Effects of Photoperiod on the Developmental Duration and Reproduction of Sclerodermus sichuanensis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Inoculation of Host Larvae with Parasitoids
2.3. Examining the Behavior of Female Parasitoids
2.4. Brood Size and Male Ratio of Parasitoid Offspring
2.5. Statistical Analyses
3. Results
3.1. Parasitism Rate and Offspring Emergence Rate of Female S. sichuanensis Under Different Photoperiods
3.2. Pre-Oviposition Period of Female S. sichuanensis Under Different Photoperiods
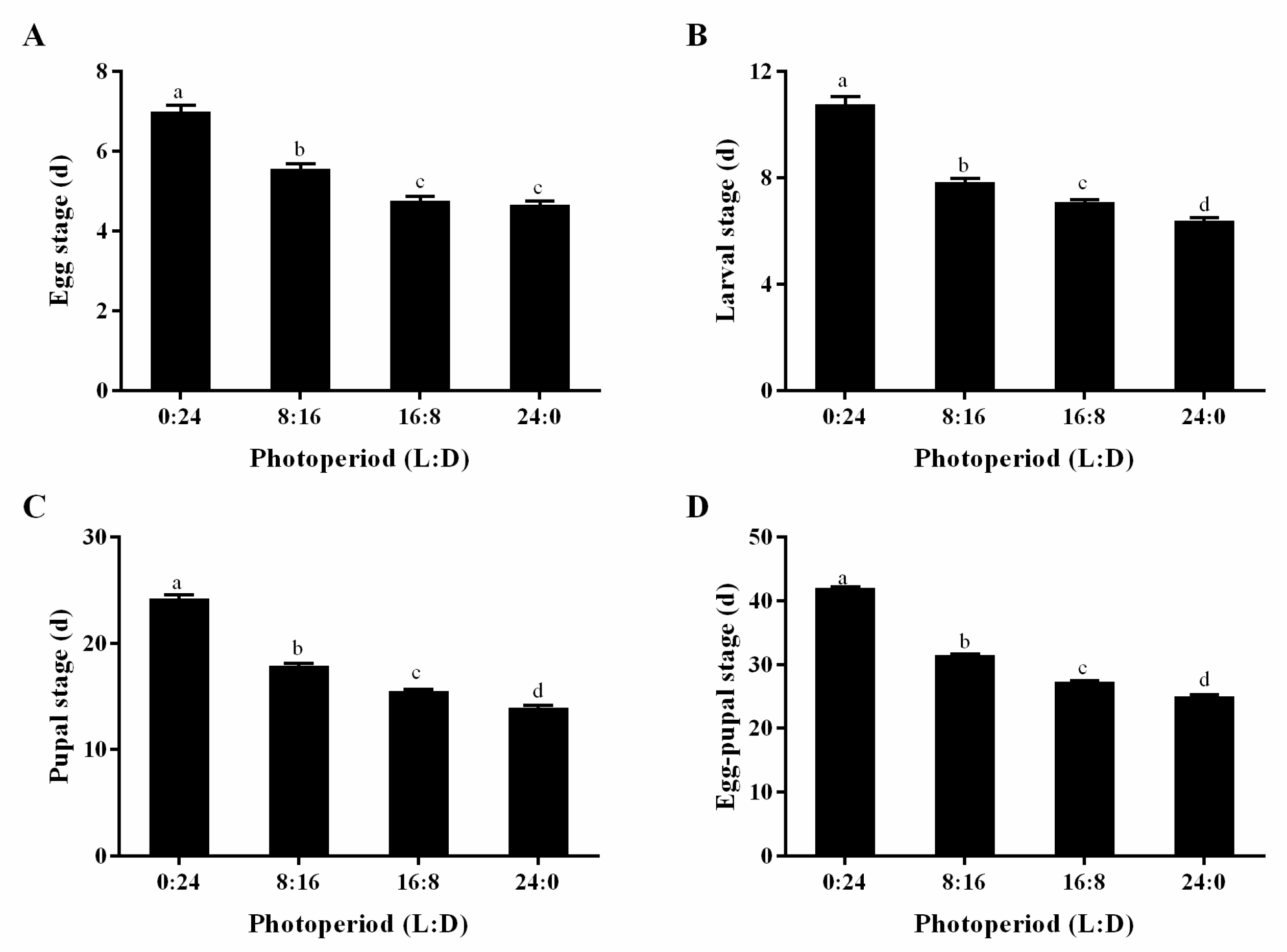
3.3. Egg Stage, Larval Stage, Pupal Stage, and Egg-to-Pupa Development Period of S. sichuanensis Offspring Under Different Photoperiods
3.4. Offspring Characteristics of S. sichuanensis Under Four Photoperiod Treatments
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LD | Long day |
| SD | Short day |
| JH | Juvenile hormone |
| Kr-h1 | Krüppel homolog 1 |
| JHAMT | JH acid O-methyltransferase |
| CA | corpora allata |
| 20E | 20-Hydroxyecdysone |
| ANOVA | Analysis of variance |
| LSD | Least significant difference |
References
- Yang, Z.Q.; Wang, X.Y.; Zhang, Y.N. Recent Advances in Biological Control of Important Native and Invasive Forest Pests in China. Biol. Control 2014, 68, 117–128. [Google Scholar] [CrossRef]
- Gao, H.; Qian, Q.; Liu, L.; Xu, D. Predicting the distribution of Sclerodermus sichuanensis (Hymenoptera: Bethylidae) under climate change in China. Insects 2023, 14, 475. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.J.; Li, J.Y.; Wang, H.; Zhang, X.L. Biological characteristics of Scleroermus sichuanensis. J. Appl. Entomol. 2000, 124, 15–21. [Google Scholar]
- Zhang, F.P.; Li, D.; Zhou, G. Photoperiod effects on Encarsia formosa. Environ. Entomol. 2018, 47, 413–420. [Google Scholar]
- Men, J.; Zhao, B.; Cao, D.D.; Wang, W.C.; Wei, J.R. Evaluating host location in three native Sclerodermus species and their ability to cause mortality in the wood borer Aromia bungii (Coleoptera: Cerambycidae) in laboratory. Biol. Control 2019, 134, 95–102. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.; Zhou, Y. Host suitability of Batocera horsfieldi for Sclerodermus sichuanensis. Insect Sci. 2007, 14, 159–166. [Google Scholar]
- Fang, R.; Xu, D.; Liu, L.; Yang, W.; Yang, H. Learning behavior of Sclerodermus sichuanensis Xiao: Habitual responses and cumulative effects. Pol. J. Environ. Stud. 2024, 33, 2027–2036. [Google Scholar] [CrossRef]
- Harvey, J.A. Host quality and reproduction in parasitic wasps. Ecol. Entomol. 2005, 30, 557–564. [Google Scholar]
- Wang, X.H.; Kang, L. Molecular Mechanisms of Phase Change in Locusts. Annu. Rev. Entomol. 2014, 59, 225–244. [Google Scholar] [CrossRef]
- Chen, L.J.; Zhang, M.; Wu, X. Temperature effects on Sclerodermus species development. Environ. Entomol. 2004, 33, 1237–1243. [Google Scholar]
