Enhancing Biological Control of Drosophila suzukii: Efficacy of Trichopria drosophilae Releases and Interactions with a Native Parasitoid, Pachycrepoideus vindemiae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
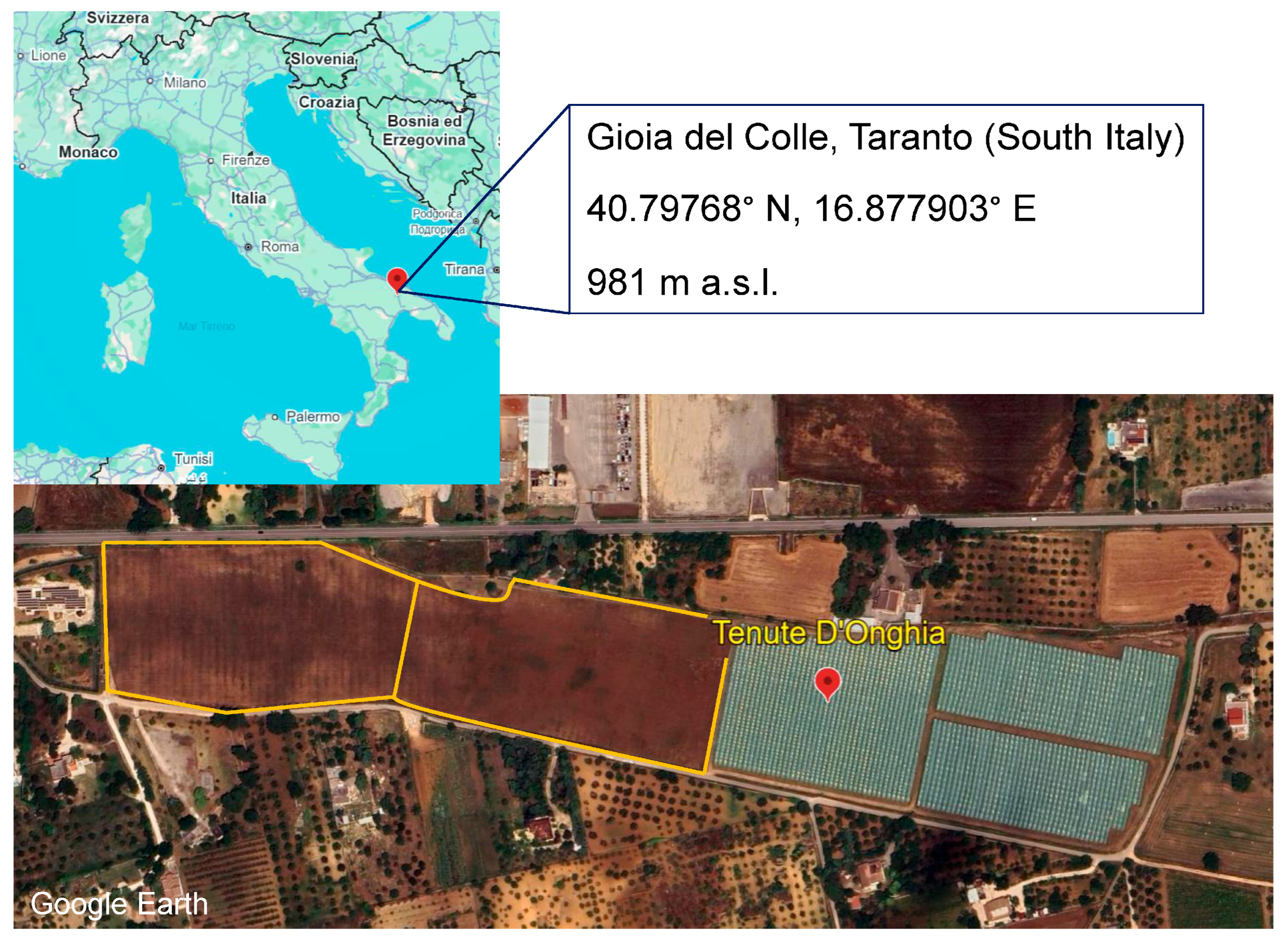
2.1. Effect of T. drosophilae and Its Interaction with P. vindemiae on the Control of D. suzukii Under Field Conditions
2.1.1. D. suzukii Rearing
2.1.2. Parasitoid Rearing
2.1.3. Trichopria drosophilae Release
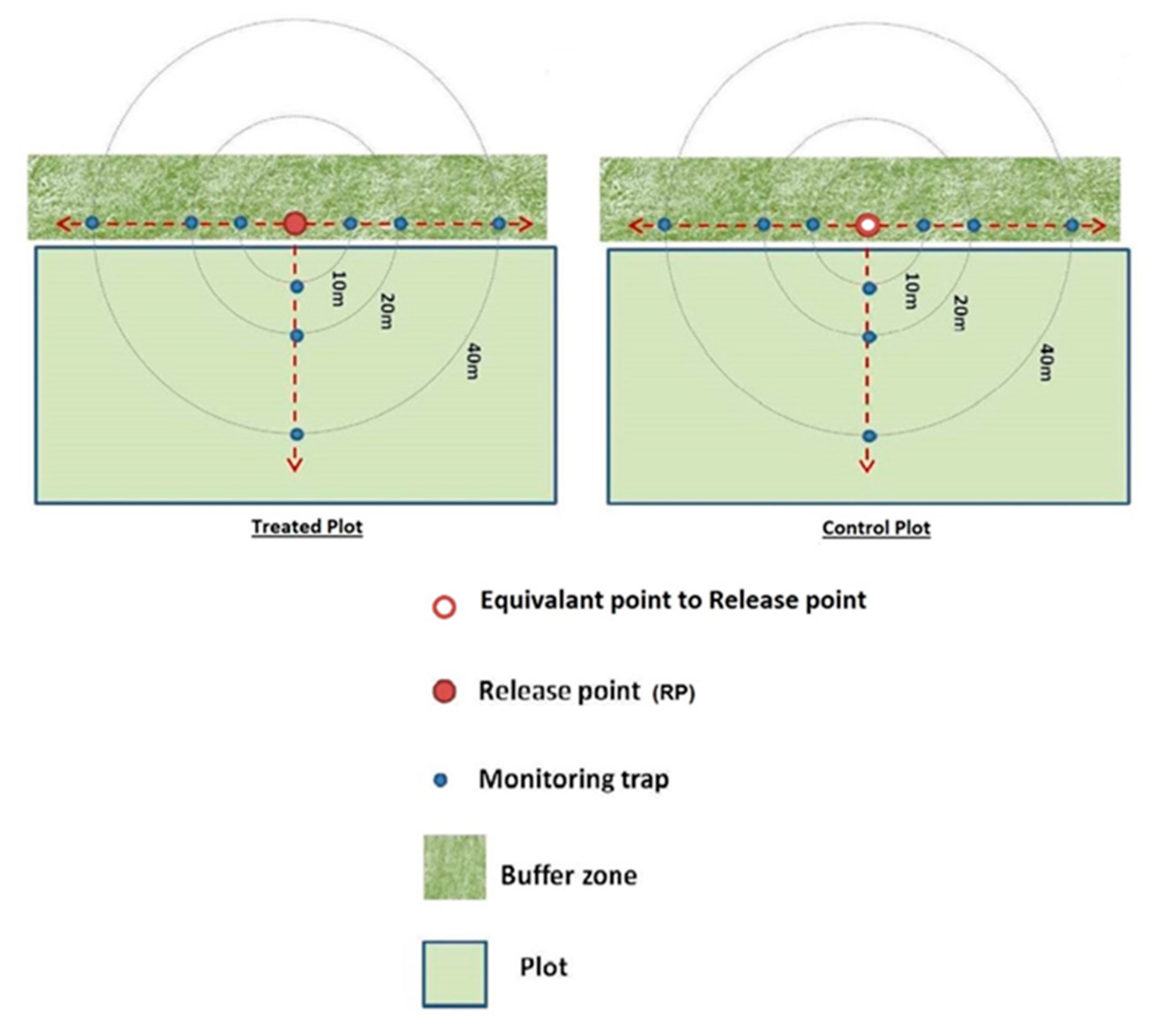
