 22, 1476–1488. [Google Scholar] [CrossRef][Green Version]
- Reitz, S.R.; Gao, Y.; Kirk, W.D.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion biology, ecology, and management of western flower thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, S.; Fathipour, Y.; Riahi, E.; Zalucki, M.P. Pollen alone or a mixture of pollen types? Assessing their suitability for mass rearing of Neoseiulus cucumeris (Acari: Phytoseiidae) over 20 generations. J. Insect Sci. 2022, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Nemati, A.; Riahi, E. Does feeding on pollen grains affect the performance of Amblyseius swirskii (Acari: Phytoseiidae) during subsequent generations? Bull. Entomol. Res. 2020, 110, 449–456. [Google Scholar] [CrossRef]
- Riahi, E.; Fathipour, Y.; Talebi, A.A.; Mehrabadi, M. Pollen quality and predator viability: Life table of Typhlodromus bagdasarjani on seven different plant pollens and two-spotted spider mite. Syst. Appl. Acarol. 2016, 21, 1399–1412. [Google Scholar] [CrossRef]
- Cook, S.M.; Awmack, C.S.; Murray, D.A.; Williams, I.H. Are honey bees’ foraging preferences affected by pollen amino acid composition? Ecol. Entomol. 2003, 28, 622–627. [Google Scholar] [CrossRef]
- Elmoghazy, M.M.E. Life history and life table parameters of the predatory mite Phytoseiulus macropilis (banks) (acari: Phytoseidae) at different temperatures. Scientia 2016, 13, 74–79. [Google Scholar]
- Mortazavi, N.; Fathipour, Y.; Talebi, A.A.; Riahi, E. Suitability of monotypic and mixed diets for development, population growth and predation capacity of Typhlodromus bagdasarjani (Acari: Phytoseiidae). Bull. Entomol. Res. 2023, 113, 107–117. [Google Scholar] [CrossRef]
- Metwally, A.; Abou-Awad, B.; Al-Azzazy, M. Life table and prey consumption of the predatory mite Neoseiulus cydnodactylon Shehata and Zaher (Acari: Phytoseiidae) with three mite species as prey. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 2005, 112, 276–286. [Google Scholar]
- Elmoghazy, M.M.E. Influence of temperature on the life history and life table parameters of predatory mite Phytoseiulus persimilis Athias-Henriot when fed on two spotted spider mite Tetranychus urticae koch (Acari: Phytoseidae, Tetranychidae). J. Plant Prot. Pathol. 2012, 3, 943–949. [Google Scholar] [CrossRef]
- Sugawara, R.; Ullah, M.S.; Ho, C.-C.; Gökçe, A.; Chi, H.; Gotoh, T. Temperature-dependent demography of two closely related predatory mites Neoseiulus womersleyi and N. longispinosus (Acari: Phytoseiidae). J. Econ. Entomol. 2017, 110, 1533–1546. [Google Scholar] [CrossRef]
- Tsolakis, H.; Sinacori, M.; Ragusa, E.; Lombardo, A. Biological parameters of Neoseiulus longilaterus (Athias-Henriot)(Parasitiformes, Phytoseiidae) fed on prey and pollen in laboratory conditions. Syst. Appl. Acarol. 2019, 24, 1757–1768. [Google Scholar]
