Compartmentalization of Free Fatty Acids in Blood-Feeding Tabanus bovinus Females
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Extraction of Free Fatty Acids (FFAs)
2.3. Derivatization Method
2.4. GC–MS Analyses
2.5. Statistics
3. Results
3.1. Appearance of T. bovinus Females
3.2. Extracts from T. bovinus Females
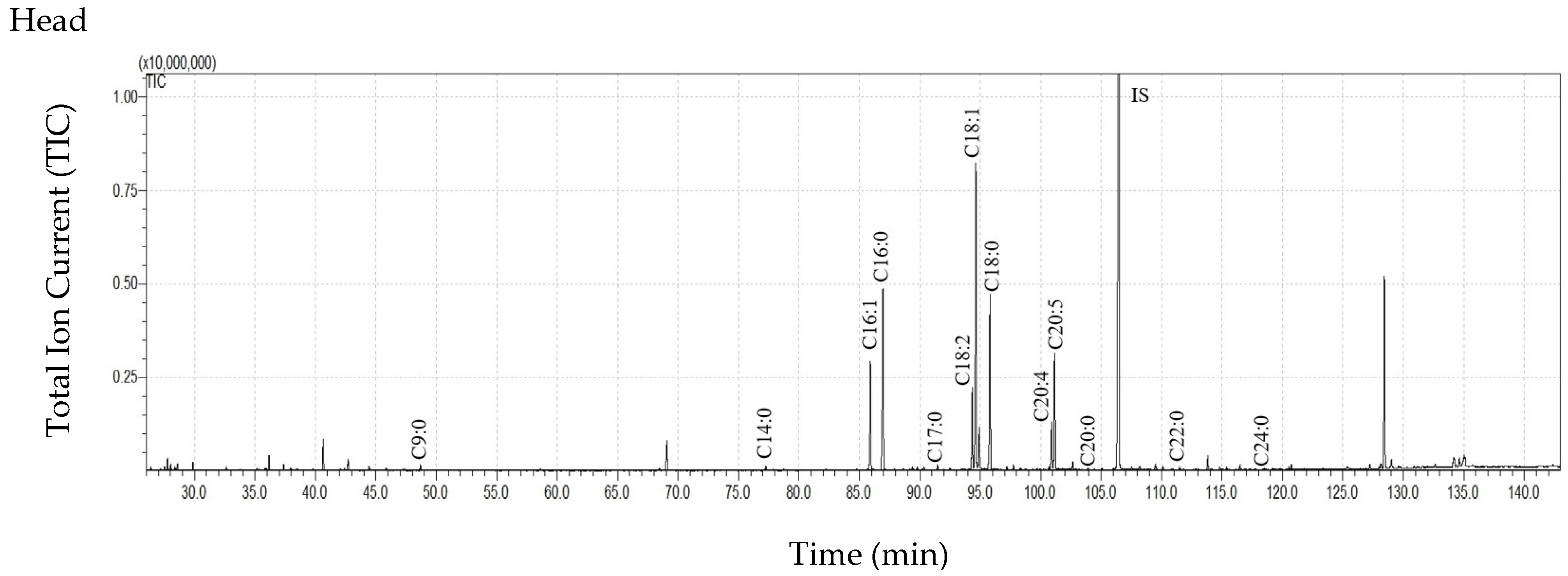
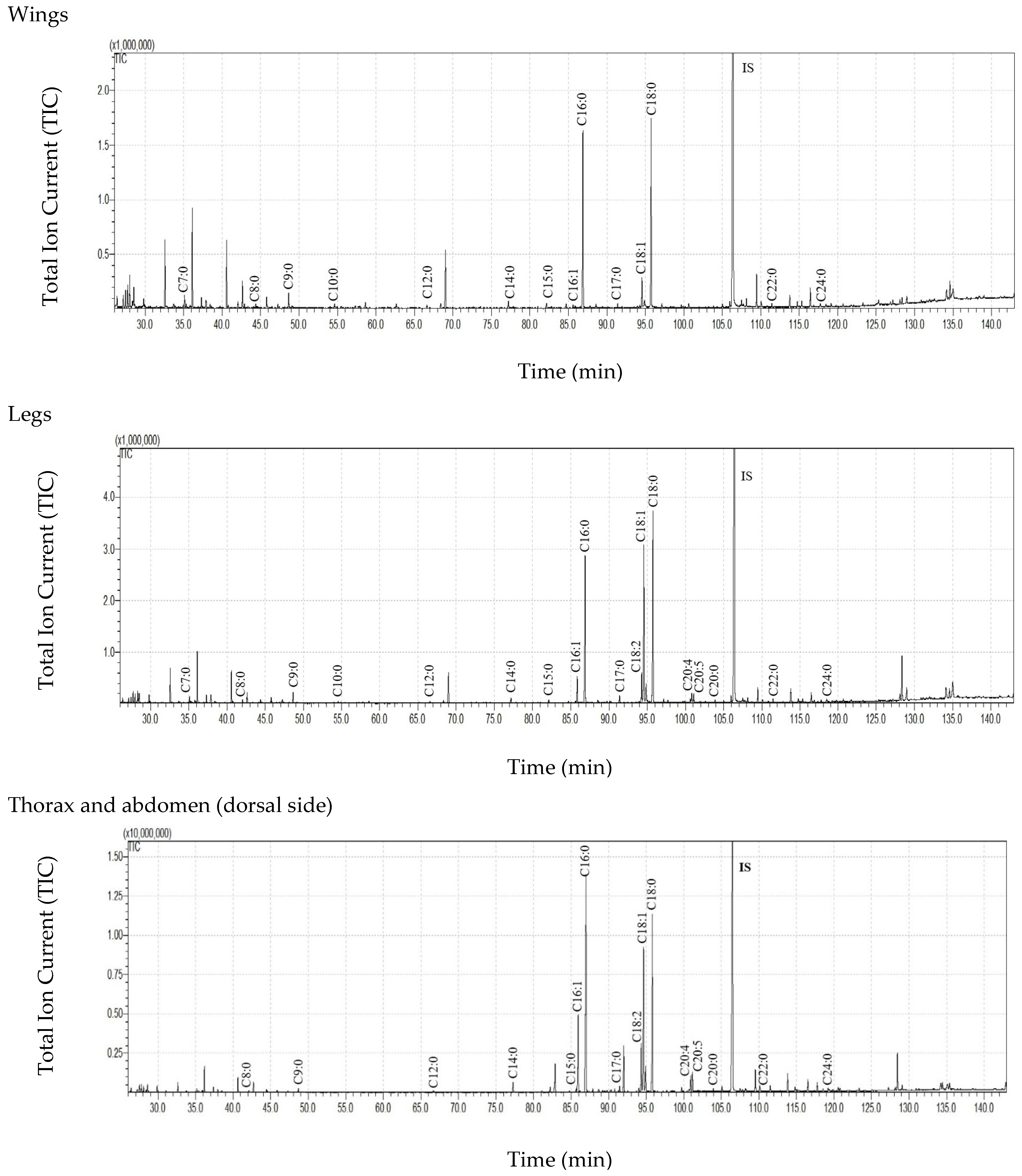
3.3. Free Fatty Acid (FFA) Content in Cuticular and Internal Lipids of T. bovinus Females
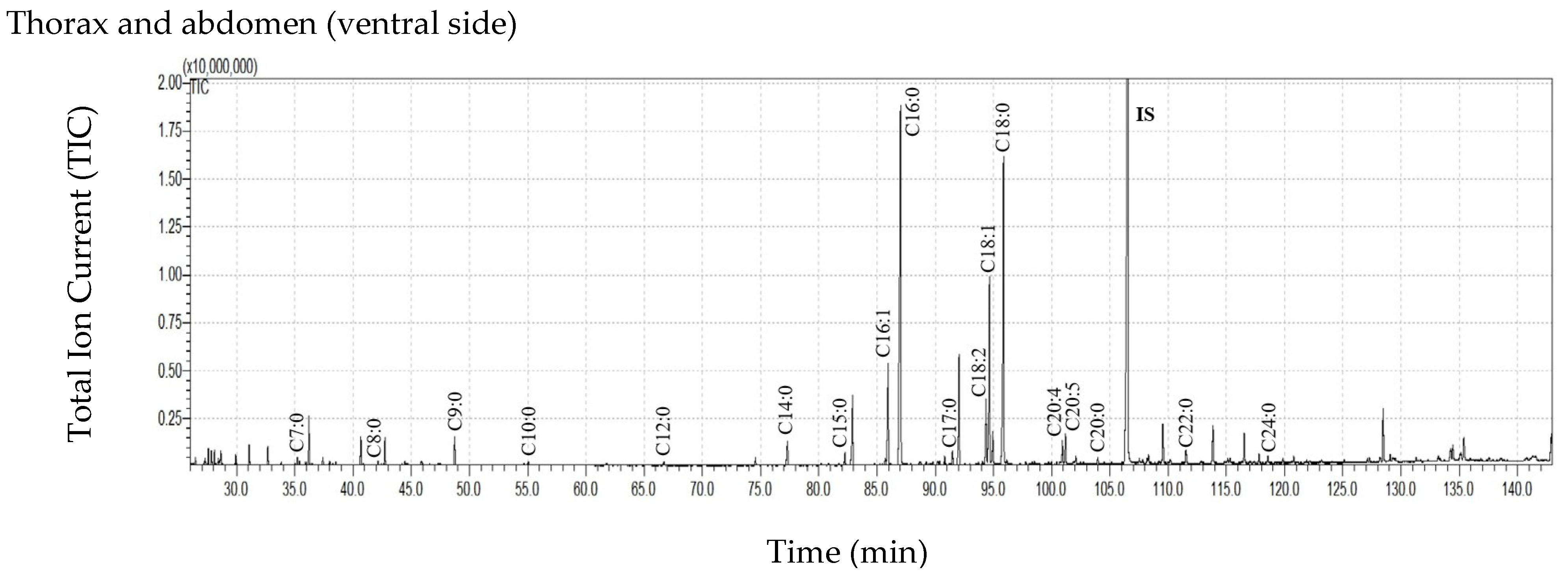
3.4. Cuticular Lipids of the Dorsal and the Ventral Sides of the Thorax and Abdomen
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parle, E.; Larmon, H.; Taylor, D. Biomechanical factors in the adaptations of Insect tibia cuticle. PLoS ONE 2016, 11, e0159262. [Google Scholar] [CrossRef] [PubMed]
- Stamm, K.; Saltin, B.D.; Dirks, J.H. Biomechanics of insect cuticle: An interdisciplinary experimental challenge. Appl. Phys. A 2021, 127, 329. [Google Scholar] [CrossRef]
- Zill, S.; Frazier, S.F.; Neff, D.; Quimby, L.; Carney, M.; DiCaprio, R.; Thuma, J.; Norton, M. Three-dimensional graphic reconstruction of the insect exoskeleton through confocal imaging of endogenous fluorescence. Microsc. Res. Tech. 2000, 48, 367–384. [Google Scholar] [CrossRef]
- Vincent, J.F.; Wegst, U.G. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef]
- Rajabi, H.; Jafarpour, M.; Darvizeh, A.; Dirks, J.H.; Gorb, S.N. Stiffness distribution in insect cuticle: A continuous or a discontinuous profile? J. R. Soc. Interface. 2017, 14, 20170310. [Google Scholar] [CrossRef]
- Laiolo, P.; Pato, J.; Illera, J.C.; Obeso, J.R. Selection for functional performance in the evolution of cuticle hardening mechanisms in insects. Evolution 2021, 75, 1132–1142. [Google Scholar] [CrossRef]
- Botella-Cruz, M.; Pallarés, S.; Millán, A.; Velasco, J. Role of cuticle hydrocarbons composition in the salinity tolerance of aquatic beetles. J. Insect Physiol. 2019, 117, 103899. [Google Scholar] [CrossRef]