2.1.4. Monitoring of T. drosophilae Field Behavior
2.1.5. Statistical Analysis
2.2. Interaction Between P. vindemiae and T. drosophilae Parasitism Rates on D. suzukii Under Laboratory Conditions
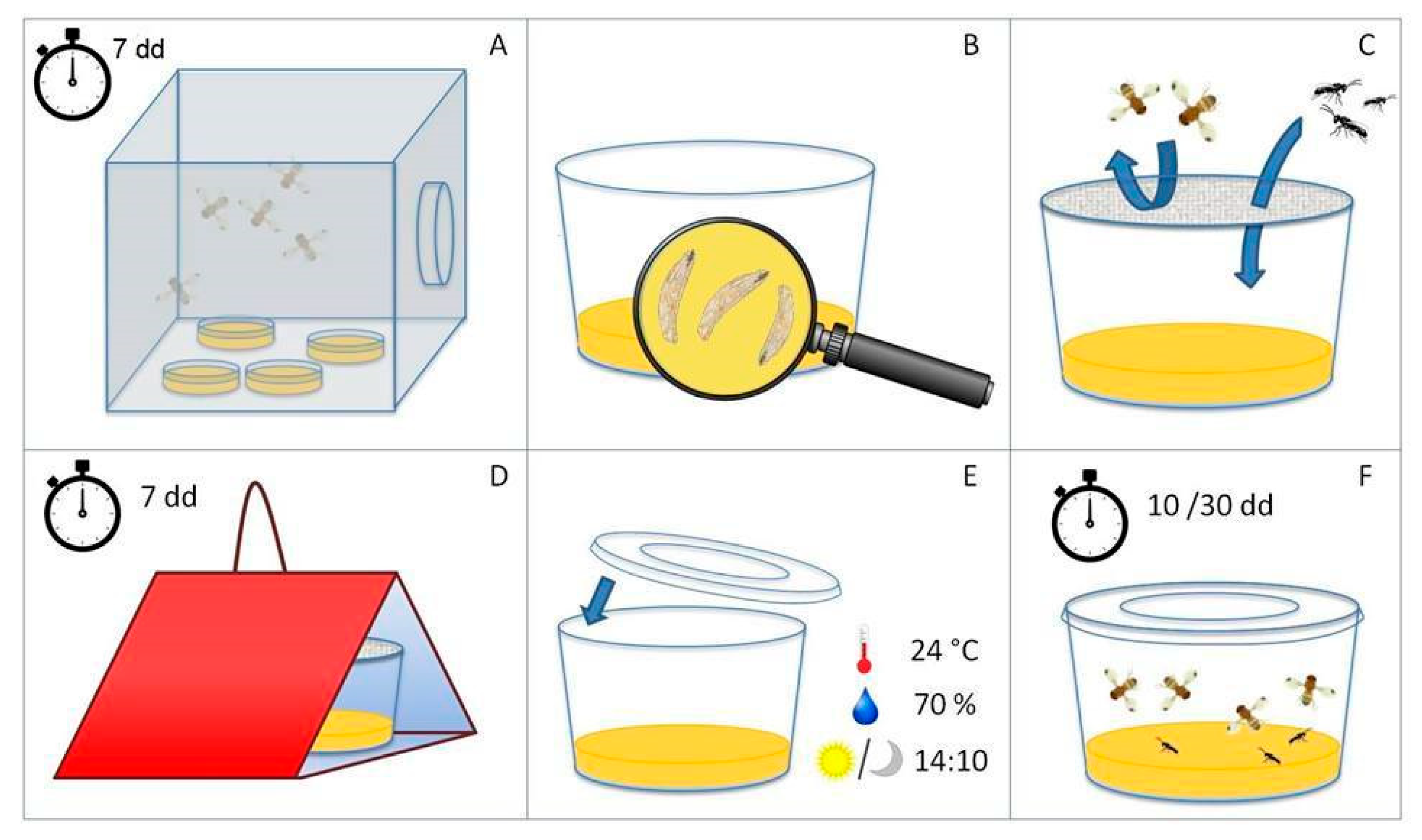
2.2.1. Insect Rearing
2.2.2. Experimental Design
- T1
- 50 pupae of D. suzukii + 3 couples of T. drosophilae.
- T2
- 50 pupae of D. suzukii + 3 couples of P. vindemiae.
- T3
- 50 pupae of D. suzukii + 3 couples of T. drosophilae + 3 couples of P. vindemiae.
- T4
- 50 pupae of D. suzukii left for 24 h with 3 couples of T. drosophilae + 3 couples of P. vindemiae.
- T5
- 50 pupae of D. suzukii left for 24 h with 3 couples of P. vindemiae + 3 couples of T. drosophilae.
- T6
- 50 pupae of D. suzukii left for 24 h with 3 couples of P. vindemiae + 50 pupae of D. suzukii + 3 couples of T. drosophilae
- T7
- 50 pupae D. suzukii left for 24 h with 3 couples of T. drosophilae + 50 pupae D. suzukii + 3 couples of P. vindemiae.
