Physiological Response of Tribolium castaneum to CO2 Controlled Atmosphere Stress Under Trehalose Feeding
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source and Feeding Method
2.2. Main Experimental Instruments
2.3. Cytochrome Oxidase P450 Enzyme Activity Detection
2.4. Carboxyesterase Detection
2.5. Determination of Sugar Content Such as Glycogen, Glucose, Trehalose, etc.
2.6. Determination of Trehalase Activity
2.7. Data Analysis and Plotting
3. Results
3.1. Preference of T. castaneum for Trehalose Intake
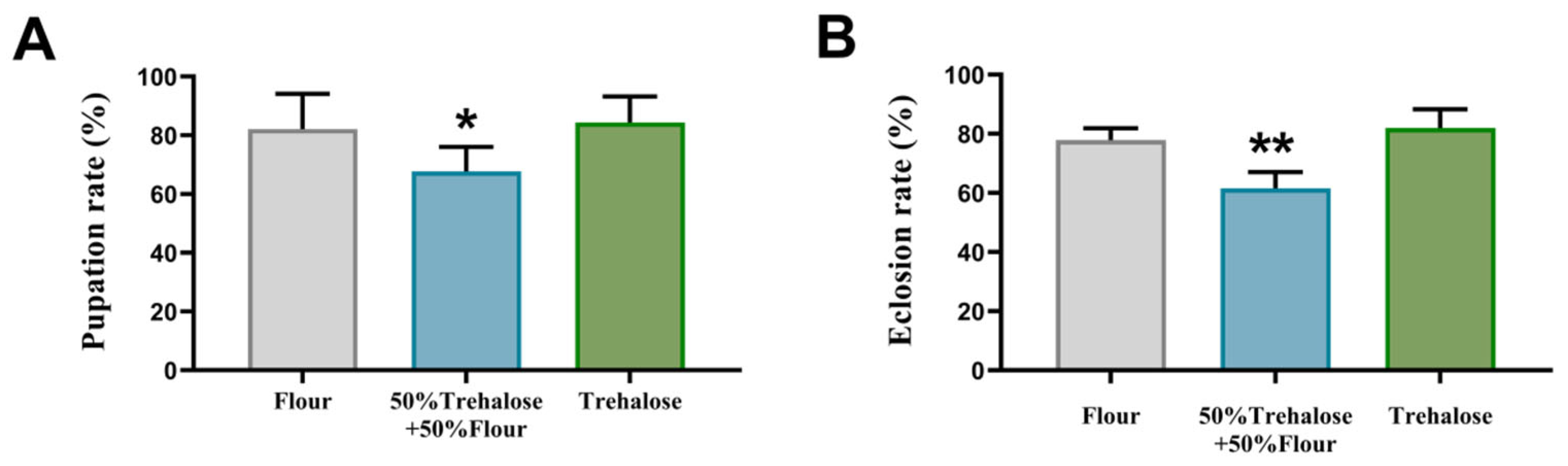
3.2. Effects of Feeding Trehalose on Pupation and Eclosion of T. castaneum
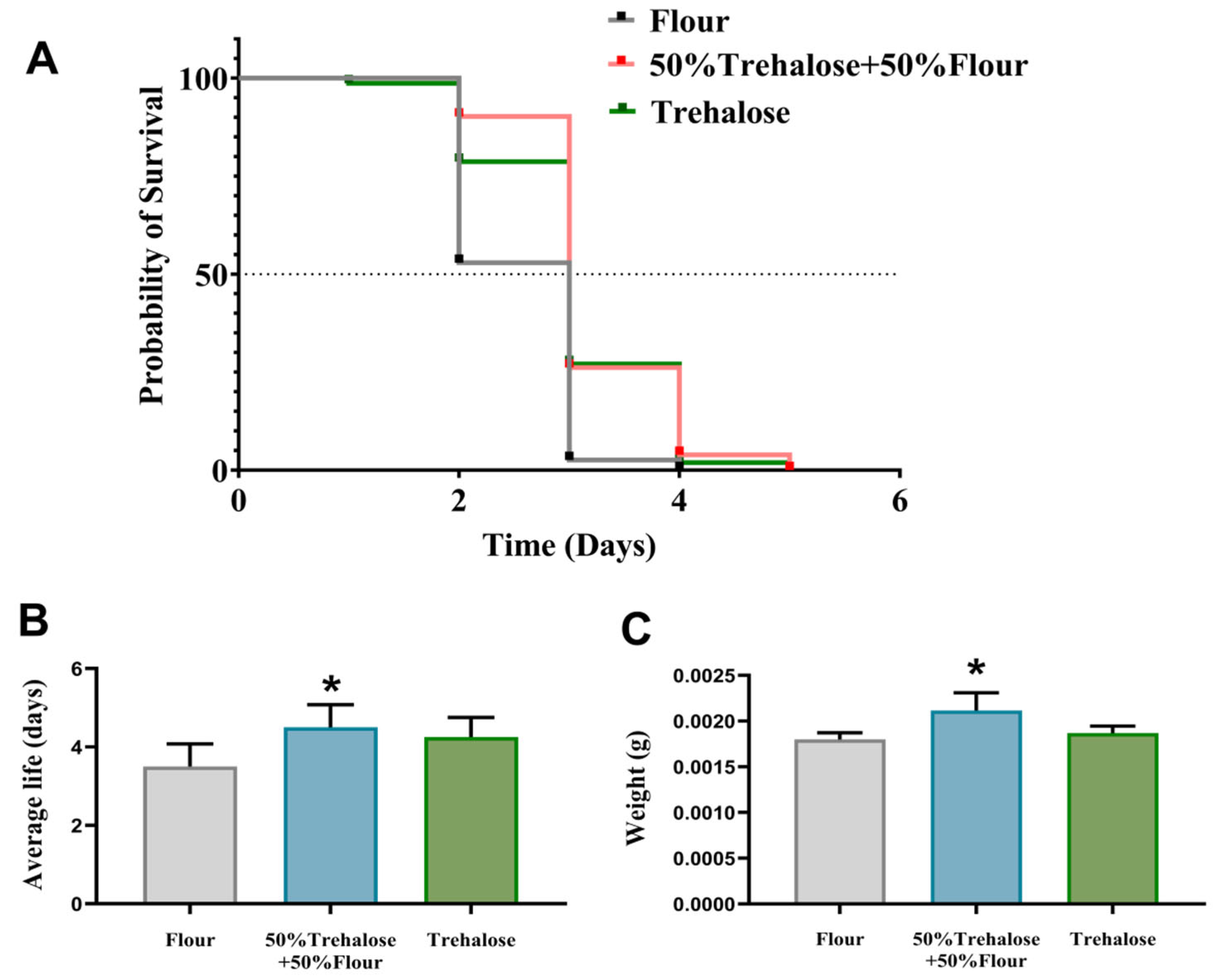
3.3. The Effect of a Trehalose Diet on T. castaneum Under Controlled Atmosphere Treatment
3.4. Effects of Trehalose Diet Under Controlled Atmosphere on Detoxification Enzyme Activity of T. castaneum
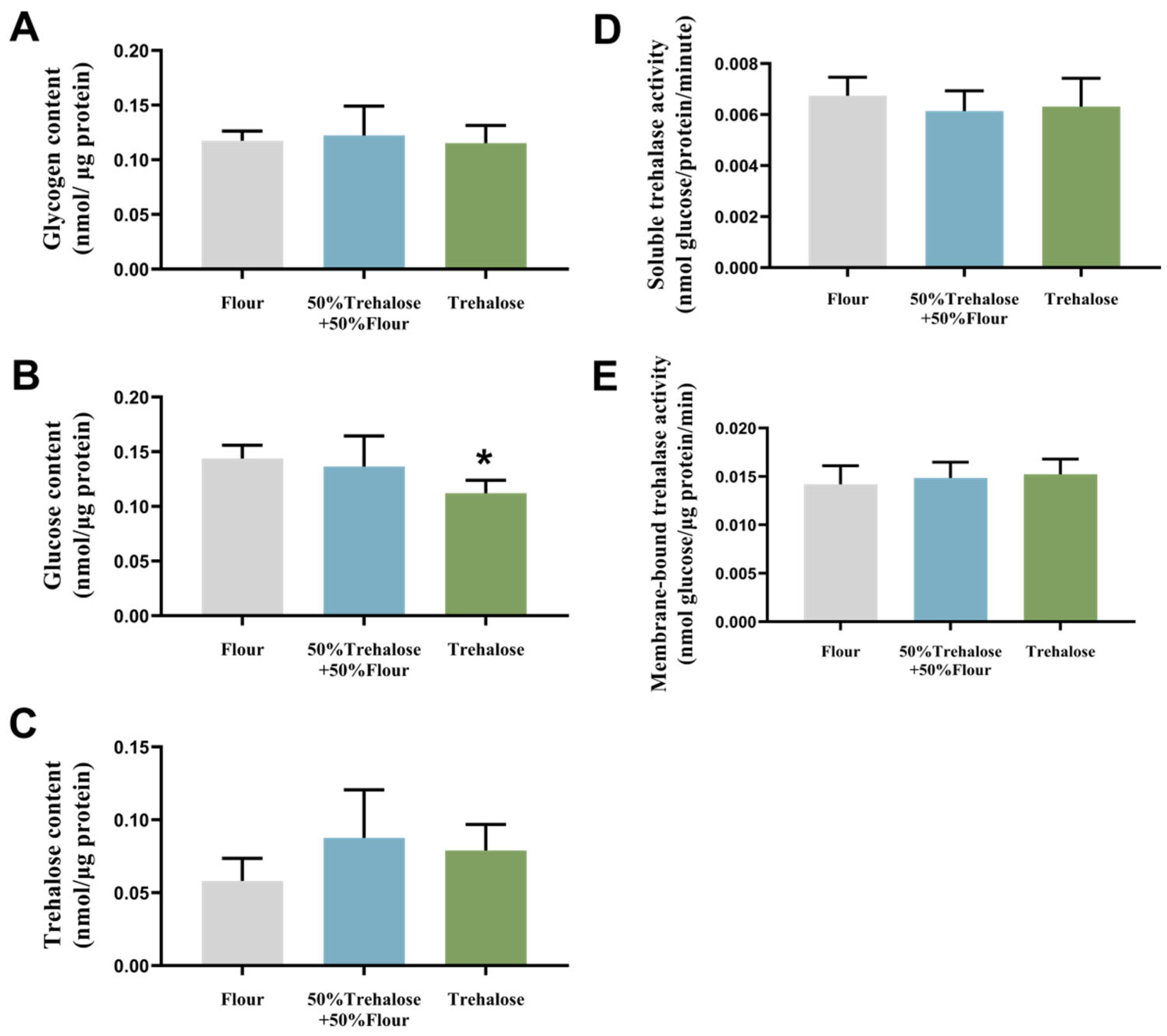
3.5. The Effect of Trehalose Diet on Trehalose Metabolism in T. castaneum Under Controlled Atmosphere Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TPS | Trehalose-6-phosphate synthase |
| TRE | Trehalase |
| AKH | Adipokinetic hormone |
| PBS | Phosphate buffered saline |
| CYP450 | Cytochrome P450 |
| CarE | Carboxylesterase |
| T6P | Trehalose-6-phosphate |
| IIS | Insulin/IGF-1 signaling pathway |
References
- Mehmood, K.; Husain, M.; Aslam, M.; Ahmedani, M.S.; Aulakh, A.M.; Shaheen, F.A. Changes in the nutritional composition of maize flour due to Tribolium castaneum infestation and application of carbon dioxide to manage this pest. Environ. Sci. Pollut. Control Ser. 2018, 25, 18540–18547. [Google Scholar] [CrossRef]
- Manandhar, A.; Milindi, P.; Shah, A. An overview of the post-harvest grain storage practices of smallholder farmers in developing countries. Agriculture 2018, 8, 57. [Google Scholar] [CrossRef]
- Rösner, J.; Wellmeyer, B.; Merzendorfer, H. Tribolium castaneum: A model for investigating the mode of action of insecticides and mechanisms of resistance. Curr. Pharm. Des. 2020, 26, 3554–3568. [Google Scholar] [CrossRef]
- Huang, Z.W.; Lv, J.H.; Xu, J.Y. Effects of Different Initial Densities of Insect Pests on Volatile Compounds from Wheat Flour. Chin. J. Grain Oil 2022, 37, 29–35. [Google Scholar]