- Ersin, F.; Turanli, F.; Cakmak, I. Development and life history parameters of Typhlodromus recki (Acari: Phytoseiidae) feeding on Tetranychus urticae (Acari: Tetranychidae) at different temperatures. Syst. Appl. Acarol. 2021, 26, 496–508. [Google Scholar] [CrossRef]
- Hassan, D.; Mikhail, W.Z.; Rizk, M.A.; Sobhy, H.M.; Nada, M.S. Evaluate the Feeding Preference of Some Predator Mites Towards Red Spider Mites Untreated and Treated with Beauveria bassiana. Egypt. Acad. J. Biol. Sci. Entomol. 2017, 10, 11–20. [Google Scholar]
- Kongchuensin, M.; Charanasri, V. Suitable host plant and optimum initial ratios of predator and prey for mass-rearing the predatory mite, Neoseiulus longispinosus (Evans). J. Acarol. Soc. Jpn. 2006, 15, 145–150. [Google Scholar] [CrossRef]
- Piyani, A.R.; Shishehbor, P.; Kocheili, F.; Riddick, E.W. Comparison of natural prey Tetranychus turkestani, date palm pollen, and bee pollen diets on development, reproduction, and life table parameters of the predator Amblyseius swirskii. Acarologia 2021, 61, 890–900. [Google Scholar] [CrossRef]
- De Courcy Williams, M.E.; Kravar-Garde, L.; Fenlon, J.S.; Sunderland, K.D. Phytoseiid mites in protected crops: The effect of humidity and food availability on egg hatch and adult life span of Iphiseius degenerans, Neoseiulus cucumeris, N. californicus and Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2004, 32, 1–13. [Google Scholar] [CrossRef]
- Yazdanpanah, S.; Fathipour, Y.; Riahi, E.; Zalucki, M.P. Modeling temperature-dependent development rate of Neoseiulus cucumeris (Acari: Phytoseiidae) fed on two alternative diets. Environ. Entomol. 2022, 51, 145–152. [Google Scholar] [CrossRef]
- Abou-Setta, M.M.; Sorrell, R.; Childers, C. Life 48: A BASIC computer program to calculate life table parameters for an insect or mite species. Fla. Entomol. 1986, 69, 690–697. [Google Scholar] [CrossRef]
- Van Rijn, P.C.; Tanigoshi, L.K. Pollen as food for the predatory mites Iphiseius degenerans and Neoseiulus cucumeris (Acari: Phytoseiidae): Dietary range and life history. Exp. Appl. Acarol. 1999, 23, 785–802. [Google Scholar] [CrossRef]
- Delisle, J.; Brodeur, J.; Shipp, L. Evaluation of various types of supplemental food for two species of predatory mites, Amblyseius swirskii and Neoseiulus cucumeris (Acari: Phytoseiidae). Exp. Appl. Acarol. 2015, 65, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Y.; Pattison, N.; Zhang, Z.-Q. Immature development and survival of Neoseiulus cucumeris (Oudemans)(Acari: Phytoseiidae) on eggs of Tyrophagus curvipenis (Fain & Fauvel)(Acari: Acaridae). Acarologia 2021, 61, 84–93. [Google Scholar]
- Yazdanpanah, S.; Fathipour, Y.; Riahi, E.; Zalucki, M.P. Mass production of Neoseiulus cucumeris (Acari: Phytoseiidae): An assessment of 50 generations reared on almond pollen. J. Econ. Entomol. 2021, 114, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Vangansbeke, D.; De Clercq, P. Performance of four species of phytoseiid mites on artificial and natural diets. Biol. Control. 2015, 80, 56–62. [Google Scholar] [CrossRef]
