Advances in Forensic Entomotoxicology for Decomposed Corpses: A Review
Simple Summary
Abstract
1. Introduction
2. Retrospective Analysis of Forensic Entomotoxicology
2.1. Impact of Pesticides on Succession Patterns and Development of Necrophagous Flies
2.2. Effect of Psychoactive Drugs on the Development of Necrophagous Flies
2.3. Effects of Antibiotics on the Development of Necrophagous Flies
2.4. Effect of Heavy Metals on the Development of Necrophagous Flies
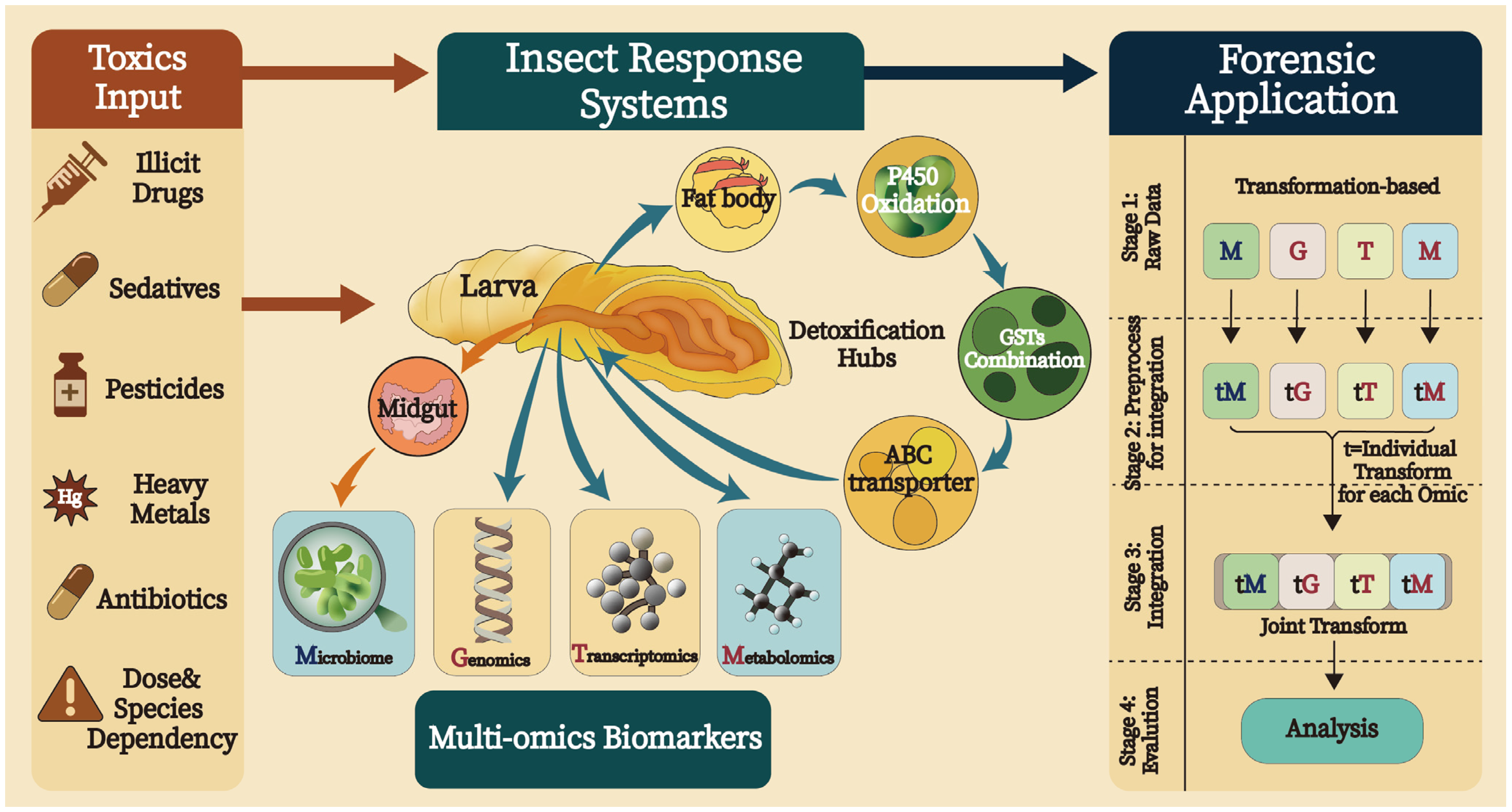
3. Importance of Multi-Omics Technologies in Entomotoxicology
4. Potential Applications of Machine Learning Methods in Multi-Omics Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goff, M.L.; Lord, W.D. Entomotoxicology. A new area for forensic investigation. Am. J. Forensic Med. Pathol. 1994, 15, 51–57. [Google Scholar] [CrossRef]
- Archer, M.S.; Elgar, M.A.; Briggs, C.A.; Ranson, D.L. Fly pupae and puparia as potential contaminants of forensic entomology samples from sites of body discovery. Int. J. Leg. Med. 2006, 120, 364–368. [Google Scholar] [CrossRef]
- Bugelli, V.; Papi, L.; Fornaro, S.; Stefanelli, F.; Chericoni, S.; Giusiani, M.; Vanin, S.; Campobasso, C.P. Entomotoxicology in burnt bodies: A case of maternal filicide-suicide by fire. Int. J. Leg. Med. 2017, 131, 1299–1306. [Google Scholar] [CrossRef]
- Hu, G.; Li, L.; Zhang, Y.; Shao, S.; Gao, Y.; Zhang, R.; Wang, Y.; Zhang, Y.; Guo, Y.; Kang, C.; et al. A global perspective of forensic entomology case reports from 1935 to 2022. Int. J. Leg. Med. 2023, 137, 1535–1553. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, M.; Wille, S.M.; Fernandez Mdel, M.; Di Fazio, V.; Samyn, N.; De Boeck, G.; Bourel, B. Entomotoxicology, experimental set-up and interpretation for forensic toxicologists. Forensic Sci. Int. 2011, 208, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chophi, R.; Sharma, S.; Sharma, S.; Singh, R. Forensic entomotoxicology: Current concepts, trends and challenges. J. Forensic Leg. Med. 2019, 67, 28–36. [Google Scholar] [CrossRef]
- Dawoud, K.; Dawoud, K.; Yavar, R.; Yavar, R.; Kourosh, A.; Kourosh, A.; Mohammad Ali, O.; Mohammad Ali, O.; Sayena, R.; Sayena, R.; et al. Analysis of the effect of methadone and temperature on the development rate of Calliphora vicina (Diptera: Calliphoridae): A forensically important fly. Nusant. Biosci. 2020, 12, 87–91. [Google Scholar] [CrossRef]
- Mullany, C.; Keller, P.A.; Nugraha, A.S.; Wallman, J.F. Effects of methamphetamine and its primary human metabolite, p-hydroxymethamphetamine, on the development of the Australian blowfly Calliphora stygia. Forensic Sci. Int. 2014, 241, 102–111. [Google Scholar] [CrossRef]
- Wood, T.; Pyper, K.; Casali, F. Effects of cocaine and heroin, and their combination, on the development rate of Calliphora vomitoria (Diptera: Calliphoridae). Sci. Justice 2022, 62, 471–475. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, M.; Xu, W.; Zhang, Y.; Wang, J. Forensic entomology in china and its challenges. Insects 2021, 12, 230. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The role of cytochrome p450s in insect toxicology and resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef]
- Lu, K.; Song, Y.; Zeng, R. The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Vogel, H.; Heckel, D.G. Comparative analysis of the UDP-glycosyltransferase multigene family in insects. Insect Biochem. Mol. Biol. 2012, 42, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Dermauw, W.; Van Leeuwen, T. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 2014, 45, 89–110. [Google Scholar] [CrossRef]
- Pu, J.; Chung, H. New and emerging mechanisms of insecticide resistance. Curr. Opin. Insect Sci. 2024, 63, 101184. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Nelliot, A.; Bond, N.; Hoshizaki, D.K. Fat-body remodeling in Drosophila melanogaster. Genesis 2006, 44, 396–400. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Feng, Q. Fat body biology in the last decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef]
- Introna, F.; Campobasso, C.P.; Goff, M.L. Entomotoxicology. Forensic Sci. Int. 2001, 120, 42–47. [Google Scholar] [CrossRef]
- Bhardwaj, T.; Sharma, S.; Dalal, J.; Verma, K. The implication of morphometrics and growth rate of dipteran flies in forensic entomotoxicology research: A review. Naturwissenschaften 2020, 107, 50. [Google Scholar] [CrossRef]