- Schmüser, L.; Zhang, W.; Marx, M.T.; Encinas, N.; Vollmer, D.; Gorb, S.; Baio, J.E.; Räder, H.J.; Weidner, T. Role of surface chemistry in the superhydrophobicity of the springtail Orchesella cincta (Insecta: Collembola). ACS Appl. Mater. Interfaces 2020, 12, 12294–12304. [Google Scholar] [CrossRef]
- Whyte, B.A.; Sandidge, R.; Buellesbach, J.; Cash, E.I.; Scheckel, K.J.; Gibson, J.D.; Tsutsui, N.D. The role of body size and cuticular hydrocarbons in the desiccation resistance of invasive Argentine ants (Linepithema humile). J. Exp. Biol. 2023, 226, jeb245578. [Google Scholar] [CrossRef]
- Chung, H.; Carroll, S.B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. Bioessays 2015, 37, 822–830. [Google Scholar] [CrossRef]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Debecker, S.; Sommaruga, R.; Maes, T.; Stoks, R. Larval UV exposure impairs adult immune function through a trade-off with larval investment in cuticular melanin. Funct. Ecol. 2015, 28, 1292–1299. [Google Scholar] [CrossRef]
- Mallick, S.; Eleftherianos, I. Role of cuticular genes in the insect antimicrobial immune response. Front. Cell. Infect. Microbiol. 2024, 14, 1456075. [Google Scholar] [CrossRef] [PubMed]
- Sirasoonthorn, P.; Kamiya, K.; Miura, K. Antifungal roles of adult-specific cuticular protein genes of the red flour beetle, Tribolium castaneum. J. Invertebr. Pathol. 2021, 186, 107674. [Google Scholar] [CrossRef]
- Wu, W.; Mao, Q.; Ye, Z.X.; Liao, Z.; Shan, H.W.; Li, J.M.; Zhang, C.X.; Chen, J.P. Brochosomes as an antireflective camouflage coating for leafhoppers. Elife 2025, 13, RP99639. [Google Scholar] [CrossRef]
- Tajiri, R. Cuticle itself as a central and dynamic player in shaping cuticle. Curr. Opin. Insect Sci. 2017, 19, 30–35. [Google Scholar] [CrossRef]
- Asano, T.; Seto, Y.; Hashimoto, K.; Kurushima, H. Mini-review an insect-specific system for terrestrialization: Laccase-mediated cuticle formation. Insect Biochem. Mol. Biol. 2019, 108, 61–70. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Ju, Y.; Zhang, W.; Wang, Y. Insect cuticle and insecticide development. Arch. Insect Biochem. Physiol. 2023, 114, e22057. [Google Scholar] [CrossRef]