- Smith, T.R.; Tay, A.; Koprivnikar, J. Effects of insect host chemical secretions on the entomopathogenic nematode Steinernema carpocapsae. J. Helminthol. 2023, 97, e63. [Google Scholar] [CrossRef]
- Guo, Y.; Lv, J.; Bai, C.; Gu, C.; Guo, C. Transcriptome analysis reveals adaptation mechanism of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) adults to benzoquinone stress. J. Stored Prod. Res. 2023, 101, 102083. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Wang, F.; Liu, Y.; Cai, F.; Wang, J.; Shu, Y. Trophic transfer and effects of soil cadmium in maize–fall armyworm–stinkbugs. Entomol. Gen. 2024, 44, 1481–1491. [Google Scholar] [CrossRef]
- Sabita, R.; Yunus, E.A.; Christos, G.A.; Kun, Y.Z.; William, R.M. Efficacy of long-lasting insecticide-incorporated netting in controlling preharvest and postharvest pest insects: A meta-analysis study. Entomol. Gen. 2024, 44, 1441–1458. [Google Scholar]
- Wong-Corra, F.J.; Castane, C.; Riudavets, J. Lethal effects of CO2-modified atmospheres for the control of three Bruchidae species. Stored Prod. Res. 2013, 55, 62–67. [Google Scholar] [CrossRef]
- Boyer, S.; Zhang, H.; Lempérière, G. A review of control methods and resistance mechanisms in stored-product insects. Bull. Entomol. Res. 2021, 102, 213–229. [Google Scholar] [CrossRef]
- Laure, J.; Bernard, M. Focussing on the contact: The insect tarsi and their environment. Entomol. Gen. 2024, 44, 1409–1426. [Google Scholar]
- Kim, D.; Lee, E.S. Proteomic evaluation of pathways associated with phosphine-induced mitochondrial dysfunction and resistance mechanisms in Tribolium castaneum against phosphine fumigation: Whole and partial proteome identification. Ecotoxicol. Environ. Saf. 2025, 289, 117652. [Google Scholar] [CrossRef]
- Yang, W.Q.; Lei, G.M.; Liu, X.; Li, X.H. Effects of controlled atmospheres with high CO2 concentrations on eating quality of rice. Storage Process 2012, 12, 20–22. [Google Scholar]
- Wang, L.; Cui, S.; Liu, Z.; Ping, Y.; Qiu, J.; Geng, X. Inhibition of mitochondrial respiration under hypoxia and increased antioxidant activity after reoxygenation of Tribolium castaneum. PLoS ONE 2018, 13, e0199056. [Google Scholar] [CrossRef]