- Groth, O.C.; Pi, A.; Jensen, A.E.; Reckel, F.; Hodecek, J.; Kori Yahia, A.; Rahaus, S.; Villet, M.H.; Graw, M. Evaluating the value of entomotoxicology in forensic toxicology casework using the first minipig model. Forensic Toxicol. 2025, 43, 333–348. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Di Vella, G.; Introna, F. Factors affecting decomposition and diptera colonization. Forensic Sci. Int. 2001, 120, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Charabidze, D.; Hedouin, V. Temperature: The weak point of forensic entomology. Int. J. Leg. Med. 2019, 133, 633–639. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.I.T.; Wilhelmi, B.; Villet, M.H. Forensic entomotoxicology revisited-towards professional standardisation of study designs. Int. J. Leg. Med. 2017, 131, 1399–1412. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.J.; Liu, M.F.; Li, N.; Dang, L.H.; An, G.S.; Lu, X.J.; Wang, L.L.; Du, Q.X.; Cao, J.; et al. Multi-omics integration strategy in the post-mortem interval of forensic science. Talanta 2024, 268, 125249. [Google Scholar] [CrossRef] [PubMed]
- Dawidowska, J.; Krzyżanowska, M.; Markuszewski, M.J.; Kaliszan, M. The application of metabolomics in forensic science with focus on forensic toxicology and time-of-death estimation. Metabolites 2021, 11, 801. [Google Scholar] [CrossRef]
- Moitas, B.; Caldas, I.M.; Sampaio-Maia, B. Microbiology and postmortem interval: A systematic review. Forensic Sci. Med. Pathol. 2024, 20, 696–715. [Google Scholar] [CrossRef]
- Jain, S.; Parrott, J.J.; Javan, G.T. Exploring the impact of xenobiotic drugs on forensic entomology for accurate post-mortem interval estimation. Front. Insect Sci. 2024, 4, 1411342. [Google Scholar] [CrossRef]
- Rosli, M.A.F.; Syed Jaafar, S.N.; Azizan, K.A.; Yaakop, S.; Aizat, W.M. Omics approaches to unravel insecticide resistance mechanism in Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). PeerJ 2024, 12, e17843. [Google Scholar] [CrossRef]
- Binson, V.A.; Thomas, S.; Subramoniam, M.; Arun, J.; Naveen, S.; Madhu, S. A review of machine learning algorithms for biomedical applications. Ann. Biomed. Eng. 2024, 52, 1159–1183. [Google Scholar] [CrossRef] [PubMed]
- Pien, K.; Laloup, M.; Pipeleers-Marichal, M.; Grootaert, P.; De Boeck, G.; Samyn, N.; Boonen, T.; Vits, K.; Wood, M. Toxicological data and growth characteristics of single post-feeding larvae and puparia of Calliphora vicina (Diptera: Calliphoridae) obtained from a controlled nordiazepam study. Int. J. Leg. Med. 2004, 118, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Goff, M.L.; Omori, A.I.; Goodbrod, J.R. Effect of cocaine in tissues on the development rate of Boettcherisca peregrina (Diptera: Sarcophagidae). J. Med. Entomol. 1989, 26, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Boulkenafet, F.; Dob, Y.; Karroui, R.; Al-Khalifa, M.; Boumrah, Y.; Toumi, M.; Mashaly, A. Detection of benzodiazepines in decomposing rabbit tissues and certain necrophagic dipteran species of forensic importance. Saudi J. Biol. Sci. 2020, 27, 1691–1698. [Google Scholar] [CrossRef]
- Parkhideh, S.Z.; Forouzesh, M.; Akhgari, M.; Bahmanabadi, L.; Jokar, F.; Valiyari, S.; Akbarzadeh, K.; Rahimi, S.; Alimohammadi, A.M.; Rafizadeh, S.; et al. Preliminary analysis of methamphetamine detection in Lucilia sericata (Diptera: Calliphoridae) reared in methamphetamine-treated meat at various developmental stages. J. Arthropod Borne Dis. 2023, 17, 229–240. [Google Scholar] [CrossRef]
- Groth, O.C.; Strassberger, A.; Höft, V.; Schusterbauer, I.; Rahaus, S.; Adetimehin, A.D.; Graw, M.; Villet, M.H. Exploring unified methods of killing and storing insect samples for forensic entomotoxicology using diazepam in Lucilia sericata (Meigen, 1826) (Diptera: Calliphoridae) larvae. Forensic Sci. Int. 2024, 365, 112255. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Chen, W.; Ren, L.; Ling, J.; Shang, Y.; Guo, Y. Effects of Methamphetamine on the development and its determination in Aldrichina grahami (Diptera: Calliphoridae). J. Med. Entomol. 2020, 57, 691–696. [Google Scholar] [CrossRef]
- de Carvalho, L.M.; Linhares, A.X.; Badan Palhares, F.A. The effect of cocaine on the development rate of immatures and adults of Chrysomya albiceps and Chrysomya putoria (Diptera: Calliphoridae) and its importance to postmortem interval estimate. Forensic Sci. Int. 2012, 220, 27–32. [Google Scholar] [CrossRef]