- Chrissian, C.; Stawski, M.L.; Williams, A.P.; Stark, R.E. Elucidating structure and metabolism of insect biomaterials by solid-state NMR. Solid State Nucl. Magn. Reson. 2024, 134, 101974. [Google Scholar] [CrossRef]
- Stamm, K.; Dirks, J.H. Semi-automated differentiation of insect exo- and endocuticle in X-ray microtomography. Arthropod Struct. Dev. 2022, 66, 101139. [Google Scholar] [CrossRef]
- Rong, J.; Lin, Y.; Sui, Z.; Wang, S.; Wei, X.; Xiao, J.; Huang, D. Amorphous calcium phosphate in the pupal cuticle of Bactrocera dorsalis Hendel (Diptera: Tephritidae): A new discovery for reconsidering the mineralization of the insect cuticle. J. Insect Physiol. 2019, 119, 103964. [Google Scholar] [CrossRef] [PubMed]
- Casey, C.; Yager, C.; Jankauski, M.; Heveran, C.M. The flying insect thoracic cuticle is heterogenous in structure and in thickness-dependent modulus gradation. Acta Biomater. 2022, 138, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Wrońska, A.K.; Kaczmarek, A.; Boguś, M.I.; Kuna, A. Lipids as a key element of insect defense systems. Front. Genet. 2023, 14, 1183659. [Google Scholar] [CrossRef] [PubMed]
- Krupp, J.J.; Nayal, K.; Wong, A.; Millar, J.G.; Levine, J.D. Desiccation resistance is an adaptive life-history trait dependent upon cuticular hydrocarbons, and influenced by mating status and temperature in D. melanogaster. J. Insect Physiol. 2020, 121, 103990. [Google Scholar] [CrossRef]
- Palm, W.; Rodenfels, J. Understanding the role of lipids and lipoproteins in development. Development 2020, 147, dev186411. [Google Scholar] [CrossRef]
- Gondim, K.C.; Atella, G.C.; Pontes, E.G.; Majerowicz, D. Lipid metabolism in insect disease vectors. Insect Biochem. Mol. Biol. 2018, 101, 108–123. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Ginzel, M.D. Chemical ecology, biochemistry, and molecular biology of insect hydrocarbons. Annu. Rev. Entomol. 2021, 66, 45–60. [Google Scholar] [CrossRef]
- Gibbs, A.G. Lipid melting and cuticular permeability: New insights into an old problem. J. Insect Physiol. 2002, 48, 391–400. [Google Scholar] [CrossRef]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef]