2.2.3. Statistical Analysis
3. Results
3.1. Effect of T. drosophilae and Its Interaction with P. vindemiae on D. suzukii Under Field Conditions
3.1.1. Effect of T. drosophilae Releases on D. suzukii Suppression During the Release Period
3.1.2. Influence of Distance on T. drosophilae Dispersal and Effectiveness
3.1.3. Relationship Between T. drosophilae and P. vindemiae in D. suzukii Population Reduction
3.1.4. Interaction Between T. drosophilae and P. vindemiae
3.1.5. Post-Release Monitoring: Assessing T. drosophilae Adaptation and Its Long-Term Impact on D. suzukii and P. vindemiae
Parasitoid Presence in Treated and Control Plots and Persistence of T. drosophilae in the Field
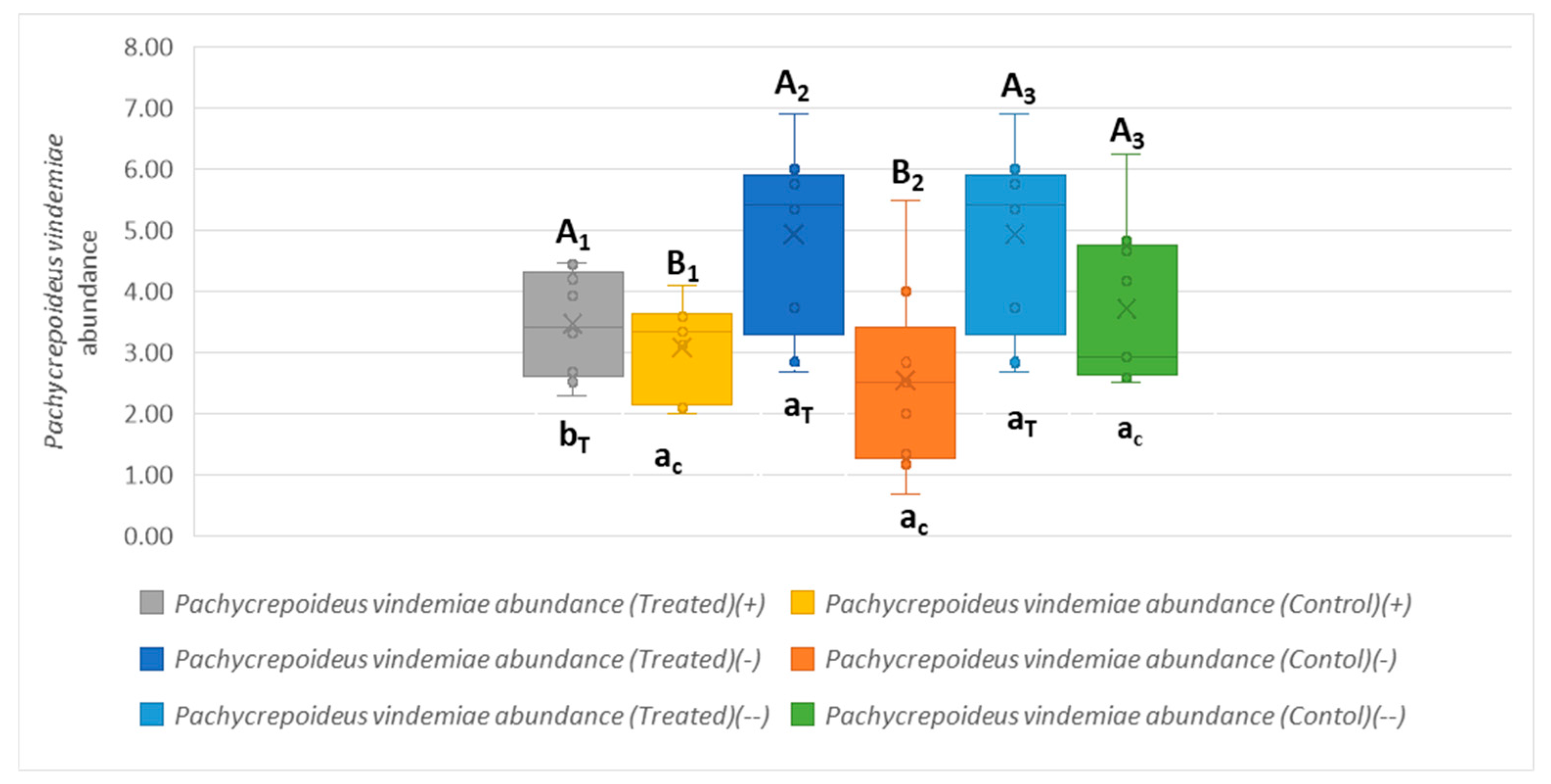
Changes in P. vindemiae Populations During the Post-Release Period
Role of P. vindemiae in D. suzukii Population Reduction in the Absence of T. drosophilae
3.2. Effect of the Interaction Between P. vindemiae and T. drosophilae on the Control of D. suzukii and their Parasitism Rates Under Laboratory Conditions