- Saunders, D.S. Insect photoperiodism: Seeing the light. Physiol. Entomol. 2012, 37, 207–218. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wei, K.; Yang, Z.Q.; Jennings, D.E.; Duan, J.J. Effects of biotic and abiotic factors on phenotypic partitioning of wing morphology and development in Sclerodermus pupariae (Hymenoptera: Bethylidae). Sci. Rep. 2016, 6, 26408. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, X.Y.; Yang, Z.Q.; Duan, J.J. Effects of photoperiod and light intensity on wing dimorphism and development in the parasitoid Sclerodermus pupariae (Hymenoptera: Bethylidae). Biol. Control 2019, 133, 117–122. [Google Scholar] [CrossRef]
- Denlinger, D.L. Why study diapause? Entomol. Res. 2008, 38, 1–9. [Google Scholar] [CrossRef]
- Prado, S.G.; Jandricic, S.E.; Frank, S.D. Ecological interactions affecting the efficacy of Aphidius colemani in greenhouse crops. Insects 2015, 6, 538–575. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.G.; Chen, J.Y.; Wang, J.Y.; Zhu, J.H.; Zhang, F.P. Effects of temperature and photoperiod on the development and reproduction of endoparasitoid wasp Coccophagus japonicus Compere. J. Plant Prot. 2021, 48, 848–854. (In Chinese) [Google Scholar]
- Takada, Y. Reproductive biology of Trichogramma dendrolimi. Jpn. J. Appl. Entomol. Zool. 1994, 29, 91–95. [Google Scholar] [CrossRef]
- Stokkebo, S.; Jensen, A.B.; Jespersen, J.B. Effects of light on Anisopteromalus calandrae. Biol. Control 2011, 58, 159–166. [Google Scholar]
- Wang, S. Effects of photoperiod on behavior and expression patterns of genes related to biological clock of Trichogramma japonicum. Master’s Thesis, Yangtze University, Jingzhou, China, 2022. [Google Scholar]
- Ji, X.Y.; Hua, L.D.; Jiang, J.X.; Wan, N.F.; Yang, J.J. The influence of temperature and photoperiod on parasitism and female ratio of Microplitis pallidipes. Chin. J. Appl. Entomol. 2011, 48, 370–374. [Google Scholar]
- Goto, S.G. Photoperiodic time measurement, photoreception, and circadian clocks in insect photoperiodism. Appl. Entomol. Zool. 2022, 5, 193–212. [Google Scholar] [CrossRef]
- Stehlík, J.; Závodská, R.; Shimada, K.; Sauman, I.; Kostál, V. Photoperiodic induction of diapause requires regulated transcription of timeless in the larval brain of Chymomyza costata. J. Biol. Rhythm. 2008, 23, 129–139. [Google Scholar] [CrossRef]
- Sakamoto, T.; Uryu, O.; Tomioka, K. The clock gene period plays an essential role in photoperiodic control of nymphal development in the cricket Modicogryllus siamensis. J. Biol. Rhythm. 2009, 24, 379–390. [Google Scholar] [CrossRef]
- Ikeno, T.; Numata, H.; Goto, S.G. Circadian clock genes period and cycle regulate photoperiodic diapause in the bean bug Riptortus pedestris males. J. Insect Physiol. 2011, 57, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Shinohara, T.; Chafino, S.; Noji, S.; Tomiok, K. Photoperiod and temperature separately regulate nymphal development through JH and insulin/TOR signaling pathways in an insect. Proc. Natl. Acad. Sci. USA 2020, 117, 201922747. [Google Scholar] [CrossRef]
- Shinoda, T.; Itoyama, K. Juvenile hormone acid methyltransferase: A key regulatory enzyme for insect metamorphosis. Proc. Natl. Acad. Sci. USA 2003, 100, 11986–11991. [Google Scholar] [CrossRef] [PubMed]