- Hadley, N.F. Cuticular lipids of terrestrial plants and arthropods: A comparison of their structure, composition, and waterproofing function. Biol. Rev. 1981, 56, 23–47. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Boguś, M.I.; Paszkiewicz, M.; Stepnowski, P. Cuticular lipids of insects as potential biofungicides: Methods of lipid composition analysis. Anal. Bioanal. Chem. 2011, 399, 3177–3191. [Google Scholar] [CrossRef] [PubMed]
- Azeez, O.I.; Meintjes, R.; Chamunorwa, J.P. Fat body, fat pad and adipose tissues in invertebrates and vertebrates: The nexus. Lipids Health Dis. 2014, 13, 71. [Google Scholar] [CrossRef]
- Beenakkers, A.M.; Van der Horst, D.J.; Van Marrewijk, W.J. Insect lipids and lipoproteins, and their role in physiological processes. Prog. Lipid Res. 1985, 24, 19–67. [Google Scholar] [CrossRef]
- Gäde, G.; Auerswald, L. Mode of action of neuropeptides from the adipokinetic hormone family. Gen. Comp. Endocrinol. 2003, 132, 10–20. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Cerkowniak, M.; Urbanek, A.; Słocińska, M.; Rosiński, G.; Stepnowski, P. Adipokinetic hormone induces changes in the fat body lipid composition of the beetle Zophobas atratus. Peptides 2014, 58, 65–73. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Prostaglandins and other eicosanoids in insects: Biosynthesis and biological actions. Front. Physiol. 2019, 9, 1927. [Google Scholar] [CrossRef]
- Fonseca, F.; Pénicaud, C.; Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Passot, S. Factors influencing the membrane fluidity and the impact on production of lactic acid bacteria starters. Appl. Microbiol. Biotechnol. 2019, 103, 6867–6883. [Google Scholar] [CrossRef]
- Florek, O.B.; Clifton, L.A.; Wilde, M.; Arnold, T.; Green, R.J.; Frazier, R.A. Lipid composition in fungal membrane models: Effect of lipid fluidity. Acta Crystallogr. D Struct. Biol. 2018, 74 Pt 12, 1233–1244. [Google Scholar] [CrossRef]
- Overgaard, J.; Sørensen, J.G.; Petersen, S.O.; Loeschcke, V.; Holmstrup, M. Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. J. Insect Physiol. 2005, 51, 1173–1182. [Google Scholar] [CrossRef]