- El-Samad, L.; Tantawi, T.; El-Ghaffar, H.; Beltagy, B.; El-Abd, E. The effect of morphine on the development rate of flies (Diptera: Calliphoridae, Sarcophagidae) reared on rabbit carcasses containing this drug and its implications to postmortem interval estimates. Swed. J. Biosci. Res. 2020, 1, 28–38. [Google Scholar] [CrossRef]
- Gosselin, M.; Di Fazio, V.; Wille, S.M.; Fernandez Mdel, M.; Samyn, N.; Bourel, B.; Rasmont, P. Methadone determination in puparia and its effect on the development of Lucilia sericata (Diptera, Calliphoridae). Forensic Sci. Int. 2011, 209, 154–159. [Google Scholar] [CrossRef]
- Yan-Wei, S.; Xiao-Shan, L.; Hai-Yang, W.; Run-Jie, Z. Effects of malathion on the insect succession and the development of Chrysomya megacephala (Diptera: Calliphoridae) in the field and implications for estimating postmortem interval. Am. J. Forensic Med. Pathol. 2010, 31, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Alanis, L.A.; Lira-Paredes, A.A.; Pedraza-Lara, C.; Quijano-Mateos, A.; Bravo-Gómez, M.E. Effect of malathion on the development of Megaselia scalaris (Loew, 1866) (Diptera: Phoridae), a Forensically Important Fly. J. Med. Entomol. 2022, 59, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, N.I.; Centeno, N.D. Forensic significance of Roundup Full® II effect on the development of Dermestes maculatus (Coleoptera: Dermestidae) and Lucilia sericata (Diptera: Calliphoridae). J. Forensic Sci. 2024, 69, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Jales, J.T.; Barbosa, T.M.; Moreira, V.R.F.; Vasconcelos, S.D.; de Paula Soares Rachetti, V.; Gama, R.A. Effects of terbufos (organophosphate) on larval behaviour of two forensically important diptera species: Contributions for entomotoxicology. Neotrop. Entomol. 2023, 52, 1155–1164. [Google Scholar] [CrossRef]
- Galil, F.; Zambare, S.P.; Al-Mekhlafi, F.A.; Al-Keridis, L.A. Effect of dimethoate on the developmental rate of forensic importance Calliphoridae flies. Saudi J. Biol. Sci. 2021, 28, 1267–1271. [Google Scholar] [CrossRef]
- Abd Al Galil, F.M.; Zambare, S.P.; Al-Mekhlafi, F.A.; Wadaan, M.A.; Al-Khalifa, M.S. Effects of insecticide dimethoate on the developmental rate of forensic importance sarcophagid flies. J. King Saud Univ.-Sci. 2021, 33, 101349. [Google Scholar] [CrossRef]
- Al-Shuraym, L.A.; Al-Mekhlafi, F.A.; Abd Al Galil, F.M.; Alhag, S.K.; Al-Keridis, L.A.; Ali El Hadi Mohamed, R.; Wadaan, M.A.; Al-Khalifa, M.S. Effect of zolpidem tartrate on the developmental rate of forensically important flies Chrysomya megacephala (Diptera: Calliphoridae) and Chrysomya saffranea. J. Med. Entomol. 2021, 58, 2101–2106. [Google Scholar] [CrossRef]
- Ahmed Al-Keridis, L.; Al Galil, F.M.A.; Al-Mekhlafi, F.A.; Wadaan, M.A.; Al-Khalifa, M.S. Impact of hypnotic drug zolpidem tartrate on the development of forensic fly Sarcophaga ruficornis (Diptera: Sarcophagidae). J. Med. Entomol. 2022, 59, 820–825. [Google Scholar] [CrossRef]
- Annasaheb Bansode, S.; Ramrao More, V. Effect of lorazepam on the development of the hairy maggot blow fly, Chrysomya rufifacies (Macquart): Implication for Forensic Entomology. J. Toxicol. 2023, 2023, 1051736. [Google Scholar] [CrossRef]
- Preußer, D.; Fischer, T.; Juretzek, T. Effects of antibiotics ceftriaxone and levofloxacin on the growth of Lucilia sericata (Diptera: Calliphoridae). Med. Vet. Entomol. 2023, 37, 805–815. [Google Scholar] [CrossRef]
- Preußer, D.; Bröring, U.; Fischer, T.; Juretzek, T. Effects of antibiotics ceftriaxone and levofloxacin on the growth of Calliphora vomitoria L. (Diptera: Calliphoridae) and effects on the determination of the post-mortem interval. J. Forensic Leg. Med. 2021, 81, 102207. [Google Scholar] [CrossRef]
- Preußer, D.; Fischer, T.; Juretzek, T. Effects of antibiotics ceftriaxone and levofloxacin on the growth of Protophormia terraenovae (Diptera: Calliphoridae). Forensic Sci. Med. Pathol. 2024, 20, 1318–1330. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Yang, F.; Zhang, C.; Ngando, F.J.; Ren, L.; Guo, Y. Effect of ciprofloxacin on the composition of intestinal microbiota in Sarcophaga peregrina (Diptera: Sarcophagidae). Microorganisms 2023, 11, 2867. [Google Scholar] [CrossRef]
- Kaur Heer, B. Effect of cadmium chloride on the development of Chrysomya Megacephala (Diptera:Calliphoridae) and its Importance to Postmortem Interval Estimate. J. Forensic Sci. Crim. Investig. 2017, 3, 555622. [Google Scholar] [CrossRef]