- Noriega, F.G. Juvenile hormone biosynthesis in insects: What is new, what do we know, and what questions remain? Int. Sch. Res. Not. 2014, 2014, 967361. [Google Scholar] [CrossRef]
- Zhou, X.F.; Coll, M.; Applebaum, S.W. Effect of temperature and photoperiod on juvenile hormone biosynthesis and sexual maturation in the cotton bollworm, Helicoverpa armigera: Implications for life history traits. Insect Biochem. Mol. Biol. 2000, 30, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.N.; Liu, H.; Wang, Z.; Zhang, T. Effects of photoperiod on the development of Agrotis ipsilon. J. Appl. Entomol. 2016, 140, 36–42. (In Chinese) [Google Scholar]
- Jia, Q.; Li, S. MMP-induced fat body cell dissociation promotes pupal development and moderately averts pupal diapause by activating lipid metabolism. Proc. Natl. Acad. Sci. USA 2023, 120, e2215214120. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Luo, M.; Guo, J.Y.; Chen, H.S.; Wan, F.H. Effect of Photoperiod on Developmental Fitness in Ophraella communa (Coleoptera: Chrysomelidae). Environ. Entomol. 2014, 43, 1435–1442. [Google Scholar] [CrossRef]
- Wei, K.; Tang, Y.L.; Wang, X.Y.; Cao, L.M.; Yang, Z.Q. The developmental strategies and related profitability of an idiobiont ectoparasitoid Sclerodermus pupariae vary with host size. Ecol. Entomol. 2014, 39, 101–108. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Duan, Z.; Zhang, Y.; Zhang, Y.; Cao, L.; Wei, K. Sclerodermus alternatus (Hymenoptera: Bethylidae), a new species from China, parasitizing Monochamus alternatus (Coleoptera: Cerambycidae). Zool. Syst. 2024, 49, 258–266. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Z. Controlling Monochamus alternatus by domestication of Sclerodermus sichuanensis. J. Sichuan For. Sci. Technol. 2007, 28, 16–20. [Google Scholar]
- Jing, Y.P.; Wen, X.P.; Li, L.J.; Zhou, S.T. The vitellogenin receptor functionality of the migratory locust depends on its phosphorylation by juvenile hormone. Proc. Natl. Acad. Sci. USA 2021, 118, e2106908118. [Google Scholar] [CrossRef]
- Luo, W.; Liu, S.; Zhang, W.; Yang, L.; Huang, J.; Zhou, S.; Feng, Q.; Palli, S.R.; Wang, J.; Roth, S.; et al. Juvenile hormone signaling promotes ovulation and maintains egg shape by inducing expression of extracellular matrix genes. Proc. Natl. Acad. Sci. USA 2021, 118, e2104461118. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Sheng, Z.T.; Sun, Z.Y.; Palli, S.R. Ecdysteroid regulation of ovarian growth and oocyte maturation in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.X.; Spradling, A.C. Steroid signaling within Drosophila ovarian epithelial cells sex-specifically modulates early germ cell development and meiotic entry. PLoS ONE 2012, 7, e46109. [Google Scholar] [CrossRef]
