The Effects of Six Brassica napus Cultivars on the Life Table Parameters of the Green Peach Aphid Myzus persicae (Sulzer) (Hemiptera: Aphididae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plants Cultures
2.2. Life Table Study of M. persicae
2.3. Data Analysis
3. Results
3.1. Survival Rate of M. persicae on Six B. napus Cultivars
3.2. Developmental Duration of M. persicae on Six B. napus Cultivars
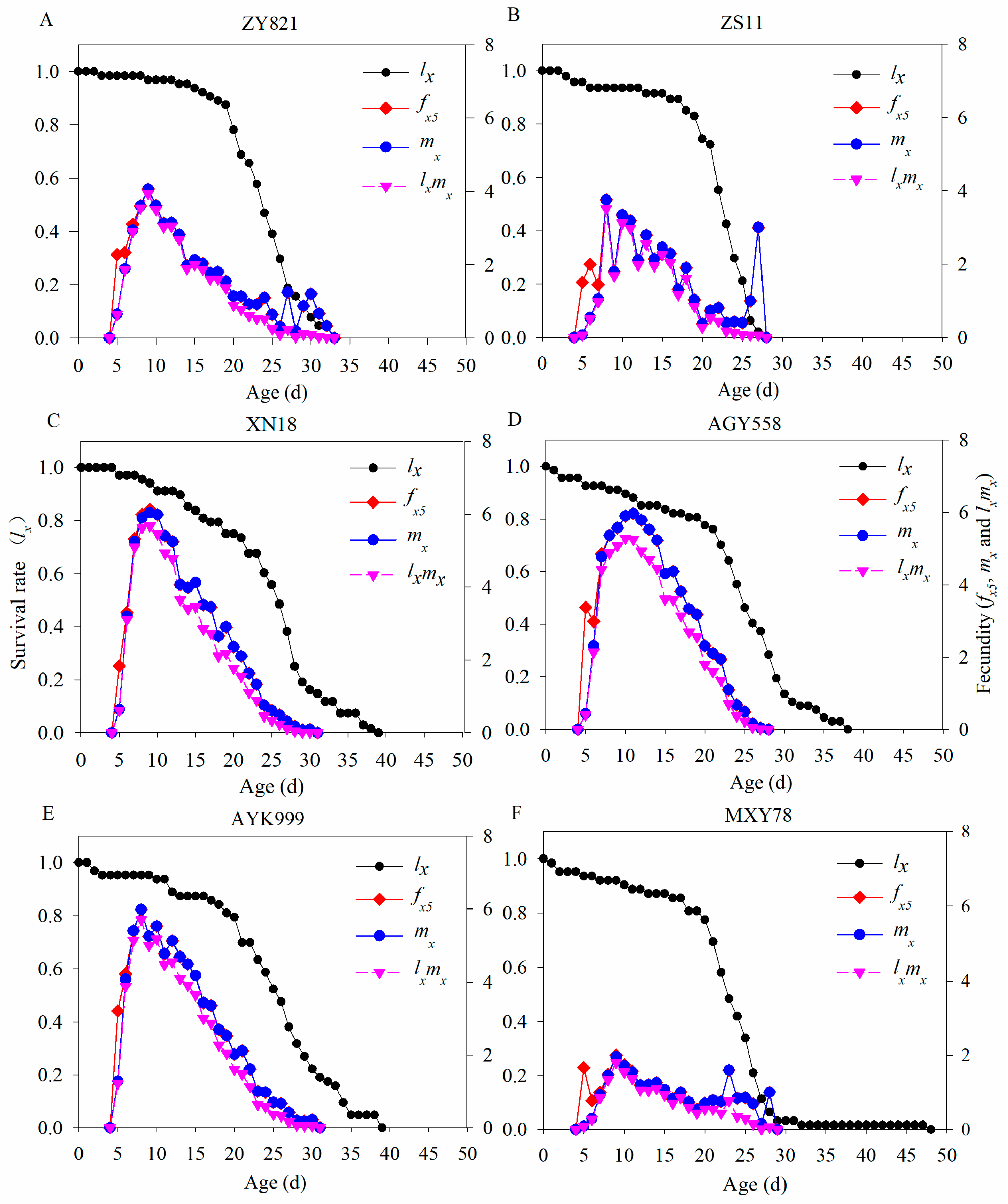
3.3. The Population Survival Rate and Fecundity of M. persicae in Six B. napus Cultivars
3.4. The Population Parameters of M. persicae in Six B. napus Cultivars
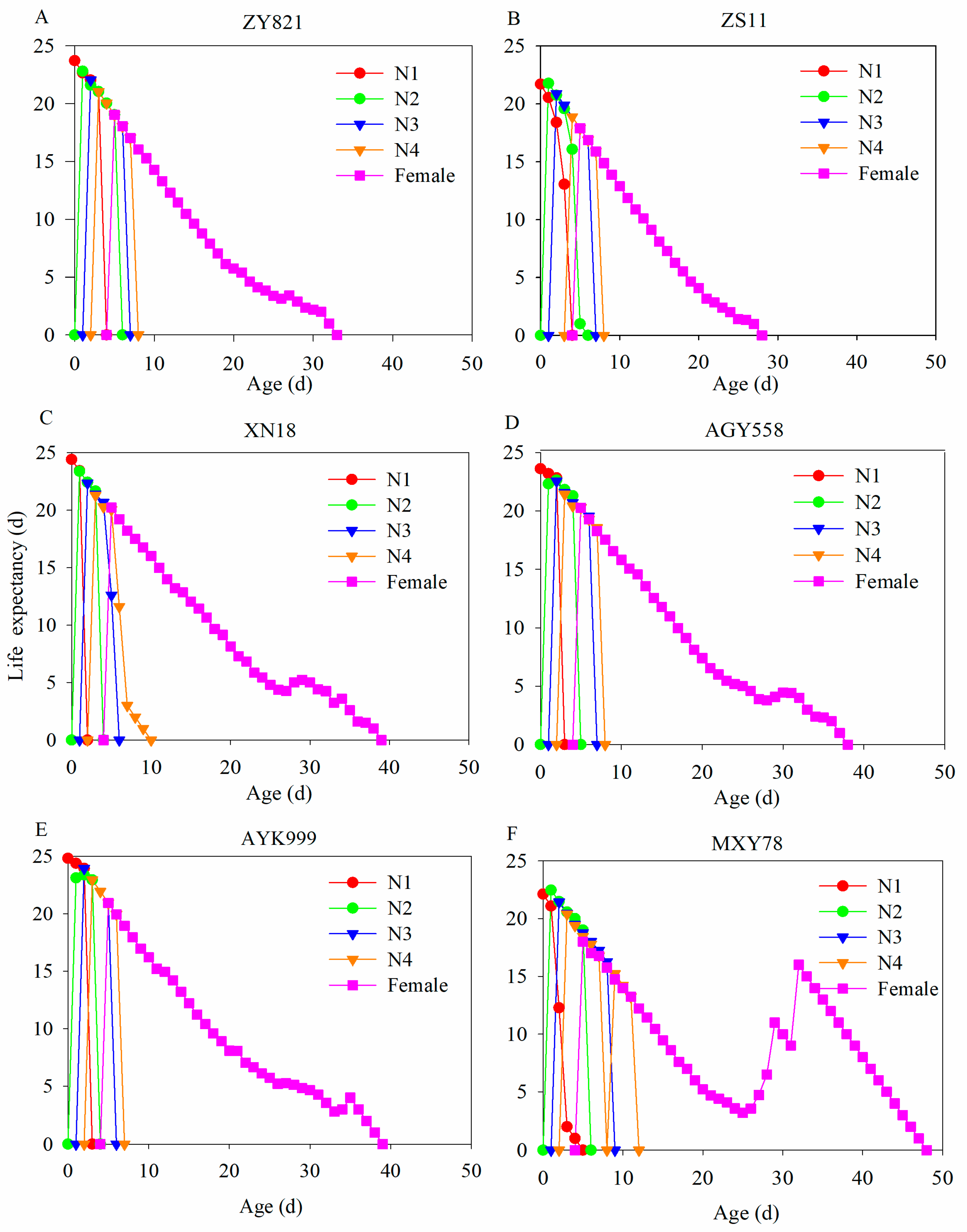
3.5. Life Expectancy of M. persicae in Six B. napus Cultivars
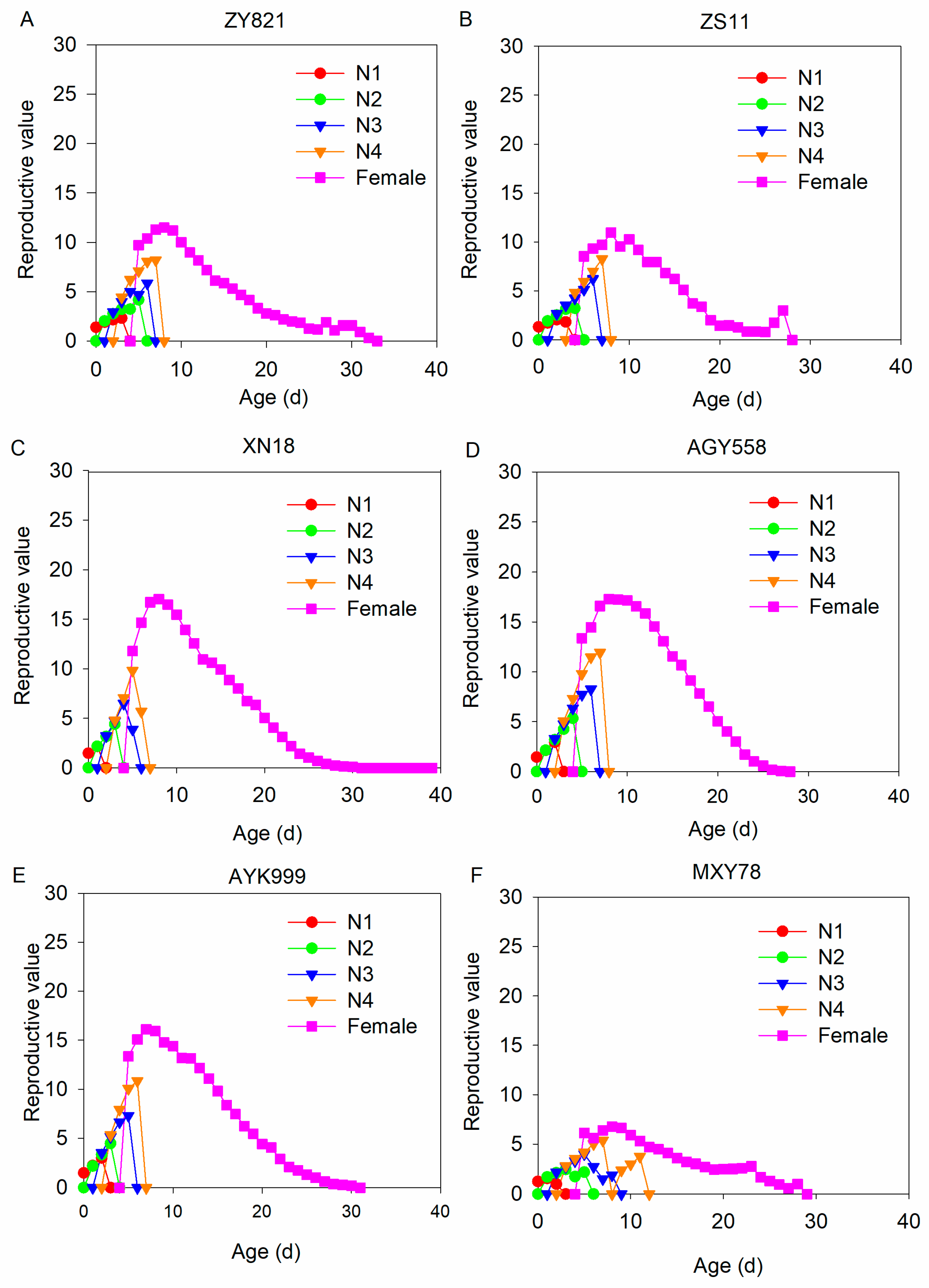
3.6. Reproductive Value of M. persicae in Six B. napus Cultivars
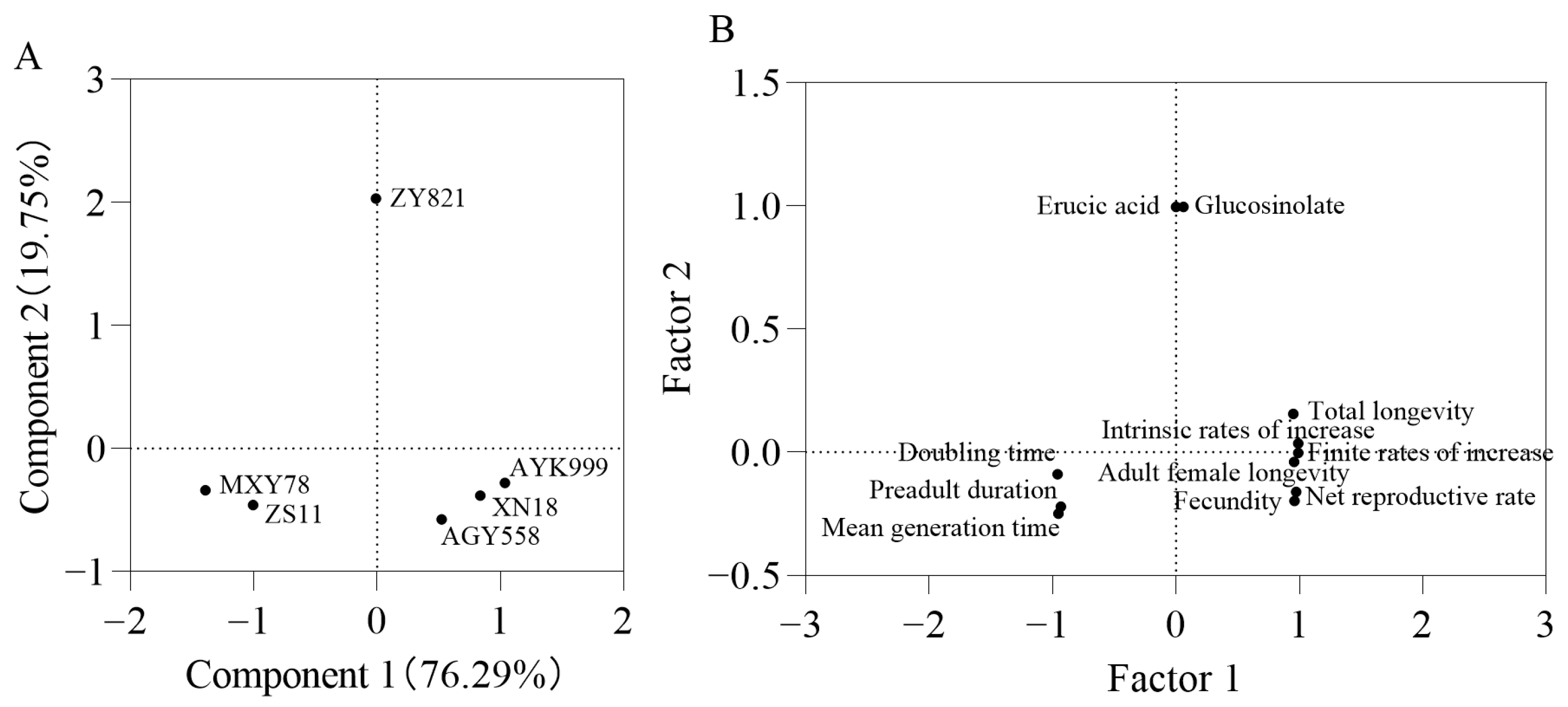