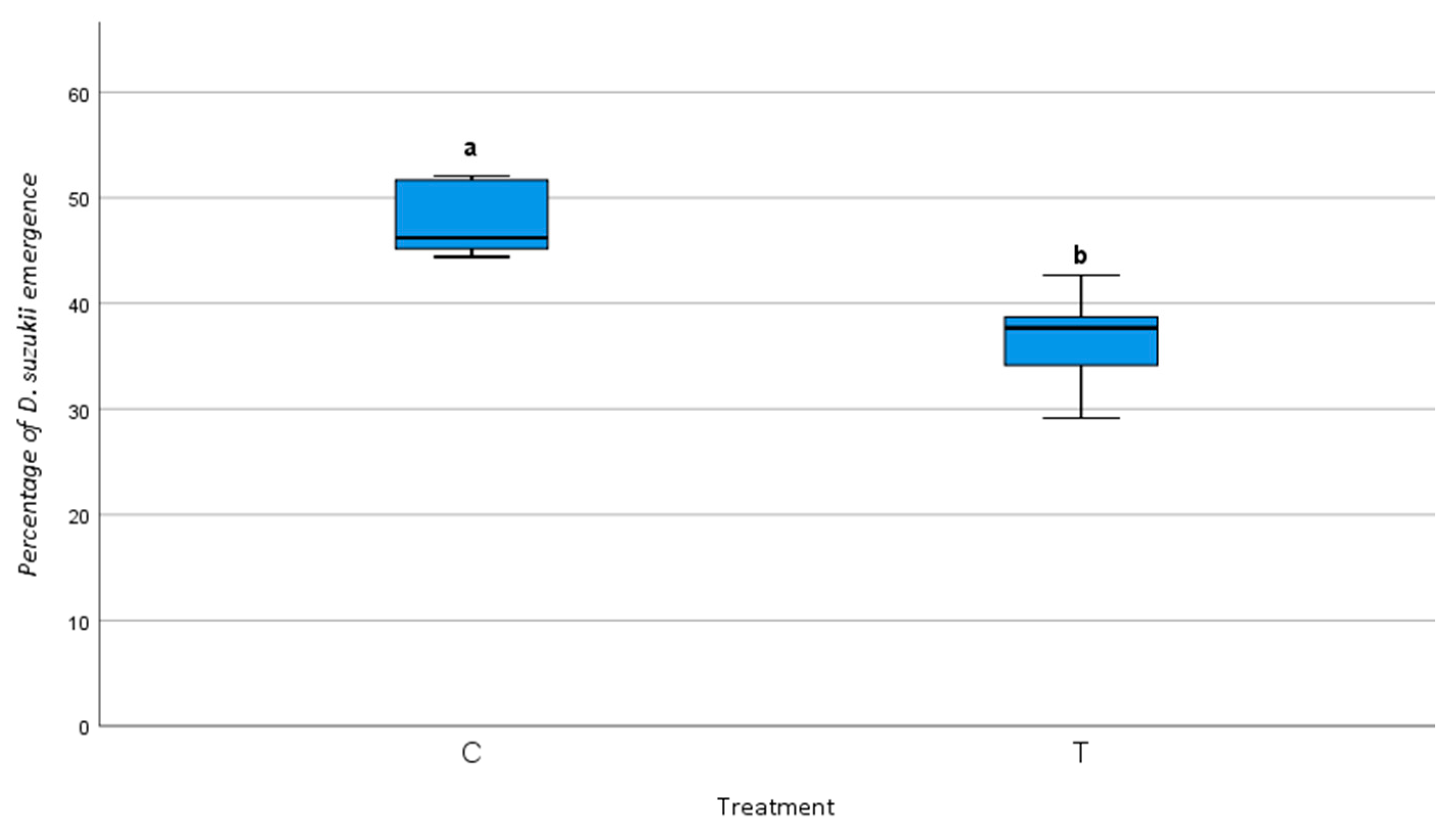
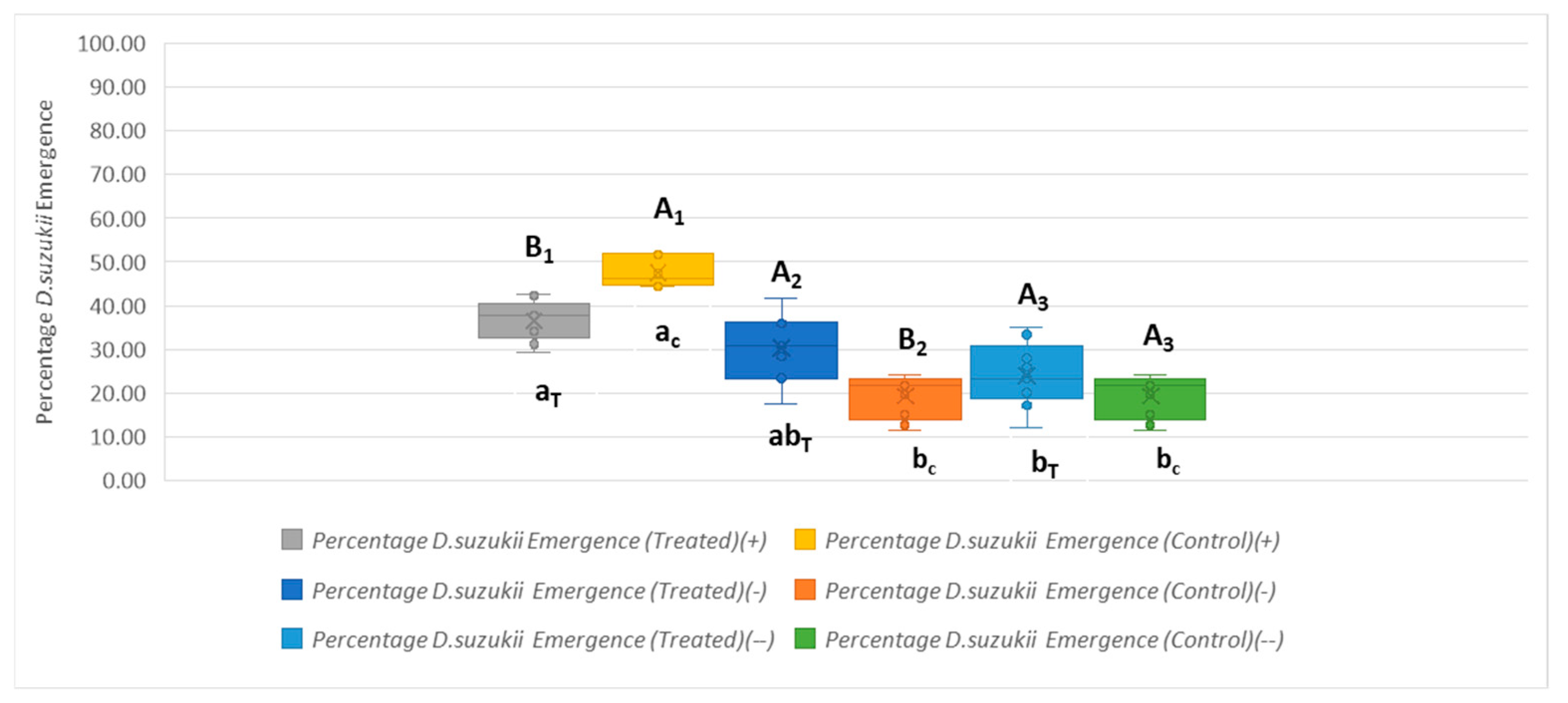
Drosophila suzukii Emergence Across Different Parasitoid Combinations
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research Towards a Sustainable Integrated Pest Management Program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-G.; Hogg, B.N.; Hougardy, E.; Nance, A.H.; Daane, K.M. Potential competitive outcomes among three solitary larval endoparasitoids as candidate agents for classical biological control of Drosophila suzukii. Biol. Control 2020, 130, 18–26. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S. Biological control of Drosophila suzukii: Challenges and opportunities. CAB Rev. 2020, 15. [Google Scholar] [CrossRef]
- da Costa Oliveira, D.; Stupp, P.; Martins, L.N.; Wollmann, J.; Geisler, F.C.S.; Cardoso, T.D.N.; Bernardi, D.; Garcia, F.R.M. Interspecific competition in Trichopria anastrephae parasitism (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae) parasitism on pupae of Drosophila suzukii (Diptera: Drosophilidae). Phytoparasitica 2021, 49, 207–215. [Google Scholar] [CrossRef]
- Buonocore-Biancheri, M.J.; del Carmen Suárez, L.; Ponssa, M.D.; Kirschbaum, D.S.; Garcia, F.R.M. Assessing Natural Incidence of Resident Pupal Parasitoids on the Drosophila suzukii (Diptera: Drosophilidae) Population in Non-crop Fruits. Neotrop. Entomol. 2024, 53, 225–235. [Google Scholar] [CrossRef]
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectology 2012, 65, 149–160. [Google Scholar]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Anfora, G. Drosophila suzukii (Matsumura), Una Nuova Specie Invasiva Dannosa Alle Colture di Piccoli Frutti; Fondazione Edmund Mach: Trento, Italy, 2012; Available online: https://openpub.fmach.it/bitstream/10449/21779/1/CresoRicerca_447emb_02.pdf (accessed on 7 July 2025).