- Kharel, K.; Mason, J.L.; Murdock, L.L.; Baributsa, D. Efficacy of hypoxia against Tribolium castaneum (Coleoptera: Tenebrionidae) throughout ontogeny. J. Econ. Entomol. 2020, 112, 1463–1468. [Google Scholar] [CrossRef]
- Mortimer, N.T.; Moberg, K.H. Regulation of Drosophila embryonic tracheogenesis by dVHL and hypoxia. Dev. Biol. 2009, 329, 294–305. [Google Scholar] [CrossRef]
- Sang, W.; Ji, R.; Lei, C.L.; Zhu-Salzman, K. Parental hypoxic exposure influences performance of offspring in Callosobruchus maculatus. Pest Manag. Sci. 2019, 75, 2810–2819. [Google Scholar] [CrossRef]
- Lu, C.; Ding, D.; Chen, B. Adaptation to environmental hypoxia in insects: Progresses in physiological and molecular mechanisms. J. Environ. Entomol. 2020, 42, 82–93. (In Chinese) [Google Scholar]
- Jian, Y.L.; Jian, W.Z.; Yan, T.C.; Fei, R.; Jian, W.F.; Meng, Z.S. Juvenile hormone receptor Methoprene-tolerant (Met) gene regulates the reproduction of Agasicles hygrophila under elevated CO2. Entomol. Gen. 2024, 44, 1569–1577. [Google Scholar]
- Chi, H.Y.; Ahn, J.; Yun, D.; Lee, S.; Liu, T.; Zhu-Salzman, K. Changes in oxygen and carbon dioxide environment alter gene expression of cowpea bruchids. J. Insect Physiol. 2010, 57, 220–230. [Google Scholar] [CrossRef]
- Kostal, V.; Zahradnickova, H.; Simek, P. Hyperprolinemic larvae of the drosophilid fly, Chymomyza costata, survive cryopreservation in liquid nitrogen. Proc. Natl. Acad. Sci. USA 2011, 108, 13041–13046. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Zhou, M.; Wang, Y.Y.; Jiang, X.Y.; Zhang, P.; Xu, K.K.; Tang, B.; Li, C. Characterization of the TcCYPE2 gene and its role in regulating trehalose metabolism in response to high CO2 stress. Agronomy 2023, 13, 2263. [Google Scholar] [CrossRef]
- Maibeche, C.M.; Nikonov, A.A.; Ishida, Y.; Jacquin, J.E.; Leal, W.S. Pheromone anosmia in a scarab beetle induced by in vivo inhibition of a pheromone-degrading enzyme. Proc. Natl. Acad. Sci. USA 2004, 101, 11459–11464. [Google Scholar] [CrossRef]
- Nadeau, J.A.; Petereit, J.; Tillett, R.L.; Jung, K.; Fotoohi, M.; Maclean, M.; Young, S.; Schlauch, K.; Blomquist, G.J.; Tittiger, C. Comparative transcriptomics of mountain pine beetle pheromone-biosynthetic tissues and functional analysis of CYP6DE3. BMC Genom. 2017, 18, 311. [Google Scholar] [CrossRef]