3.7. Principal Component Analysis of Different Brassica napus Seeds and Developmental Duration and Fecundity of Other M. persicae in Six B. napus Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.P.; Liu, W.C.; Huang, C. Statistics and analysis of oilseed rape losses caused by main diseases and insect pests in recent 10 years. Plant Prot. 2018, 44, 24–30. [Google Scholar]
- Wu, X.M.; Chen, B.Y.; Lu, G.Y.; Cao, J.; Zhang, L.; Zhang, J.; Li, Z. Descriptors and Data Standard for Rapeseed (Brassca spp.); China Agriculture Press: Beijing, China, 2007; pp. 11–12. [Google Scholar]
- Zheng, X.; Koopmann, B.; Ulber, B.; von Tiedemann, A. A global survey on diseases and pests in oilseed rape—Current challenges and innovative strategies of control. Front. Agron. 2020, 2, 590908. [Google Scholar] [CrossRef]
- Hausmann, J.; Heimbach, U.; Gabriel, D.; Brandes, M. Effects of regional crop rotations on autumn insect pests in winter oilseed rape. Pest Manag. Sci. 2024, 80, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.P.; Zhan, H.X.; Gao, L.L.; Huang, F.; Zhu, L.N.; Hou, S.M. Possible effects of leaf tissue characteristics of oilseed rape Brassica napus on probing and feeding behaviors of cabbage aphids Brevicoryne brassicae. Arthropod-Plant Interact. 2020, 14, 733–744. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 353, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Stará, F.; Horská, T.; Zuskováb, E.; Kocourek, F. Pyrethroid and carbamate resistance in Czech populations of Myzus persicae (Sulzer) from oilseed rape. Pest Manag. Sci. 2024, 80, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Arena, G.D.; Ray, S.; Flannigan, S.; Casteel, C.L. The potyviral protein 6K1 reduces plant proteases activity during Turnip mosaic virus infection. Viruses 2022, 14, 1341. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Clark, R.E.; Bera, S.; Casteel, C.L.; Crowder, D.W. Responses of pea plants to multiple antagonists are mediated by order of attack and phytohormone crosstalk. Mol. Ecol. 2021, 30, 4939–4948. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, L.S.; Babineau, M.; Ward, S.E.; Van Rooyen, A.R.; Chirgwin, E.; Mata, L.; Umina, P.A. Assessing the risk of resistance to flonicamid and afidopyropen in green peach aphid (Hemiptera: Myzus persicae) via in-vivo selection. Crop Prot. 2024, 184, 106783. [Google Scholar] [CrossRef]
- Zhang, K.X.; Lin, C.Y.; Liu, H.P.; Haq, I.U.; Quandahor, P.; Gou, Y.P.; Liu, C.Z. Drought reduced the adaptability of Myzus persicae on drought-tolerant potato cultivars. Entomol. Gen. 2024, 44, 563–571. [Google Scholar] [CrossRef]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J.A. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Lou, Y.G. Insect-Plant Interactions; Science Publishing Press: Beijing, China, 2023; pp. 433–454. [Google Scholar]
- Jaleel, W.; Saeed, S.; Saeed, Q.; Naqqash, M.N.; Sial, M.U.; Aine, Q.U.; Lu, L. Effects of three different cultivars of cruciferous plants on the age-stage, two-sex life table traits of Plutella xylostella (L.) (Lepidoptera: Plutellidae). Entomol. Res. 2019, 49, 151–157. [Google Scholar] [CrossRef]
- Liu, N.; Tang, T.Z.; Fan, Q.X.; Meng, D.; Li, Z.; Li, Y.; Zhang, T. Breeding and application of Mianyou series of high-erucic acid rapeseed varieties. Agric. Sci. Technol. 2017, 18, 2049–2052. [Google Scholar]
- Tian, E.T.; Liang, H.L.; Wang, J.J.; Guo, J.; Cao, H.; Lin, S. Variation and correlation of erucic acid, oleic acid and glucosinolate contents in Brassica rapa seeds. Guizhou Agric. Sci. 2017, 45, 22–25. [Google Scholar]
- Wang, T.Y.; Yu, K.J.; Wang, W.; Ye, B.T.; Khattak, A.N.; Yang, R.Q.; Tian, E.T. Variation and correlation analysis of erucic acid and glucoside content in Germplasm Groups of Brassica napus. Seed 2020, 39, 59–62. [Google Scholar]
- NY/T 1795-2009; Grades and Specifications of Double Low Rapeseed. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2009.
- NY/T 1990-2011; High Erucic acid Rapeseed. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2011.