- Grassi, A.; Palmieri, L.; Giongo, L. Nuovo fitofago per i piccoli frutti in Trentino. Terra Trent. 2009, 10, 19–23. [Google Scholar]
- Antonacci, R.; Tritto, P.; Cappucci, U.; Fanti, L.; Piacentini, L.; Berloco, M. Drosophilidae monitoring in Apulia (Italy) reveals Drosophila suzukii as one of the four most abundant species. Bull. Insectology 2017, 70, 139–146. [Google Scholar]
- Baser, N.; Ouantar, M.B.; Broutou, O.; Lamaj, F.; Verrastro, V.; Porcelli, F. First finding of Drosophila suzukii Matsumura Diptera Drosophilidae in Apulia, Italy, and its population dynamics throughout the year. Fruits 2015, 70, 225–230. [Google Scholar] [CrossRef]
- Dalton, D.T.; Walton, V.M.; Shearer, P.W.; Walsh, D.B.; Caprile, J.; Isaacs, R. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag. Sci. 2011, 67, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa, T. Studies on Drosophila suzukii Mats. J. Plant Prot. 1939, 23, 66–70. [Google Scholar]
- Landolt, P.J.; Adams, T.; Rogg, H. Trapping spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. J. Appl. Entomol. 2012, 136, 148–154. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In Focus: Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef]
- Tochen, S.; Dalton, D.T.; Wiman, N.; Hamm, C.; Shearer, P.W.; Walton, V.M. Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ. Entomol. 2014, 43, 501–510. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Stahl, J.M.; Wang, X.; Abram, P.K.; Biondi, A.; Buffington, M.L.; Hoelmer, K.A.; Kenis, M.; Lisi, F.; Rossi-Stacconi, M.V.; Seehausen, M.L.; et al. Ganaspis kimorum (Hymenoptera: Figitidae), a promising parasitoid for biological control of Drosophila suzukii (Diptera: Drosophilidae). J. Integr. Pest Manag. 2024, 15, 44. [Google Scholar] [CrossRef]
- Giunti, G.; Benelli, G.; Messing, R.H.; Canale, A. Early adult learning affects host preferences in the tephritid parasitoid Psyttalia concolor (Hymenoptera: Braconidae). J. Pest Sci. 2016, 89, 529–537. [Google Scholar] [CrossRef]
- Cossentine, J.; Robertson, M.; Buitenhuis, R. Impact of acquired entomopathogenic fungi on adult Drosophila suzukii survival and fecundity. Biol. Control 2016, 103, 129–137. [Google Scholar] [CrossRef]
- Woltz, J.M.; Lee, J.C. Effects of fungal entomopathogens on mortality and behavior of adult Drosophila suzukii. Biol. Control 2017, 114, 39–45. [Google Scholar] [CrossRef]
- Piscitelli, L.; Baser, N. Efficacy of an Autochthonous Strain of Entomopathogenic Fungi for the Control of Drosophila suzukii Infestation in an Apulian Cherry Orchard. Acta Sci. Agric. 2022, 6, 20–27. [Google Scholar]
- Ibouh, K.; Oreste, M.; Bubici, G.; Tarasco, E.; Stacconi, M.V.R.; Ioriatti, C.; Verrastro, V.; Anfora, G.; Baser, N. Biological control of Drosophila suzukii: Efficacy of parasitoids, entomopathogenic fungi, nematodes, and deterrents of oviposition in laboratory assays. Crop Prot. 2019, 125, 104897. [Google Scholar] [CrossRef]
- Wolf, S.; Boycheva-Woltering, S.; Romeis, J.; Collatz, J. Trichopria drosophilae parasitizes Drosophila suzukii in seven common non-crop fruits. J. Pest Sci. 2019, 92, 1165–1174. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Rogg, H.; Landolt, P.J. Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing Drosophila, Drosophila suzukii. J. Chem. Ecol. 2012, 38, 1419–1431. [Google Scholar] [CrossRef]
- Gardiner, M.M.; Landis, D.A.; Gratton, C.; Schmidt, N.; O’Neal, M.; Mueller, E.; Chacon, J.; Heimpel, G.E.; Difonzo, C.D. Landscape composition influences patterns of native and exotic lady beetle abundance. Divers. Distrib. 2009, 15, 554–564. [Google Scholar] [CrossRef]
- Hamby, K.A.; Hernández, A.; Boundy-Mills, K.; Zalom, F.G. Associations of Yeasts with Spotted-Wing Drosophila (Drosophila suzukii; Diptera: Drosophilidae) in Cherries and Raspberries. Appl. Environ. Microbiol. 2012, 78, 4869. [Google Scholar] [CrossRef]
- Harris, D.W.; Hamby, K.A.; Wilson, H.E.; Zalom, F.G. Seasonal monitoring of Drosophila suzukii (Diptera: Drosophilidae) in a mixed fruit production system. J. Asia-Pac. Entomol. 2014, 17, 857–864. [Google Scholar] [CrossRef]
- Kimura, M.T. Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia 2004, 140, 442–449. [Google Scholar] [CrossRef]