- Namiki, T.; Niwa, R.; Sakudoh, T.; Shirai, K.I.; Takeuchi, H.; Kataoka, H. Cytochrome P450 CYP307A1/Spook: A regulator for ecdysone synthesis in insects. Biochem. Biophys. Res. Commun. 2005, 337, 367–374. [Google Scholar] [CrossRef]
- Haas, J.; Beck, E.; Troczka, B.J.; Hayward, A.; Hertlein, G.; Zaworra, M.; Lueke, B.; Buer, B.; Maiwald, F.; Beck, M.E.; et al. A conserved hymenopteran-specific family of cytochrome P450s protects bee pollinators from toxic nectar alkaloids. Sci. Adv. 2023, 9, eadg0885. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Tian, Z.; Li, Y.; Ye, X.; Li, R.; Li, X.; Zheng, S.; Liu, J.; Zhang, Y. Identification of key residues of carboxylesterase PxEst-6 involved in pyrethroid metabolism in Plutella xylostella (L.). J. Hazard. Mater 2021, 407, 124612. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17r–27r. [Google Scholar] [CrossRef]
- Tellis, M.B.; Gujar, N.N.; Joshi, R.S. Evolutionary and structure-function analysis elucidates diversification of prokaryotic and eukaryotic trehalases. J. Biomol. Struct. Dyn. 2019, 37, 2926–2937. [Google Scholar] [CrossRef]
- Magalhaes, R.S.S.; Popova, B.; Braus, G.H.; Outeiro, T.F.; Eleutherio, E.C.A. The trehalose protective mechanism during thermal stress in Saccharomyces cerevisiae: The roles of Ath1 and Agt1. FEMS Yeast Res. 2018, 18, foy066. [Google Scholar] [CrossRef]
- Zhu, F.; Li, M.; Sun, M.; Jiang, X.; Qiao, F. Plant hormone signals regulate trehalose accumulation against osmotic stress in watermelon cells. Protoplasma 2022, 259, 1351–1369. [Google Scholar] [CrossRef]
- Thorat, L.; Mani, K.P.; Thangaraj, P.; Chatterjee, S.; Nath, B.B. Downregulation of dTps1 in Drosophila melanogaster larvae confirms involvement of trehalose in redox regulation following desiccation. Cell Stress Chaperones 2016, 21, 285–294. [Google Scholar] [CrossRef]
- Wen, X.; Wang, S.; Duman, J.G.; Arifin, J.F.; Juwita, V.; Goddard, W.A., III; Rios, A.; Liu, F.; Maiwald, F.; Beck, M.E.; et al. Antifreeze proteins govern the precipitation of trehalose in a freezing-avoiding insect at low temperature. Proc. Natl. Acad. Sci. USA 2016, 113, 6683–6688. [Google Scholar] [CrossRef]