- Wang, P.D.; Xiong, X.J.; Zhang, X.B.; Wu, G.; Liu, F. A review of erucic acid production in Brassicaceae oilseeds: Progress and prospects for the genetic engineering of high and low-erucic acid rapeseeds (Brassica napus). Front. Plant Sci. 2022, 13, 899076. [Google Scholar] [CrossRef] [PubMed]
- Galanty, A.; Grudzinska, M.; Pazdziora, W.; Paśko, P. Erucic acid–both sides of the story: A concise review on its beneficial and toxic properties. Molecules 2023, 28, 1924. [Google Scholar] [CrossRef] [PubMed]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; You, M.S.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Gökçe, A. Age-stage two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull Inst Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rate among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. Academy of Agricultural Sciences, Fujian, China, 2024. Available online: https://www.faas.cn/cms/sitemanage/index.shtml?siteId=810640925913080000 (accessed on 24 December 2024).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall Press: New York, NY, USA, 1993. [Google Scholar]
- Huang, Y.B.; Chi, H. Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): With an invalidation of the jackknife technique. J. Appl. Entomol. 2013, 137, 327–339. [Google Scholar] [CrossRef]
- Li, J.Y.; Chen, Y.T.; Fu, J.W.; Shi, M.Z.; Chi, H.; You, M.S. Application of the bootstrap technique and the multinomial theorem in the research of age-stage, two-sex life table. Acta Entomol. Sin. 2022, 65, 1389–1400. [Google Scholar]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813, Erratum in Pest Manag. Sci. 2014, 70, 1936. [Google Scholar]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Acetamiprid resistance and fitness costs of melon aphid, Aphis gossypii: An age-stage, two-sex life table study. Pestic. Biochem. Physiol. 2021, 171, 104729. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Cai, L.; Ding, T.; Tian, E.; Yan, X.; Wang, X.; Chen, Z. Comparative transcriptome analysis reveals the molecular basis of Brassica napus in response to aphid stress. Plants 2023, 12, 2855. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Naseem, T.; Arshad, M.; Ashraf, I.; Rizwan, M.; Tahir, M.; Liu, T.X. Host-plant variations affect the biotic potential, survival, and population projection of Myzus persicae (Hemiptera: Aphididae). Insects 2021, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Mewis, I.; Appel, H.M.; Hom, A.; Raina, R.; Schultz, J.C. Major signaling pathways modulate arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 2005, 138, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Liu, L.; Zhu, B.F.; Wang, C.Z.; Wang, H.X.; Ma, Y.X.; Wang, S.S. Effects of glucosinolates on the growth, survival and protective enzymes of pea aphid (Acythosiphon pisum). Acta Agrestia Sin. 2021, 29, 1675–1681. [Google Scholar]
- Zhao, Y.G.; Jiang, M.X.; Zhang, W.; Yang, J.; Lu, G.; Cheng, Y.; Zhang, X. Bird damage and glucosinolates of canola (Brassica napus L.). Chin. J. Oil Crop Sci. 2016, 38, 111–115. [Google Scholar]
- Li, P.W. Glucosinolate and Their Relationship Between Leaves and Seeds in Brassica napus. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2006. [Google Scholar]
- Ahmed, N.; Darshanee, H.L.C.D.; Khan, I.A.; Zhang, Z.F.; Liu, T.X. Host selection behavior of the green peach aphid, Myzus persicae, in response to volatile organic compounds and nitrogen contents of cabbage cultivars. Front. Plant Sci. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Darshanee, H.L.C.; Fu, W.Y.; Hu, X.S.; Fan, Y.; Liu, T.X. Resistance of seven cabbage cultivars to green peach aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2018, 111, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; De Alwis, M.; Bak, A.; Dong, H.; Whitham, S.A.; Jander, G. Disruption of ethylene responses by Turnip mosaic virus mediates suppression of plant defense against the green peach aphid vector. Plant Physiol. 2015, 69, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Parizad, S.; Bera, S. The effect of organic farming on water reusability, sustainable ecosystem, and food toxicity. Environ. Sci. Pollut. Res. 2023, 30, 71665–71676. [Google Scholar] [CrossRef] [PubMed]