- Rossi Stacconi, M.V.; Amiresmaeili, N.; Biondi, A.; Carli, C.; Caruso, S.; Dindo, M.L.; Francati, S.; Gottardello, A.; Grassi, A.; Lupi, D.; et al. Host location and dispersal ability of the cosmopolitan parasitoid Trichopria drosophilae released to control the invasive spotted wing Drosophila. Biol. Control 2017, 117, 188–196. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Mills, N.J. Biological Control: Ecology and Applications; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Batchelor, T.P.; Hardy, I.C.W.; Barrera, J.F.; Pérez-Lachaud, G. Insect gladiators II: Competitive interactions within and between bethylid parasitoid species of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Biol. Control 2005, 33, 194–202. [Google Scholar] [CrossRef]
- Snyder, W.E.; Ives, A.R. Interactions between specialist and generalist natural enemies: Parasitoids, predators, and pea aphid biocontrol. Ecology 2003, 84, 91–107. [Google Scholar] [CrossRef]
- Rossi Stacconi, M.V.; Buffington, M.; Daane, K.M.; Dalton, D.T.; Grassi, A.; Kaçar, G.; Miller, B.; Miller, J.C.; Baser, N.; Ioriatti, C.; et al. Host stage preference, efficacy and fecundity of parasitoids attacking Drosophila suzukii in newly invaded areas. Biol. Control 2015, 84, 28–35. [Google Scholar] [CrossRef]
- Wang, X.-G.; Kaçar, G.; Biondi, A.; Daane, K.M. Foraging efficiency and outcomes of interactions of two pupal parasitoids attacking the invasive spotted wing drosophila. Biol. Control 2016, 96, 64–71. [Google Scholar] [CrossRef]
- Bono Rosselló, N.; Rossini, L.; Speranza, S.; Garone, E. Towards pest outbreak predictions: Are models supported by field monitoring the new hope? Ecol. Inform. 2023, 78, 102310. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Sun, S.; Xi, X. Competitive superiority switches between larval and adult stages reducing the fitness difference between competing parasitoids. Entomol. Exp. Appl. 2024, 173, 166–173. [Google Scholar] [CrossRef]
- Wang, H.; Liu, T.; Sun, S.; Lewis, O.T.; Xi, X. Temporal variability in host availability alters the outcome of competition between two parasitoid species. J. Anim. Ecol. 2024, 93, 1845–1853. [Google Scholar] [CrossRef]
- Lee, J.C.; Dreves, A.J.; Cave, A.M.; Kawai, S.; Isaacs, R.; Miller, J.C.; Van Timmeren, S.; Bruck, D.J. Infestation of Wild and Ornamental Noncrop Fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann. Entomol. Soc. Am. 2015, 108, 117–129. [Google Scholar] [CrossRef]
- Chabert, S.; Allemand, R.; Poyet, M.; Eslin, P.; Gibert, P. Ability of European parasitoids (Hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii. Biol. Control 2012, 63, 40–47. [Google Scholar] [CrossRef]
- Chen, S.; Fleischer, S.J.; Saunders, M.C.; Thomas, M.B. The influence of diurnal temperature variation on degree-day accumulation and insect life history. PLoS ONE 2015, 10, e0120772. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baser, N.; Matar, C.; Rossini, L.; Ibn Amor, A.; Šunjka, D.; Bošković, D.; Gualano, S.; Santoro, F. Enhancing Biological Control of Drosophila suzukii: Efficacy of Trichopria drosophilae Releases and Interactions with a Native Parasitoid, Pachycrepoideus vindemiae. Insects 2025, 16, 715. https://doi.org/10.3390/insects16070715
Baser N, Matar C, Rossini L, Ibn Amor A, Šunjka D, Bošković D, Gualano S, Santoro F. Enhancing Biological Control of Drosophila suzukii: Efficacy of Trichopria drosophilae Releases and Interactions with a Native Parasitoid, Pachycrepoideus vindemiae. Insects. 2025; 16(7):715. https://doi.org/10.3390/insects16070715
Chicago/Turabian StyleBaser, Nuray, Charbel Matar, Luca Rossini, Abir Ibn Amor, Dragana Šunjka, Dragana Bošković, Stefania Gualano, and Franco Santoro. 2025. "Enhancing Biological Control of Drosophila suzukii: Efficacy of Trichopria drosophilae Releases and Interactions with a Native Parasitoid, Pachycrepoideus vindemiae" Insects 16, no. 7: 715. https://doi.org/10.3390/insects16070715
APA StyleBaser, N., Matar, C., Rossini, L., Ibn Amor, A., Šunjka, D., Bošković, D., Gualano, S., & Santoro, F. (2025). Enhancing Biological Control of Drosophila suzukii: Efficacy of Trichopria drosophilae Releases and Interactions with a Native Parasitoid, Pachycrepoideus vindemiae. Insects, 16(7), 715. https://doi.org/10.3390/insects16070715