- Elmoghazy, M.M.E. Tetranychus urticae density on variety of plant leaves influencing predatory mite Euseius scutalis functional response. Int. J. Acarol. 2022, 48, 114–120. [Google Scholar] [CrossRef]
- Grenier, S.; Clercq, P.D. Comparison of artificially vs. naturally reared natural enemies and their potential for use in biological control. In Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; CABI Publishing: Wallingford UK, 2003; pp. 115–131. [Google Scholar]
- Callebaut, B.; Van Baal, E.; Vandekerkhove, B.; Bolckmans, K.; De Clercq, P. A fecundity test for assessing the quality of Macrolophus caliginosus reared on artificial diets. Parasitica 2004, 60, 9–14. [Google Scholar]
- Bonde, J. Biological studies including population growth parameters of the predatory mite Amblyseius barkeri (Acarina.: Phytoseiidae) at 25° C in the laboratory. Entomophaga 1989, 34, 275–287. [Google Scholar] [CrossRef]
- Park, H.-H.; Shipp, L.; Buitenhuis, R.; Ahn, J.J. Life history parameters of a commercially available Amblyseius swirskii (Acari: Phytoseiidae) fed on cattail (Typha latifolia) pollen and tomato russet mite (Aculops lycopersici). J. Asia-Pac. Entomol. 2011, 14, 497–501. [Google Scholar] [CrossRef]
- Xia, B.; Zou, Z.; Li, P.; Lin, P. Effect of temperature on development and reproduction of Neoseiulus barkeri (Acari: Phytoseiidae) fed on Aleuroglyphus ovatus. Exp. Appl. Acarol. 2012, 56, 33–41. [Google Scholar] [CrossRef]
- Faraji, F.; Fathipour, Y.; Jafari, S. The influence of temperature on the functional response and prey consumption of Neoseiulus barkeri (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). J. Entomol. Soc. Iran. 2012, 31, 39–52. [Google Scholar]
- Samaras, K.; Pappas, M.L.; Fytas, E.; Broufas, G.D. Pollen suitability for the development and reproduction of Amblydromalus limonicus (Acari: Phytoseiidae). BioControl 2015, 60, 773–782. [Google Scholar] [CrossRef]
- Rezaie, M.; Askarieh, S. Effect of different pollen grains on life table parameters of Neoseiulus barkeri (Acari: Phytoseiidae). Persian J. Acarol. 2016, 5, 239–253. [Google Scholar]
- Marisa, C.; Sauro, S. Biological observations and life table parameters of Amblyseius cucumeris (Oud.)(Acarina: Phytoseiidae) reared on different diets. Redia 1990, 73, 569–583. [Google Scholar]
- Zhang, Y.; Zhang, Z.-Q.; Lin, J.; Ji, J. Potential of Amblyseius cucumeris (Acari: Phytoseiidae) as a biocontrol agent against Schizotetranychus nanjingensis (Acari: Tetranychidae) in Fujian, China. Syst. Appl. Acarol. Spec. Publ. 2000, 4, 109–124. [Google Scholar] [CrossRef]
- Sarwar, M.; Wu, K.; Xu, X. Evaluation of biological aspects of the predacious mite, Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) due to prey changes using selected arthropods. Int. J. Acarol. 2009, 35, 503–509. [Google Scholar] [CrossRef]