- Shulman, M.V.; Pakhomov, O.Y.; Brygadyrenko, V.V. Effect of lead and cadmium ions upon the pupariation and morphological changes in Calliphora vicina (Diptera, Calliphoridae). Folia Oecologica 2017, 44, 28–37. [Google Scholar] [CrossRef]
- Kökdener, M.; Gündüz, N.E.A.; Zeybekoğlu, Ü.; Aykut, U.; Yılmaz, A.F. The effect of different heavy metals on the development of Lucilia sericata (Diptera: Calliphoridae). J. Med. Entomol. 2022, 59, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Essarras, A.; Pazzi, M.; Dadour, I.R.; Magni, P.A. The effect of antifreeze (ethylene glycol) on the survival and the life cycle of two species of necrophagous blowflies (Diptera: Calliphoridae). Sci. Justice 2018, 58, 85–89. [Google Scholar] [CrossRef]
- Cavalcante, K.; Peniche, T.; Façanha, B.L.B.; Araújo, C.M.; Lobato, T.A.S.; Souto, R.N.P. Effect of diazinon (organophosphate) on the composition and succession of Calliphoridae assemblages in rabbit carcasses in the Eastern Amazon. Int. J. Leg. Med. 2023, 137, 1253–1261. [Google Scholar] [CrossRef]
- Jales, J.T.; Barbosa, T.M.; Soares, V.P.; Gama, R.A.; Brundage, A. Effect of Terbufos (Organophosphate) on the cadaveric colonization process: Implications for postmortem interval calculation. J. Med. Entomol. 2021, 58, 1056–1063. [Google Scholar] [CrossRef]
- Eulalio, A.D.M.d.M.; Paula-Silva, M.C.d.; Michelutti, K.B.; Oliveira, F.C.d.; Brum, A.C.d.S.; Lima-Junior, S.E.; Cardoso, C.A.L.; Antonialli-Junior, W.F. Effect of thiamethoxam (organophosphate) on the flies and beetle visitation and cadaveric decomposition process. Rev. Bras. Entomol. 2023, 67, e20220049. [Google Scholar] [CrossRef]
- El-Samad, L.; Hussein, H.; Toto, N.; Mahmoud, D.; Radwan, E. Variation of insect succession in summer on decomposing rabbit carrions treated with aluminum phosphide in Beheira governorate, Egypt. Swed. J. Biosci. Res. 2021, 2, 91–102. [Google Scholar] [CrossRef]
- Saber, T.M.; Hassanen, E.A.A.; Anter, R.G.A.; Farag, M.R.; Saber, T.; Imam, T.S. Identification of forensically important insects on atrazine-intoxicated rat carcasses at different decomposition stages dur-ing summer season. Slov. Vet. Res. 2021, 58, 373–387. [Google Scholar] [CrossRef]
- Al-Qahtni, A.; Mashaly, A.; Haddadi, R.; Al-Khalifa, M.; Byrd, J. Seasonal impact of heroin on rabbit carcass decomposition and insect succession. J. Med. Entomol. 2021, 58, 567–575. [Google Scholar] [CrossRef]
- Al-Khalifa, M.; Mashaly, A.; Al-Qahtni, A. Impacts of antemortem ingestion of alcoholic beverages on insect successional patterns. Saudi J. Biol. Sci. 2021, 28, 685–692. [Google Scholar] [CrossRef]
- Aneyo, I.; Alafia, O.; Doherty, F.; Udoma, R.; Balogun, B.; Adeola, A. Aerobic microbe community and necrophagous insects associated with decomposition of pig carrion poisoned with lead. Leg. Med. 2020, 42, 101638. [Google Scholar] [CrossRef]
- Jales, J.T.; Barbosa, T.M.; Dos Santos, L.C.; Rachetti, V.P.S.; Gama, R.A. Carrion decomposition and assemblage of necrophagous dipterans associated with Terbufos (Organophosphate) intoxicated rat carcasses. Acta Trop. 2020, 212, 105652. [Google Scholar] [CrossRef]
- El-Ashram, S.; Toto, N.A.; El Wakil, A.; Augustyniak, M.; El-Samad, L.M. Reduced body length and morphological disorders in Chrysomya albiceps (Diptera: Calliphoridae) larvae reared on aluminum phosphide-treated rabbits. Sci. Rep. 2022, 12, 8358. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, V.; Venkataraman, S.; Rajendran, D.S.; Vinoth Kumar, V.; Kumar, V.V.; Rangasamy, G. Acetylcholinesterase biosensors for electrochemical detection of neurotoxic pesticides and acetylcholine neurotransmitter: A literature review. Environ. Res. 2023, 227, 115724. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, T.; Sharma, S.; Dalal, J.; Tanwar, R. Effects of aluminium phosphide on larval morphometry of two important Chrysomya species. Int. J. Leg. Med. 2024, 138, 73–83. [Google Scholar] [CrossRef]
- Büyükgüzel, E.; Büyükgüzel, K.; Snela, M.; Erdem, M.; Radtke, K.; Ziemnicki, K.; Adamski, Z. Effect of boric acid on antioxidant enzyme activity, lipid peroxidation, and ultrastructure of midgut and fat body of Galleria mellonella. Cell Biol. Toxicol. 2013, 29, 117–129. [Google Scholar] [CrossRef]
- World Drug Report. Available online: https://www.unodc.org/documents/data-and-analysis/WDR_2024/WDR24_Key_findings_and_conclusions.pdf (accessed on 6 February 2025).