- Wan, S.; He, J.; Chao, L.; Shi, Z.; Wang, S.; Yu, W.; Huang, Z.; Wang, S.; Wang, S.; Zhang, Z. Regulatory role of trehalose metabolism in cold stress of Harmonia axyridis laboratory and overwinter populations. Agronomy 2023, 13, 148. [Google Scholar] [CrossRef]
- Singh, V.; Louis, J.; Ayre, B.G.; Reese, J.C.; Shah, J. Trehalose Phosphate Synthase11-dependent trehalose metabolism promotes Arabidopsis thaliana defense against the phloem-feeding insect Myzus persicae. Plant J. 2011, 67, 94–104. [Google Scholar] [CrossRef]
- Bao, J.; Wang, X.; Feng, C.; Li, X.; Jiang, H. Trehalose metabolism in the Chinese mitten crab Eriocheir sinensis: Molecular cloning of trehalase and its expression during temperature stress. Aquac. Rep. 2021, 20, 100770. [Google Scholar] [CrossRef]
- Tang, B.; Wei, P.; Zhao, L.; Shi, Z.; Shen, Q.; Yang, M.; Xie, G.; Wang, S. Knockdown of five trehalase genes using RNA interference regulates the gene expression of the chitin biosynthesis pathway in Tribolium castaneum. BMC Biotechnol. 2016, 16, 67. [Google Scholar] [CrossRef]
- Pan, B.; Xu, K.; Luo, Y.; Wang, Y.; Zhou, M.; Li, C.; Tang, B. The sequence characteristics and functions on regulating trehalose metabolism of two PTP genes in Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2020, 89, 101692. [Google Scholar] [CrossRef]
- Wu, Z.; Guan, L.; Pan, B.; Xu, H.; Luo, Y.; Zhou, M.; Zhang, J.; Wang, S.; Li, C.; Tang, B. Mechanism of HIF1-α-mediated regulation of Tribolium castaneum metabolism under high CO2 concentration elucidated. J. Stored Prod. Res. 2022, 99, 102030. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Zhang, W.; Zhang, X.; Wang, S.; Cui, M.; Zhao, X.; Fan, D.; Dai, C. Knockdown of the expression of two trehalase genes with RNAi disrupts the trehalose and Chitin metabolism pathways in the Oriental Armyworm, Mythimna separata. Insects 2024, 15, 142. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, J.; Li, Y.; Gou, Y.; Quandahor, P.; Liu, C. Trehalose and glucose levels regulate feeding behavior of the phloem-feeding insect, the pea aphid Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 15864. [Google Scholar] [CrossRef]