- Koštál, V.; Urban, T.; Rimnáčová, L.; Berková, P.; Simek, P. Seasonal changes in minor membrane phospholipid classes, sterols and tocopherols in overwintering insect, Pyrrhocoris apterus. J. Insect Physiol. 2013, 59, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, R.; Van Antwerpen, R. Lipid uptake by insect oocytes. Insect Biochem. Mol. Biol. 2006, 36, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Rosas-Oliveira, R.; Saraiva, F.B.; Majerowicz, D.; Gondim, K.C. Lipid accumulation and utilization by oocytes and eggs of Rhodnius prolixus. Arch. Insect Biochem. Physiol. 2011, 77, 1–16. [Google Scholar] [CrossRef]
- Leyria, J.; Orchard, I.; Lange, A.B. What happens after a blood meal? A transcriptome analysis of the main tissues involved in egg production in Rhodnius prolixus, an insect vector of Chagas disease. PLoS Negl. Trop. Dis. 2020, 14, e0008516. [Google Scholar] [CrossRef]
- Krcmar, S.; Maric, S. The role of blood meal in the life of haematophagous horse flies (Diptera: Tabanidae). Period. Biol. 2010, 112, 207–210. [Google Scholar]
- Cerkowniak, M.; Puckowski, A.; Stepnowski, P.; Gołębiowski, M. The use of chromatographic techniques for the separation and the identification of insect lipids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 937, 67–78. [Google Scholar] [CrossRef]
- Kazek, M.; Kaczmarek, A.; Wrońska, A.K.; Boguś, M.I. Diet influences the bacterial and free fatty acid profiles of the cuticle of Galleria mellonella larvae. PLoS ONE 2019, 14, e0211697. [Google Scholar] [CrossRef]
- Kazek, M.; Kaczmarek, A.; Wrońska, A.; Boguś, M. Dodecanol, metabolite of entomopathogenic fungus Conidiobolus coronatus, affects fatty acid composition and cellular immunity of Galleria mellonella and Calliphora vicina. Sci. Rep. 2021, 11, 15963. [Google Scholar] [CrossRef]
- Trojan, P. Tabanidae ślepaki (Insecta: Diptera). In Fauna Polski—Fauna Poloniae; Riedel, A., Ed.; PWN: Warsaw, Poland, 1979; Volume 8, ISBN 83-01-00251-4. Available online: http://rcin.org.pl (accessed on 10 June 2025).