| Cultivar Name | Abbreviation Name | Erucic Acid Content (%) | Oil Content (%) | Glucosinolate (µmol/g/ Rapeseed Cake) |
|---|---|---|---|---|
| ‘Zhongyou 821’ | ZY821 | 45.45% | _ | 113.5 |
| ‘Zhongshuang 11’ | ZS11 | 0 | 49.04% | 18.84 |
| ‘Xinong 18’ | XN18 | 0 | 41.20% | 23.19 |
| ‘Aiganyou 558’ | AGY558 | 0.2% | 49.23% | 21.07 |
| ‘Aiyouku 999’ | AYK999 | 1.08% | 41.04% | 28.04 |
| ‘Mianxingyou 78’ | MXY78 | 0.4% | 46.18% | 20.31 |
| Duration (d) | ZY821 | ZS11 | XN18 | AGY558 | AYK999 | MXY78 |
|---|---|---|---|---|---|---|
| 1st-instar duration/d | 1.66 ± 0.08 bc | 2.36 ± 0.12 a | 1.38 ± 0.06 d | 1.86 ± 0.08 b | 1.57 ± 0.07 c | 1.72 ± 0.06 bc |
| 2nd-instar duration/d | 1.33 ± 0.06 ab | 1.41 ± 0.08 ab | 1.35 ± 0.06 ab | 1.30 ± 0.06 bc | 1.15 ± 0.05 c | 1.52 ± 0.08 a |
| 3rd-instar duration/d | 1.60 ± 0.07 a | 1.45 ± 0.08 ab | 1.43 ± 0.06 ab | 1.60 ± 0.07 a | 1.32 ± 0.07 b | 1.59 ± 0.08 a |
| 4th-instar duration/d | 1.37 ± 0.06 cd | 1.75 ± 0.11 ab | 1.52 ± 0.07 bc | 1.32 ± 0.06 d | 1.58 ± 0.06 b | 1.88 ± 0.07 a |
| Preadult duration/d | 5.95 ± 0.10 b | 6.95 ± 0.13 a | 5.66 ± 0.06 c | 6.11 ± 0.08 b | 5.63 ± 0.87 c | 6.71 ± 0.13 a |
| Adult female longevity/d | 18.10 ± 0.59 bc | 15.93 ± 0.47 d | 19.55 ± 0.86 ab | 19.16 ± 0.82 ab | 20.30 ± 0.06 a | 16.76 ± 0.68 cd |
| Total longevity/d | 23.72 ± 0.67 ab | 21.70 ± 0.79 b | 24.40 ± 0.95 a | 23.61 ± 1.04 ab | 24.81 ± 1.04 a | 22.11 ± 0.96 ab |
| Parameters | ZY821 | ZS11 | XN18 | AGY558 | AYK999 | MXY78 |
|---|---|---|---|---|---|---|
| Intrinsic rates of increase (r)/d−1 | 0.34 ± 0.005 c | 0.28 ± 0.006 d | 0.39 ± 0.006 ab | 0.37 ± 0.007 b | 0.40 ± 0.006 a | 0.23 ± 0.007 e |
| Finite rates of increase (λ)/d−1 | 1.40 ± 0.008 c | 1.33 ± 0.008 d | 1.47 ± 0.009 ab | 1.45 ± 0.010 b | 1.49 ± 0.008 a | 1.26 ± 0.008 e |
| Net reproductive rate (R0) | 39.69 ± 1.83 b | 29.04 ± 1.67 c | 62.16 ± 3.14 a | 63.19 ± 3.57 a | 63.43 ± 2.99 a | 17.19 ± 1.33 d |
| Mean generation time/d | 10.92 ± 0.17 bc | 11.97 ± 0.20 a | 10.71 ± 0.15 cd | 11.22 ± 0.14 b | 10.47 ± 0.13 d | 12.47 ± 0.25 a |
| Fecundity | 40.32 ± 1.74 b | 31.02 ± 1.33 c | 65.03 ± 2.80 a | 68.29 ± 3.04 a | 66.60 ± 2.52 a | 18.38 ± 1.28 d |
| Doubling time (DT) | 2.06 | 2.46 | 1.80 | 1.88 | 1.75 | 3.04 |
| Relative fitness (Rf) | 1 | 0.73 | 1.57 | 1.59 | 1.60 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.; Li, L.-K.; Zhu, F.; Zhang, S.-Z. The Effects of Six Brassica napus Cultivars on the Life Table Parameters of the Green Peach Aphid Myzus persicae (Sulzer) (Hemiptera: Aphididae). Insects 2025, 16, 726. https://doi.org/10.3390/insects16070726
Tian M, Li L-K, Zhu F, Zhang S-Z. The Effects of Six Brassica napus Cultivars on the Life Table Parameters of the Green Peach Aphid Myzus persicae (Sulzer) (Hemiptera: Aphididae). Insects. 2025; 16(7):726. https://doi.org/10.3390/insects16070726
Chicago/Turabian StyleTian, Mi, Lin-Kui Li, Feng Zhu, and Shi-Ze Zhang. 2025. "The Effects of Six Brassica napus Cultivars on the Life Table Parameters of the Green Peach Aphid Myzus persicae (Sulzer) (Hemiptera: Aphididae)" Insects 16, no. 7: 726. https://doi.org/10.3390/insects16070726
APA StyleTian, M., Li, L.-K., Zhu, F., & Zhang, S.-Z. (2025). The Effects of Six Brassica napus Cultivars on the Life Table Parameters of the Green Peach Aphid Myzus persicae (Sulzer) (Hemiptera: Aphididae). Insects, 16(7), 726. https://doi.org/10.3390/insects16070726