- Chen, X.; Yao, H.; Ye, G. Research advances on insulin-like peptides and their functions in insects. Chin. J. Biol. Control 2017, 33, 699–712. [Google Scholar]
- Du, B.; Ding, D.; Ma, C.; Guo, W.; Kang, L. Locust density shapes energy metabolism and oxidative stress resulting in divergence of flight traits. Proc. Natl. Acad. Sci. USA 2022, 119, e2115753118. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Li, C.R.; Momen, B.; Kohanski, R.A.; Pick, L. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc. Natl. Acad. Sci. USA 2009, 106, 19617–19622. [Google Scholar] [CrossRef]
- Song, Y.; Gu, F.; Li, Y.; Zhou, W.; Wu, F.; Wang, J.; Sheng, S. Host trehalose metabolism disruption by validamycin A results in reduced fitness of parasitoid offspring. Pestic. Biochem. Physiol. 2023, 195, 105570. [Google Scholar] [CrossRef]
- Sampedro, J.G.; Uribe, S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition. The role of viscosity. Mol. Cell. Biochem. 2004, 256–257, 319–327. [Google Scholar] [CrossRef]
- Ilhan, S.; Ozdemir, F.; Bor, M. Contribution of trehalose biosynthetic pathway to drought stress tolerance of Capparis ovata Desf. Plant Biol. 2015, 17, 402–407. [Google Scholar] [CrossRef]
- Sebollela, A.; Louzada, P.R.; Sola, P.M.; Sarone, W.V.; Coelho, S.T.; Ferreira, S.T. Inhibition of yeast glutathione reductase by trehalose: Possible implications in yeast survival and recovery from stress. Int. J. Biochem. Cell Biol. 2004, 36, 900–908. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, M.; Huang, C.; Zhang, L.; Zhang, J. Trehalose alleviates high-temperature stress in Pleurotus ostreatus by affecting central carbon metabolism. Microb. Cell Factories 2021, 20, 82. [Google Scholar] [CrossRef]
- Jin, J.; Zhu, K.; Tang, S.; Xiang, Y.; Mao, M.; Hong, X.; Chen, A.; Zhang, X.; Lu, H.; Chen, Z.; et al. Trehalose promotes functional recovery of keratinocytes under oxidative stress and wound healing via ATG5/ATG7. Burns 2023, 49, 1382–1391. [Google Scholar] [CrossRef]
- Chen, Q.; Behar, K.L.; Xu, T.; Fan, C.; Haddad, G.G. Expression of Drosophila trehalose-phosphate synthase in HEK-293 cells increases hypoxia tolerance. J. Biol. Chem. 2003, 278, 49113–49118. [Google Scholar] [CrossRef]