- Matsuo, T.; Mochizuki, M.; Yara, K.; Mitsunaga, T.; Mochizuki, A. Suitability of pollen as an alternative diet for Amblyseius cucumeris (Oudeman). Jpn. J. Appl. Entomol. Zool. 2003, 74, 153–158. [Google Scholar] [CrossRef]
- Ranabhat, N.B.; Goleva, I.; Zebitz, C.P. Life tables of Neoseiulus cucumeris exclusively fed with seven different pollens. BioControl 2014, 59, 195–203. [Google Scholar] [CrossRef]
- Flechtmann, C.H.; McMurtry, J.A. Studies of cheliceral and deutosternal morphology of some Phytoseiidae (Acari: Mesostigmata) by scanning electron microscopy. Int. J. Acarol. 1992, 18, 163–169. [Google Scholar] [CrossRef]
- Dicke, M.; Takabayashi, J.; Posthumus, M.A.; Schütte, C.; Krips, O.E. Plant—Phytoseiid interactions mediated by herbivore-induced plant volatiles: Variation in production of cues and in responses of predatory mites. Exp. Appl. Acarol. 1998, 22, 311–333. [Google Scholar] [CrossRef]
- Goleva, I.; Zebitz, C.P. Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii (Acari, Phytoseiidae). Exp. Appl. Acarol. 2013, 61, 259–283. [Google Scholar] [CrossRef]
- Massaro, M.; Martin, J.P.I.; de Moraes, G.J. Factitious food for mass production of predaceous phytoseiid mites (Acari: Phytoseiidae) commonly found in Brazil. Exp. Appl. Acarol. 2016, 70, 411–420. [Google Scholar] [CrossRef]
- Broufas, G.; Koveos, D. Effect of different pollens on development, survivorship and reproduction of Euseius finlandicus (Acari: Phytoseiidae). Environ. Entomol. 2000, 29, 743–749. [Google Scholar] [CrossRef]
- Mohamed, K.; Abd–El–Wahed, N.; El Ghobashy, M.S. Laboratory trails to evaluate the predatory mite Neoseiulus cucumeris (oudeman) when fed on european red mite Panonychus ulmi (koch) under different degrees of temperatures. J. Plant Prot. Pathol. 2008, 33, 519–523. [Google Scholar] [CrossRef]
- Rahman, V.J.; Babu, A.; Roobakkumar, A.; Perumalsamy, K. Life table and predation of Neoseiulus longispinosus (Acari: Phytoseiidae) on Oligonychus coffeae (Acari: Tetranychidae) infesting tea. Exp. Appl. Acarol. 2013, 60, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Joharchi, O.; Döker, I.; Khaustov, V.A.; Salavatulin, V.M.; Popov, D.A.; Belyakova, N.A. Effects of temperature on life table parameters of a newly described phytoseiid predator, Neoseiulus neoagrestis (Acari: Phytoseiidae) fed on Tyrophagus putrescentiae (Acari: Acaridae). Acarologia 2023, 63, 31–40. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Y.-X.; Lin, J.-Z.; Chen, X.; Sun, L.; Saito, Y. Life histories of three predatory mites feeding upon Carpoglyphus lactis (Acari, Phytoseiidae; Carpoglyphidae). Syst. Appl. Acarol. 2015, 20, 491–496. [Google Scholar] [CrossRef]
- Gotoh, T.; Yamaguchi, K.; Mori, K. Effect of temperature on life history of the predatory mite Amblyseius (Neoseiulus) californicus (Acari: Phytoseiidae). Exp. Appl. Acarol. 2004, 32, 15–30. [Google Scholar] [CrossRef]
- Sabelis, M.W.; Janssen, A. Evolution of life-history patterns in the Phytoseiidae. In Mites: Ecological and Evolutionary Analyses of Life-History Patterns; Springer: Boston, MA, USA, 1994; pp. 70–98. [Google Scholar]