- Goff, M.L.; Brown, W.A.; Omori, A.I. Preliminary observations of the effect of methamphetamine in decomposing tissues on the development rate of Parasarcophaga ruficornis (Diptera: Sarcophagidae) and implications of this effect on the estimations of postmortem intervals. J. Forensic Sci. 1992, 37, 867–872. [Google Scholar] [CrossRef]
- George, K.A.; Archer, M.S.; Green, L.M.; Conlan, X.A.; Toop, T. Effect of morphine on the growth rate of Calliphora stygia (Fabricius) (Diptera: Calliphoridae) and possible implications for forensic entomology. Forensic Sci. Int. 2009, 193, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Goff, M.L.; Brown, W.A.; Hewadikaram, K.A.; Omori, A.I. Effect of heroin in decomposing tissues on the development rate of Boettcherisca peregrina (Diptera, Sarcophagidae) and implications of this effect on estimation of postmortem intervals using arthropod development patterns. J. Forensic Sci. 1991, 36, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V.M.; Gandhi, T.N.; Chopra, V.; Petty, L.A.; Giesler, D.L.; Malani, A.N.; Bernstein, S.J.; Hsaiky, L.M.; Pogue, J.M.; Dumkow, L.; et al. Antibiotic overuse after hospital discharge: A multi-hospital cohort study. Clin. Infect. Dis. 2021, 73, e4499–e4506. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef]
- Denk-Lobnig, M.; Wood, K.B. Antibiotic resistance in bacterial communities. Curr. Opin. Microbiol. 2023, 74, 102306. [Google Scholar] [CrossRef]
- Ruan, L.; Ye, K.; Wang, Z.; Xiong, A.; Qiao, R.; Zhang, J.; Huang, Z.; Cai, M.; Yu, C. Characteristics of gut bacterial microbiota of black soldier fly (Diptera: Stratiomyidae) larvae effected by typical antibiotics. Ecotoxicol. Environ. Saf. 2024, 270, 115861. [Google Scholar] [CrossRef]
- Luo, M.; Cao, H.M.; Fan, Y.Y.; Zhou, X.C.; Chen, J.X.; Chung, H.; Wei, H.Y. Bioaccumulation of cadmium affects development, mating behavior, and fecundity in the asian corn borer, Ostrinia furnacalis. Insects 2019, 11, 7. [Google Scholar] [CrossRef]
- Zhan, H.; Zhang, J.; Chen, Z.; Huang, Y.; Ruuhola, T.; Yang, S. Effects of Cd2+ exposure on key life history traits and activities of four metabolic enzymes in Helicoverpa armigera (Lepidopteran: Noctuidae). Chem. Ecol. 2017, 33, 325–338. [Google Scholar] [CrossRef]
- Boulkenafet, F.; Bouhayene, S.; Benzazia, S.; Lambiase, S.; Al-Khalifa, M.S.; Toumi, M.; Al-Mekhlafi, F.A. Heavy metal bioaccumulation in rabbit organs and Chrysomya albiceps (wiedemann, 1819) larvae: Implications for forensic entomology. Indian J. Anim. Res. 2024. [Google Scholar] [CrossRef]
- Pölkki, M.; Kangassalo, K.; Rantala, M.J. Effects of interaction between temperature conditions and copper exposure on immune defense and other life-history traits of the blow fly Protophormia terraenovae. Environ. Sci. Technol. 2014, 48, 8793–8799. [Google Scholar] [CrossRef]
- Dabour, K.; Al Naggar, Y.; Masry, S.; Naiem, E.; Giesy, J.P. Cellular alterations in midgut cells of honey bee workers (Apis millefera L.) exposed to sublethal concentrations of CdO or PbO nanoparticles or their binary mixture. Sci. Total Environ. 2019, 651, 1356–1367. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yang, X.; Sun, L.; Han, X.; Xu, L.; Gu, W.; Zhang, M. Effects of cadmium on oxidative stress and cell apoptosis in Drosophila melanogaster larvae. Sci. Rep. 2022, 12, 4762. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Mo, F.; Han, Y.; Gu, W.; Zhang, M. Digital gene expression profiling (DGE) of cadmium-treated Drosophila melanogaster. Environ. Toxicol. Pharmacol. 2015, 39, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, D.; Wang, C.; Chi, X.; Liu, Z.; Wang, Y.; Wang, H.; Guo, X.; Wang, N.; Xu, B.; et al. Toxic effects of the heavy metal Cd on Apis cerana cerana (Hymenoptera: Apidae): Oxidative stress, immune disorders and disturbance of gut microbiota. Sci. Total Environ. 2024, 912, 169318. [Google Scholar] [CrossRef]
- Ziller, A.; Fraissinet-Tachet, L. Metallothionein diversity and distribution in the tree of life: A multifunctional protein. Metallomics 2018, 10, 1549–1559. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Gao, Z.; Wen, Y.; Wang, W.; Liu, W.; Wang, X.; Zhu, F. Identification of three metallothioneins in the black soldier fly and their functions in Cd accumulation and detoxification. Environ. Pollut. 2021, 286, 117146. [Google Scholar] [CrossRef]
- Tony, M.; Ashry, M.; Tanani, M.M.A.; Abdelreheem, A.M.A.; Abdel-Samad, M.R.K. Bio-efficacy of aluminum phosphide and cypermethrin against some physiological and biochemical aspects of Chrysomya megacephala maggots. Sci. Rep. 2023, 13, 4407. [Google Scholar] [CrossRef]
- Yang, X.; Gong, J.; Zhang, X.; Zhang, W.; Li, D.; Lin, J.; Li, X.; Chai, Y.; Liu, J. The responses of the growth, cytochrome P450 isoenzymes activities and the metabolomics in earthworms to sublethal doses of dichlorvos in soil. Ecotoxicol. Environ. Saf. 2021, 207, 111547. [Google Scholar] [CrossRef]