- O’Hara, L.E.; Paul, M.J.; Wingler, A. How do sugars regulate plant growth and development? new insight into the role of trehalose-6-phosphate. Mol. Plant 2013, 6, 261–274. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Liu, Y.; Lu, Y.; Zhou, M.; Wang, S. The effect of different dietary sugars on the development and fecundity of Harmonia axyridis. Front. Physiol. 2020, 11, 574851. [Google Scholar] [CrossRef]
- Dogan, C.; Guney, G.; Guzel, K.K.; Can, A.; Hegedus, D.D.; Toprak, U. What you eat matters: Nutrient inputs alter the metabolism and neuropeptide expression in egyptian cotton leaf worm, Spodoptera littoralis (Lepidoptera: Noctuidae). Front. Physiol. 2021, 12, 773688. [Google Scholar] [CrossRef]
- Tellis, M.B.; Kotkar, H.M.; Joshi, R.S. Regulation of trehalose metabolism in insects: From genes to the metabolite window. Glycobiology 2023, 33, 262–273. [Google Scholar] [CrossRef]
- Yang, J.; Cui, M.; Zhao, X.; Zhang, C.; Hu, Y.; Fan, D. Trehalose-6-phosphate synthase regulates chitin synthesis in Mythimna separata. Front. Physiol. 2023, 14, 1109661. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Mu, X.; Li, X.; Zhou, M.; Song, Y.; Xu, K.; Li, C. Insulin-like ILP2 regulates trehalose metabolism to tolerate hypoxia/hypercapnia in Tribolium castaneum. Front. Physiol. 2022, 13, 857239. [Google Scholar] [CrossRef]
- Seo, Y.; Kingsley, S.; Walker, G.; Mondoux, M.A.; Tissenbaum, H.A. Metabolic shift from glycogen to trehalose promotes lifespan and healthspan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2018, 115, E2791–E2800. [Google Scholar] [CrossRef]
- Yoko, H.; Masashi, T.; Shuji, H. Trehalose extends longevity in the nematode Caenorhabditis elegans. Aging Cell 2010, 9, 558–569. [Google Scholar] [CrossRef]
- Zečić, A.; Braeckman, B.P. DAF-16/FoxO in Caenorhabditis elegans and its role in metabolic Remodeling. Cells 2020, 9, 109. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, E.; Behar, K.L.; Xu, T.; Haddad, G.G. Role of trehalose phosphate synthase in anoxia tolerance and development in Drosophila melanogaster. J. Biol. Chem. 2002, 277, 3274–3279. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, Z.; Palli, S.R. Juvenile hormone and insulin regulate trehalose homeostasis in the red flour beetle, Tribolium castaneum. PLoS Genet. 2013, 9, e1003535. [Google Scholar] [CrossRef]