- Zera, A.J. Evolutionary genetics of juvenile hormone and ecdysteroid regulation in Gryllus: A case study in the microevolution of endocrine regulation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, D.; Kinjoh, T.; Shinoda, T.; Hiruma, K. The role of 20-hydroxyecdysone and juvenile hormone in pupal commitment of the epidermis of the silkworm, Bombyx mori. Mech. Dev. 2008, 125, 411–420. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, S.; Ji, W.; Yang, C.; Wang, D.; Ma, R. Effects of photoperiod on growth, development and reproduction of the oriental armyworm Mythimna separata (Lepidoptera: Noctuidae). J. Plant Prot. 2019, 46, 542–548. [Google Scholar]
- Chen, Z.Z.; Li, M.G.; Guo, Y.N.; Yin, X.C.; Zhang, F.; Xu, Y.Y. Effects of photoperiod and temperature on the post-diapause biology of Chrysoperla sinica (Tjeder) adults in different overwintering periods. Sci. Agric. Sin. 2013, 46, 1610–1618. [Google Scholar]
- Zhang, F.P.; Zhu, J.H.; Han, D.Y.; Li, L.; Niu, L.M.; Fu, Y.G. Factors influencing the parasitism of Metaphycus parasaissetiae (Hymenoptera: Encyrtidae). Acta Ecol. Sin. 2015, 35, 1–8. [Google Scholar]

| Photoperiod (L:D) | Replicates (n) | Parasitism (n) | Parasitism Rate (%) | Successful Parasitism Number (n) | Successful Parasitism Rate (%) |
|---|---|---|---|---|---|
| 0:24 | 35 | 26 | 74.3 b | 25 | 96.2 a |
| 8:16 | 35 | 35 | 100.00 a | 33 | 94.3 a |
| 16:8 | 35 | 35 | 100.00 a | 33 | 94.3 a |
| 24:0 | 35 | 35 | 100.00 a | 31 | 88.6 a |
| χ2 | − | − | 81.997 | − | 5.127 |
| p | − | − | p < 0.05 | − | p > 0.05 |
| Photoperiod (L:D) | Number of Male Offspring (n) | Number of Female Offspring (n) | Total Number of Offspring (n) | Male Ratio (%) |
|---|---|---|---|---|
| 0:24 | 2.4 ± 0.3 ab | 50.8 ± 3.5 a | 53.1 ± 3.6 a | 4.8 ± 0.5 a |
| 8:16 | 2.3 ± 0.2 ab | 53.7 ± 3.1 a | 56.1 ± 3.8 a | 4.3 ± 0.4 a |
| 16:8 | 3.0 ± 0.3 a | 53.1 ± 3.6 a | 55.9 ± 3.2 a | 5.2 ± 0.4 a |
| 24:0 | 1.7 ± 0.3 b | 35.5 ± 3.1 b | 37.3 ± 3.3 b | 5.1 ± 0.4 a |
| df | 3121 | 3121 | 3121 | 3121 |
| F | 4.069 | 6.76 | 6.808 | 0.906 |
| p | 0.0086 | 0.0003 | 0.0003 | 0.4405 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, K.; Wang, L.; Xiao, Z.; Wang, S.; Wei, K.; Wang, X.; Zhang, Y.; Tang, Y. Effects of Photoperiod on the Developmental Duration and Reproduction of Sclerodermus sichuanensis. Insects 2025, 16, 701. https://doi.org/10.3390/insects16070701
Kang K, Wang L, Xiao Z, Wang S, Wei K, Wang X, Zhang Y, Tang Y. Effects of Photoperiod on the Developmental Duration and Reproduction of Sclerodermus sichuanensis. Insects. 2025; 16(7):701. https://doi.org/10.3390/insects16070701
Chicago/Turabian StyleKang, Kui, Lina Wang, Zhongjiu Xiao, Shaobo Wang, Ke Wei, Xiaoyi Wang, Yanlong Zhang, and Yanlong Tang. 2025. "Effects of Photoperiod on the Developmental Duration and Reproduction of Sclerodermus sichuanensis" Insects 16, no. 7: 701. https://doi.org/10.3390/insects16070701
APA StyleKang, K., Wang, L., Xiao, Z., Wang, S., Wei, K., Wang, X., Zhang, Y., & Tang, Y. (2025). Effects of Photoperiod on the Developmental Duration and Reproduction of Sclerodermus sichuanensis. Insects, 16(7), 701. https://doi.org/10.3390/insects16070701