- Szeremeta, M.; Pietrowska, K.; Niemcunowicz-Janica, A.; Kretowski, A.; Ciborowski, M. Applications of metabolomics in forensic toxicology and forensic medicine. Int. J. Mol. Sci. 2021, 22, 3010. [Google Scholar] [CrossRef]
- Ishak, N.; Ahmad, A.H.; Mohamad Noor, S.A.; Ahmad, A. Detection of heroin metabolites at different developmental stages of Lucilia cuprina (Diptera: Calliphoridae) reared in heroin-treated meat: A preliminary analysis. Egypt. J. Forensic Sci. 2019, 9, 65. [Google Scholar] [CrossRef]
- Buratti, E.; Mietti, G.; Cippitelli, M.; Cerioni, A.; Froldi, R.; Cingolani, M.; Scendoni, R. Detection of three opioids (morphine, codeine and methadone) and their metabolites (6-monoacetylmorphine and 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine) in larvae of lucilia sericata species by UHPLC-TF-MS and validation. Molecules 2023, 28, 4649. [Google Scholar] [CrossRef]
- Muñoz-Benavent, M.; Pérez-Cobas, A.E.; García-Ferris, C.; Moya, A.; Latorre, A. Insects’ potential: Understanding the functional role of their gut microbiome. J. Pharm. Biomed. Anal. 2021, 194, 113787. [Google Scholar] [CrossRef]
- Rupawate, P.S.; Roylawar, P.; Khandagale, K.; Gawande, S.; Ade, A.B.; Jaiswal, D.K.; Borgave, S. Role of gut symbionts of insect pests: A novel target for insect-pest control. Front. Microbiol. 2023, 14, 1146390. [Google Scholar] [CrossRef]
- Reel, P.S.; Reel, S.; Pearson, E.; Trucco, E.; Jefferson, E. Using machine learning approaches for multi-omics data analysis: A review. Biotechnol. Adv. 2021, 49, 107739. [Google Scholar] [CrossRef]
- Bersanelli, M.; Mosca, E.; Remondini, D.; Giampieri, E.; Sala, C.; Castellani, G.; Milanesi, L. Methods for the integration of multi-omics data: Mathematical aspects. BMC Bioinform. 2016, 17 (Suppl. 2), 15. [Google Scholar] [CrossRef]
- Galante, N.; Cotroneo, R.; Furci, D.; Lodetti, G.; Casali, M.B. Applications of artificial intelligence in forensic sciences: Current potential benefits, limitations and perspectives. Int. J. Leg. Med. 2023, 137, 445–458. [Google Scholar] [CrossRef]
- Vadapalli, S.; Abdelhalim, H.; Zeeshan, S.; Ahmed, Z. Artificial intelligence and machine learning approaches using gene expression and variant data for personalized medicine. Brief. Bioinform. 2022, 23, bbac191. [Google Scholar] [CrossRef]
- Zhang, X.; Long, T.; Deng, S.; Chen, Q.; Chen, S.; Luo, M.; Yu, R.; Zhu, X. Machine learning modeling based on microbial community for prediction of natural attenuation in groundwater. Environ. Sci. Technol. 2023, 57, 21212–21223. [Google Scholar] [CrossRef]
- Mason, A.R.; McKee-Zech, H.S.; Steadman, D.W.; DeBruyn, J.M. Environmental predictors impact microbial-based postmortem interval (PMI) estimation models within human decomposition soils. PLoS ONE 2024, 19, e0311906. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Dong, F.; Song, M.; Li, Z.; Khan, M.K.H.; Patterson, T.A.; Hong, H. Review of machine learning and deep learning models for toxicity prediction. Exp. Biol. Med. 2023, 248, 1952–1973. [Google Scholar] [CrossRef]
- Behnoush, B.; Bazmi, E.; Nazari, S.H.; Khodakarim, S.; Looha, M.A.; Soori, H. Machine learning algorithms to predict seizure due to acute tramadol poisoning. Hum. Exp. Toxicol. 2021, 40, 1225–1233. [Google Scholar] [CrossRef]
| Drugs/Toxins | Dose | Food Source | Analytical Methods | Sample Preparation for Toxicological Analysis | Species of Flies | Developmental Rate | References |
|---|---|---|---|---|---|---|---|
| Methamphetamine | 10 mg/kg | Kangaroo mice | HPLC–UV | The solution was extracted with CH2Cl2, and the combined organic layers were concentrated to produce p-hydroxyamphetamine. Boc2O was added. CH2Cl2 was then added, and the organic layer was extracted. | Calliphora stygia (Fabricuis, 1781) | Larval growth significantly accelerated and increased the size of all life stages. The pupal stage was prolonged 78 h. | [8] |
| Methamphetamine | 180 mg/kg | Rabbits | GC–MS | The larvae were placed in falcon tubes, and dichloromethane was added. The tube was stored at 4 °C for 48 h to fully dissolve the matrix. The salting-out effect was attained by adding NaCl. | Calliphora (Aldrichina) grahami Aldrich, 1930 | The developmental time to reach the pupal stage was slower. the mean length of larvae was longer. | [37] |
| Cocaine | 17 mg/kg | Rabbit liver | GC–MS | Liver was homogenised using methanol. The supernatant was recovered, and 1 N acetic acid was added. Ether: hexane was then added. The organic layers were taken to waste and the aqueous phases were pipetted onto cation-exchange resin columns. | Chrysomya albiceps (Wiedemann, 1819), Chrysomya putoria (Wiedemann, 1830) | The larvae developed faster than the control, indicating that the drug influences and stimulates larval growth. | [38] |
| Morphine | 3/6/12 mg/kg | Rabbits | RIA kit | Cleaned larvae were dried, weighed, and cut with scissors. To determine morphine, samples were homogenized and centrifuged for 15 min at 10,000 rpm, and the supernatant was obtained. | Lucilia sericata (Meigen, 1826), Chrysomya megacephala (Fabricius, 1794), Sarcophaga argyrostoma (Robineau-Desvoidy, 1830), C. albiceps | After morphine treatment, the larval age estimates based on the mean body length had large errors, which were 24, 27, 6, and 21 h, respectively. | [39] |