- El Taj, H.; Jung, C. Effect of temperature on the life-history traits of Neoseiulus californicus (Acari: Phytoseiidae) fed on Panonychus ulmi. Exp. Appl. Acarol. 2012, 56, 247–260. [Google Scholar] [CrossRef]
- Broufas, G.D.; Koveos, D.S. Development, survival and reproduction of Euseius finlandicus (Acari: Phytoseiidae) at different constant temperatures. Exp. Appl. Acarol. 2001, 25, 441–460. [Google Scholar] [CrossRef]
- Rencken, I.; Pringle, K. Developmental biology of Amblyseius californicus (McGregor) (Acarina: Phytoseiidae), a predator of tetranychid mites, at three temperatures. Afr. Entomol. 1998, 6, 41–45. [Google Scholar]
- Kasap, İ.; Şekeroğlu, E. Life history of Euseius scutalis feeding on citrus red mite Panonychus citri at various temperatures. BioControl 2004, 49, 645–654. [Google Scholar] [CrossRef]
- Jafari, S.; Fathipour, Y.; Faraji, F.; Bagheri, M. Demographic response to constant temperatures in Neoseiulus barkeri (Phytoseiidae) fed on Tetranychus urticae (Tetranychidae). Syst. Appl. Acarol. 2010, 15, 83–99. [Google Scholar] [CrossRef]
- Kontodimas, D.C.; Milonas, P.G.; Stathas, G.J.; Papanikolaou, N.E.; Skourti, A.; Matsinos, Y.G. Life table parameters of the aphid predators Coccinella septempunctata, Ceratomegilla undecimnotata and Propylea quatuordecimpunctata (Coleoptera: Coccinellidae). Eur. J. Entomol. 2008, 105, 427–430. [Google Scholar] [CrossRef]


| Stage | Sex | Temperature | ||
|---|---|---|---|---|
| 18 °C | 26 °C | 34 °C | ||
| Egg | ♀ | 2.79 ± 0.07 a | 2.71 ± 0.07 a | 2.54 ± 0.10 a |
| ♂ | 2.55 ± 0.09 a | 2.50 ± 0.11 a | 2.30 ± 0.08 a | |
| Larva | ♀ | 1.25 ± 0.08 a | 1.21 ± 0.07 a | 1.17 ± 0.07 a |
| ♂ | 1.20 ± 0.08 a | 1.15 ± 0.08 a | 1.10 ± 0.07 a | |
| Protonymph | ♀ | 1.88 ± 0.07 a | 1.79 ± 0.07 a | 1.71 ± 0.07 a |
| ♂ | 1.55 ± 0.09 a | 1.40 ± 0.07 a | 1.35 ± 0.08 a | |
| Deutonymph | ♀ | 2.75 ± 0.08 a | 2.42 ± 0.10 a | 2.29 ± 0.07 a |
| ♂ | 2.25 ± 0.08 a | 2.15 ± 0.08 a | 2.10 ± 0.07 a | |
| Life cycle | ♀ | 8.67 ± 0.15 a | 8.13 ± 0.15 ab | 7.71 ± 0.10 b |
| ♂ | 7.55 ± 0.20 a | 7.20 ± 0.21 a | 6.85 ± 0.13 a | |
| Longevity | ♀ | 37.54 ± 0.65 a | 31.58 ± 0.34 b | 24.04 ± 0.26 c |
| ♂ | 33.25 ± 0.75 a | 26.30 ± 0.29 b | 19.75 ± 0.29 c | |
| Life span | ♀ | 46.21 ± 0.70 a | 39.71 ± 0.40 b | 31.75 ± 0.24 c |
| ♂ | 40.80 ± 0.67 a | 33.50 ± 0.26 b | 26.60 ± 0.23 c | |
| Stage | Sex | Temperature | ||
|---|---|---|---|---|
| 18 °C | 26 °C | 34 °C | ||
| Egg | ♀ | 2.77 ± 0.08 a | 2.27 ± 0.08 a | 2.18 ± 0.08 a |
| ♂ | 2.50 ± 0.07 a | 2.14 ± 0.07 a | 2.14 ± 0.07 a | |
| Larva | ♀ | 1.23 ± 0.08 a | 1.18 ± 0.08 a | 1.14 ± 0.07 a |
| ♂ | 1.18 ± 0.08 a | 1.09 ± 0.06 a | 1.09 ± 0.06 a | |
| Protonymph | ♀ | 1.86 ± 0.07 a | 1.73 ± 0.08 a | 1.64 ± 0.07 a |
| ♂ | 1.50 ± 0.10 a | 1.18 ± 0.10 a | 1.14 ± 0.07 a | |
| Deutonymph | ♀ | 2.73 ± 0.08 a | 2.27 ± 0.08 a | 2.27 ± 0.08 a |