- Matsuda, H.; Yamada, T.; Yoshida, M.; Nishimura, T. Flies without trehalose. J. Biol. Chem. 2015, 290, 1244–1255. [Google Scholar] [CrossRef]
- Wang, G.; Gou, Y.; Guo, S.; Zhou, J.J.; Liu, C. RNA interference of trehalose-6-phosphate synthase and trehalase genes regulates chitin metabolism in two color morphs of Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 948. [Google Scholar] [CrossRef]
- Yoshida, M.; Matsuda, H.; Kubo, H.; Nishimura, T. Molecular characterization of Tps1 and treh genes in Drosophila and their role in body water homeostasis. Sci. Rep. 2016, 6, 30582. [Google Scholar] [CrossRef]
- Tetsuo, Y.; Takayuki, Y.; Takashi, N. Adaptation to dietary conditions by trehalose metabolism in Drosophila. Sci. Rep. 2017, 7, 1619. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Zhi, J.; Li, D.; Li, C. Regulatory effect of trehalose metabolism on chitin synthesis in Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) as determined using RNAi. J. Asia-Pac. Entomol. 2024, 27, 102179. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Hu, S.; Zhou, M.; Zhang, X.; Guan, L.; Zhou, Y.; Lv, J.; Tang, B. Physiological Response of Tribolium castaneum to CO2 Controlled Atmosphere Stress Under Trehalose Feeding. Insects 2025, 16, 768. https://doi.org/10.3390/insects16080768
Zhang Y, Hu S, Zhou M, Zhang X, Guan L, Zhou Y, Lv J, Tang B. Physiological Response of Tribolium castaneum to CO2 Controlled Atmosphere Stress Under Trehalose Feeding. Insects. 2025; 16(8):768. https://doi.org/10.3390/insects16080768
Chicago/Turabian StyleZhang, Yuya, Shangrong Hu, Min Zhou, Xinyi Zhang, Liwen Guan, Yanfei Zhou, Jun Lv, and Bin Tang. 2025. "Physiological Response of Tribolium castaneum to CO2 Controlled Atmosphere Stress Under Trehalose Feeding" Insects 16, no. 8: 768. https://doi.org/10.3390/insects16080768
APA StyleZhang, Y., Hu, S., Zhou, M., Zhang, X., Guan, L., Zhou, Y., Lv, J., & Tang, B. (2025). Physiological Response of Tribolium castaneum to CO2 Controlled Atmosphere Stress Under Trehalose Feeding. Insects, 16(8), 768. https://doi.org/10.3390/insects16080768