| Methadone | 4 mg/g | Beef heart | UPLC–MS/MS | Samples were ground to powder and transferred to a glass vial after addition of deionized water and saturated ammonium chloride buffer. Following centrifugation, the clear organic phase was transferred to a clean vial and evaporated to dryness in a vacuum centrifuge. | L. sericata | Development seemed to be slightly decelerated in presence of high methadone concentration. | [40] |
| Carbamazepine and clobazam | 3271.57/414.08 mg/kg | Rabbits | UHPLC/QTOF-MS | Homogenates were inserted in tubes containing methanol. Three successive stirrings were carried out to dissolve the solutes in methanol and facilitate the extraction. Then, the mixtures were centrifuged. | Lucilia silvarum (Meigen, 1826), C. albiceps, L. sericata | C. albiceps larvae fed on drugs developed faster, while the development of L. sericata and L. silvarum larvae slowed. | [34] |
| Malathion | 1530 mg/kg | Rabbits | GC-MC | Larvae were washed and homogenized with anhydrous sodium sulfate. Acetone was added to the sample. Sodium sulfate and dichloromethane were added to the extraction and homogenized. The underlayer was collected, and the residue was re-extracted with dichloromethane. | C. megacephala | The maximum length of larvae and weight of pupae were observed under increasing concentrations. The rate of development varies from 12 to 36 h. | [41] |
| Malathion | / | / | HPLC-DAD | 60 mL of acetone was added to the tested half and agitated for 45 min; the resulting extract was filtrated and washed with acetone. The acetone extracts were mixed, and anhydride sulfate sodium was added and homogenized. Extraction was perfrormed by adding dichloromethane. | Fannia scalaris (Fabricius, 1794) | It reduced the larval growth rate and increased the duration of the larval stage. | [42] |
| Glyphosate (herbicides) | 7.69 mL/kg | Pigs | / | / | L. sericata | The duration of the developmental stages remained unchanged, but all size parameters of the puparium were reduced. | [43] |
| Terbufos (organophosphate) | 20 mg/kg | Rats | / | / | Lucilia eximia (Wiedemann, 1819), Peckia chrysostoma (Wiedemann, 1830) | Larvae of L. eximia were more active, with greater frequency of body movements and lateral contractions. Immature P. chrysostoma were less active, with fewer body and lateral contractions. | [44] |
| Dimethoate | 1–4 mg/kg | Sheep liver | / | / | Chrysomya saffranea (Bigot, 1877); Chrysomya rufifacies (Macquart, 1843); Chrysomya indiana Walker, 1861; C. megacephala | Dimethoate causes a delay in development. The duration increased with an increase in concentration. | [45] |
| Dimethoate | 1–4 mg/kg | Sheep liver | / | / | Sarcophaga peregrina (Robineau-Desvoidy, 1830); Sarcophaga dux Thomson, 1869; Sarcophaga ruficornis (Fabricius, 1794) | Dimethoate delays the larval, pupal, and prepupal stages of development. | [46] |
| Benzoylecgonine and morphine | 17/34 mg/kg | Pork mince | / | / | Calliphora vomitoria (Linnaeus, 1758) | Cocaine shortened pupation and accelerated eclosion, and the insects less in length and weight. Heroin led to lengthier pupation, and the insects were smaller and lighter. | [9] |
| Zolpidem tartrate | 1–4 mg/kg | Buffalo liver | / | / | C. megacephala, C. saffranea | The weight, length, and width decreased as the concentration increased. The duration of both developmental stages increased as the concentration increased. | [47] |
| Zolpidem tartrate | 1–4 mg/kg | Buffalo liver | / | / | S. ruficornis | The total developmental durations were prolonged when the concentration increased. | [48] |
| Lorazepam | 1–4 mg/kg | Beef liver | / | / | C. rufifacies | Length, weight, and width of larvae decreased with increased concentration of lorazepam. | [49] |
| Ceftriaxone and levofloxacin | 28.57/3.57 mg/kg | Minced pork | / | / | L. sericata | The time to pupation was significantly extended, and the mortality rate increased. | [50] |
| 28.56/3.56 mg/kg | Minced pork | / | / | C. vomitoria | The maggot growth was delayed by levofloxacin but not with ceftriaxone. Pupation was delayed in both antibiotics, and mortality was reduced. | [51] | |
| 28.57/3.57 mg/kg | Minced pork | / | / | Calliphora (Protophormia) terraenovae Macquart, 1851 | The maggot development time was significantly decreased. The time to start pupation was significantly increased. The survivability of the maggots was improved. | [52] | |
| Ciprofloxacin | 1.33 mg/kg | Pork lung | / | / | S. peregrina | The length of larvae increased with higher drug concentrations, while the weight of both the pupa and adult decreased significantly. | [53] |
| Cadmium | 6.5 mg/kg (lethal) | Rats | / | / | C. megacephala | Development time was prolonged at higher concentrations, and larval mortality increased with increasing concentration. | [54] |