| ♂ | 2.23 ± 0.08 a | 2.09 ± 0.06 a | 2.05 ± 0.05 a | |
| Life cycle | ♀ | 8.59 ± 0.15 a | 7.45 ± 0.24 b | 7.23 ± 0.17 b |
| ♂ | 7.41 ± 0.18 a | 6.50 ± 0.17 b | 6.55 ± 0.13 b | |
| Longevity | ♀ | 33.91 ± 0.29 a | 28.41 ± 0.26 b | 21.27 ± 0.46 c |
| ♂ | 30.50 ± 0.28 a | 21.68 ± 0.37 b | 16.27 ± 0.19 c | |
| Life span | ♀ | 42.50 ± 0.33 a | 35.86 ± 0.28 b | 28.50 ± 0.61 c |
| ♂ | 37.91 ± 0.20 a | 28.18 ± 0.30 b | 22.68 ± 0.21 c | |
| °C | Generation Period | Pre-Oviposition | Oviposition | Post-Oviposition | No. of Eggs/Female | |
|---|---|---|---|---|---|---|
| Total Average | Daily Rate | |||||
| 18 | 11.13 ± 0.24 a | 2.46 ± 0.13 a | 29.67 ± 0.45 a | 5.42 ± 0.29 a | 33.42 ± 1.03 a | 1.13 ± 0.03 a |
| 26 | 9.96 ± 0.22 b | 1.83 ± 0.09 b | 26.83 ± 0.21 b | 2.92 ± 0.19 b | 40.17 ± 0.58 b | 1.50 ± 0.02 b |
| 34 | 9.25 ± 0.12 c | 1.54 ± 0.11 b | 20.08 ± 0.23 c | 2.42 ± 0.15 b | 39.08 ± 0.58 c | 1.95 ± 0.03 c |
| °C | Generation Period | Pre-Oviposition | Oviposition | Post-Oviposition | No. of Eggs/Female | |
|---|---|---|---|---|---|---|
| Total Average | Daily Rate | |||||
| 18 | 10.68 ± 0.18 a | 2.09 ± 0.06 a | 28.27 ± 0.19 a | 3.55 ± 0.16 a | 23.18 ± 0.40 a | 0.82 ± 0.01 a |
| 26 | 9.23 ± 0.24 b | 1.77 ± 0.08 b | 24.18 ± 0.23 b | 2.45 ± 0.16 b | 26.64 ± 0.54 b | 1.10 ± 0.02 b |
| 34 | 8.68 ± 0.23 c | 1.45 ± 0.11 b | 18.09 ± 0.31 c | 1.73 ± 0.19 c | 24.64 ± 0.97 c | 1.36 ± 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmoghazy, M.M.E.; Fahmy, E.K.; Ali, T.S.A.M.; El-Sherbiny, M.; Al-Serwi, R.H.; Abulfaraj, M.; Elsherbini, D.M.A. Demographic Parameters and Life History Traits of Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) Influenced by Different Temperatures and Two Types of Food. Insects 2025, 16, 777. https://doi.org/10.3390/insects16080777
Elmoghazy MME, Fahmy EK, Ali TSAM, El-Sherbiny M, Al-Serwi RH, Abulfaraj M, Elsherbini DMA. Demographic Parameters and Life History Traits of Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) Influenced by Different Temperatures and Two Types of Food. Insects. 2025; 16(8):777. https://doi.org/10.3390/insects16080777
Chicago/Turabian StyleElmoghazy, Mohammed M. E., Eslam Kamal Fahmy, Tagwa Salah Ahmed Mohammed Ali, Mohamed El-Sherbiny, Rasha Hamed Al-Serwi, Moaz Abulfaraj, and Dalia M. A. Elsherbini. 2025. "Demographic Parameters and Life History Traits of Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) Influenced by Different Temperatures and Two Types of Food" Insects 16, no. 8: 777. https://doi.org/10.3390/insects16080777
APA StyleElmoghazy, M. M. E., Fahmy, E. K., Ali, T. S. A. M., El-Sherbiny, M., Al-Serwi, R. H., Abulfaraj, M., & Elsherbini, D. M. A. (2025). Demographic Parameters and Life History Traits of Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) Influenced by Different Temperatures and Two Types of Food. Insects, 16(8), 777. https://doi.org/10.3390/insects16080777