| Lead and cadmium ions | / | Pork liver | / | / | Calliphora vicina Robineau-Desvoidy, 1830 | Fly larvae exhibited reduced motor activity, along with delays in puparia formation and adult emergence. | [55] |
| Cadmium, zinc, copper | 2 mg/kg | Chicken livers | / | / | L. sericata | Larval and pupal survival decreased as heavy metal concentrations increased. Pupal weight and larval length were significantly different among heavy metals and concentrations. | [56] |
| Ethylene glycol | 28 mL/kg | Beef liver | / | / | Lucilia cuprina (Wiedemann, 1830) L. sericata | Neither species can survive in high concentrations. The developmental time of both species is slower than the control; the body length of the immatures is also smaller. | [57] |
| Toxic Substances | Dose | Food Source | Location | Community Succession | Rate of Decay | References |
|---|---|---|---|---|---|---|
| Diazinon | 100/300 mg/kg | Rabbits | Eastern Amazon | The adult specimens in the control group with the highest abundance were observed only from the advanced decay stage onward. In the dry stage, abundance was higher in control than in treated carcasses. The larvae of C. albiceps (76.3%), C. putoria (1%), and L. eximia (22.7%) were identified; the number of immatures was higher in control. | Diazinon slowed down the decomposition stages. | [58] |
| Malathion | 1530 mg/kg | Rabbits | Sun Yat-Sen University, Guangzhou | C. megacephala was the most abundant adult species in all groups. Larvae of C. rufifacies were only collected from the control; the appearance of beetles on the treated carcass was later by 1 to 3 days than on the control carcass. | Malathion altered decomposition rates. | [41] |
| Terbufos | 5/10 mg/kg | Rats | Federal University of Rio Grande do Norte | C. albiceps was collected with a clearly high dose of terbufos; L. eximia S. nudiseta and P. chrysostoma were collected with low doses. | Higher doses accelerated decomposition. | [59] |
| Thiamethoxam | / | Pigs | Dourados, Mato Grosso do Sul, Brazil | 1462 specimens from Diptera and 279 Coleoptera were identified, mainly including C. megacephala and L. eximia. In the control group, 641 Diptera and 385 Coleoptera were collected, obtaining C. putoria, L. cuprina, C. albiceps, and D. maculatus. | The experimental group took longer to reach the late stage of corruption than the control group. | [60] |
| Aluminum phosphide (AIP) | 27.4 mg/kg | Rabbits | Beheira, Egypt | C. rufifacies was detected only in control. C. albiceps and C. megacephalla were presented in all groups but exhibited variations across the decomposition process. | AlP appeared to delay the decomposition process. | [61] |
| Atrazine | 3000 mg/kg | Rats | Zagazig University, Egypt | A delay in the colonization of insect fauna was observed in treatment. In the control group, Dipteran insects were the most dominant insects (57.14%), followed by Coleopteran insects (42.85%). The treatment showed 42.85% for insects of order Diptera and 57.14% for Coleoptera. | Decay of carrions was delayed in treatment. | [62] |
| Heroin | 6/12/18 mg | Rabbits | Riyadh, Saudi Arabia | Heroin did not have a significant impact on the number of insects, but during the summer, M. domestica and C. albiceps were more attracted to treated carcasses with a higher dose. Flies were more attracted to carcasses with a higher dose. | Heroin appeared to delay the decomposition process. | [63] |
| Alcohol | 25/50/75 mL | Rabbits | Riyadh, Saudi Arabia | Alcoholic beverages did not significantly affect insect succession patterns. | The treated rabbits took two days longer than the untreated ones to reach the dry stage in winter and one day longer in summer. | [64] |
| Lead (Pb) | 0.18/0.2 mg/kg | Pigs | Lagos State University, Nigeria | The decomposition rate of pigs fed with lead-contaminated feed attracted insects. C. chloropyga was the most predominant. | The decomposition rate of pigs fed with lead-contaminated feed increased the rate of hair fall. | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Liu, Z.; Su, J.; Yang, Z.; Wang, Z.; Yao, X.; Lyu, Z.; Xia, Y.; Zhang, S.; Cui, W.; et al. Advances in Forensic Entomotoxicology for Decomposed Corpses: A Review. Insects 2025, 16, 744. https://doi.org/10.3390/insects16070744
Hou S, Liu Z, Su J, Yang Z, Wang Z, Yao X, Lyu Z, Xia Y, Zhang S, Cui W, et al. Advances in Forensic Entomotoxicology for Decomposed Corpses: A Review. Insects. 2025; 16(7):744. https://doi.org/10.3390/insects16070744
Chicago/Turabian StyleHou, Sen, Zengjia Liu, Jiali Su, Zeyu Yang, Zhongjiang Wang, Xinyi Yao, Zhou Lyu, Yang Xia, Shuguang Zhang, Wen Cui, and et al. 2025. "Advances in Forensic Entomotoxicology for Decomposed Corpses: A Review" Insects 16, no. 7: 744. https://doi.org/10.3390/insects16070744
APA StyleHou, S., Liu, Z., Su, J., Yang, Z., Wang, Z., Yao, X., Lyu, Z., Xia, Y., Zhang, S., Cui, W., Wang, Y., & Ren, L. (2025). Advances in Forensic Entomotoxicology for Decomposed Corpses: A Review. Insects, 16(7), 744. https://doi.org/10.3390/insects16070744