- Ivanov, V.P. [Investigation of the sensory organs on antennae of the horseflies Hybomitra bimaculata and Tabanus bovinus (Diptera: Tabanidae) by scanning electron microscope]. Parazitologiia 2007, 41, 372–380. (In Russian) [Google Scholar]
- Wathne, A.M.; Devle, H.; Naess-Andresen, C.F.; Ekeberg, D. Identification and quantification of fatty acids in T. viridissima, C. biguttulus, and C. brunneus by GC-MS. J. Lipids 2018, 28, 3679247. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Wrońska, A.K.; Kazek, M.; Boguś, M.I. Metamorphosis-related changes in the free fatty acid profiles of Sarcophaga (Liopygia) argyrostoma (Robineau-Desvoidy, 1830). Sci. Rep. 2020, 10, 17337. [Google Scholar] [CrossRef] [PubMed]
- Cerkowniak, M.; Boguś, M.I.; Włóka, E.; Stepnowski, P.; Gołębiowski, M. The composition of lipid profiles in different developmental stages of Dermestes ater and Dermestes maculatus and their susceptibility to the entomopathogenic fungus Conidiobolus coronatus. Phytoparasitica 2020, 48, 247–260. [Google Scholar] [CrossRef]
- Palupi, E.; Nasir, S.Q.; Jayanegara, A.; Susanto, I.; Ismail, A.; Iwansyah, A.C.; Setiawan, B.; Sulaeman, A.; Damanik, M.R.M.; Filianty, F. Meta-analysis on the fatty acid composition of edible insects as a sustainable food and feed. Future Foods 2025, 11, 100529. [Google Scholar] [CrossRef]
- Pilco-Romero, G.; Chisaguano-Tonato, A.M.; Herrera-Fontana, M.E.; Chimbo-Gándara, L.F.; Sharifi-Rad, M.; Giampieri, F.; Battino, M.; Vernaza, M.G.; Álvarez-Suárez, J.M. House cricket (Acheta domesticus): A review based on its nutritional composition, quality, and potential uses in the food industry. Trends Food Sci. Technol. 2023, 142, 104226. [Google Scholar] [CrossRef]
- Ding, C.H.; Cao, F.P.; Wang, Y.H.; Yang, M.L. [Extraction of fatty constituents from Tabanus bivittatus and their analysis by GC-MS]. Zhong Yao Cai 2013, 36, 188–190. (In Chinese) [Google Scholar]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Palmitic-Acid#section=Pharmacology-and-Biochemistr (accessed on 10 June 2025).
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5281 (accessed on 10 June 2025).
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/445639 (accessed on 10 June 2025).
- Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C. Effects of fatty acid unsaturation numbers on membrane fluidity and α-secretase-dependent amyloid precursor protein processing. Neurochem. Internat. 2011, 58, 321–329. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Cerkowniak, M.; Boguś, M.I.; Włóka, E.; Dawgul, M.; Kamysz, W.; Stepnowski, P. Free fatty acids in the cuticular and internal lipids of Calliphora vomitoria and their antimicrobial activity. J. Insect Physiol. 2013, 59, 416–429. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Urbanek, A.; Oleszczak, A.; Dawgul, M.; Kamysz, W.; Boguś, M.I.; Stepnowski, P. The antifungal activity of fatty acids of all stages of Sarcophaga carnaria L. (Diptera: Sarcophagidae). Microbiol. Res. 2014, 169, 279–286. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Sosnowska, A.; Puzyn, T.; Boguś, M.I.; Wieloch, W.; Włóka, E.; Stepnowski, P. Application of two-way hierarchical cluster analysis for the identification of similarities between the individual lipid fractions of Lucilia sericata. Chem. Biodivers. 2014, 11, 733–748. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Sikora, A.; Boguś, M.I.; Włóka, E.; Stepnowski, P.; Gołębiowski, M. Effect of exposure to chlorpyrifos on the cuticular and internal lipid composition of Blattella germanica males. Insect Sci. 2016, 23, 94–104. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Gołębiowski, M.; Sychowska, J.; Boguś, M.I.; Włóka, E.; Stepnowski, P. (2016). The effect of the entomopathogenic fungus, Conidiobolus coronatus, on the composition of cuticular and internal lipids of Blatta orientalis females. Physiol. Entomol. 2016, 41, 111–120. [Google Scholar] [CrossRef]
- Sun, M.; Zhou, Z.; Dong, J.; Zhang, J.; Xia, Y.; Shu, R. Antibacterial and antibiofilm activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against periodontopathic bacteria. Microb. Pathog. 2016, 99, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid. Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P. Antimicrobial Properties of Eicosapentaenoic Acid (C20:5n−3). In Marine Microbiology; Se-Kwon, K., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2013; pp. 351–367. [Google Scholar] [CrossRef]
- Borreby, C.; Lillebæk, E.M.S.; Kallipolitis, B.H. Anti-infective activities of long-chain fatty acids against foodborne pathogens. FEMS Microbiol. Rev. 2023, 47, fuad037. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Kim, Y. Why most insects have very low proportions of C20 polyunsaturated fatty acids: The oxidative stress hypothesis. Arch. Insect Bioch. Physiol. 2020, 103, e21622. [Google Scholar] [CrossRef]
- Krcmar, S.; Durbesic, P.; Dmitrovic, B. Research of the gonotrophic cycle of the species Tabanus sudeticus Zeller 1842 (Diptera: Tabanidae) in eastern Croatia. Ecologia 2002, 21, 113–118. [Google Scholar]
- Strother, S. Genus Tabanus. Tabanids (horseflies). What is this insect and how does it affect man? Dermatol. Online J. 1999, 5, 6. [Google Scholar]
- Herczeg, T.; Száz, D.; Blahó, M.; Barta, A.; Gyurkovszky, M.; Farkas, R.; Horváth, G. The effect of weather variables on the flight activity of horseflies (Diptera: Tabanidae) in the continental climate of Hungary. Parasitol. Res. 2015, 114, 1087–1097. [Google Scholar] [CrossRef]
- Hansen, I.A.; Attardo, G.M.; Rodriguez, S.D.; Drake, L.L. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front. Physiol. 2014, 5, 103. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, L.; He, Q.; Zhou, S. Regulatory mechanisms of vitellogenesis in insects. Front. Cell Dev. Biol. 2021, 8, 593613. [Google Scholar] [CrossRef]
- Harrison, R.E.; Chen, K.; South, L.; Lorenzi, A.; Brown, M.R.; Strand, M.R. Ad libitum consumption of protein- or peptide-sucrose solutions stimulates egg formation by prolonging the vitellogenic phase of oogenesis in anautogenous mosquitoes. Parasit. Vectors 2022, 15, 127. [Google Scholar] [CrossRef] [PubMed]
- Braz, V.; Selim, L.; Gomes, G.; Costa, M.L.; Mermelstein, C.; Gondim, K.C. Blood meal digestion and changes in lipid reserves are associated with the post-ecdysis development of the flight muscle and ovary in young adults of Rhodnius prolixus. J. Insect Physiol. 2023, 146, 104492. [Google Scholar] [CrossRef] [PubMed]
- Downer, R.G.H. Lipid metabolism in insects. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 10, pp. 77–114. [Google Scholar]
- Parmar, T.P.; Kindinger, A.L.; Mathieu-Resuge, M.; Twining, C.W.; Shipley, J.R.; Kainz, M.J.; Martin-Creuzburg, D. Fatty acid composition differs between emergent aquatic and terrestrial insects—A detailed single system approach. Front. Ecol. Evol. 2022, 10, 952292. [Google Scholar] [CrossRef]
- Rajpurohit, S.; Vrkoslav, V.; Hanus, R.; Gibbs, A.G.; Cvačka, J.; Schmidt, P.S. Post-eclosion temperature effects on insect cuticular hydrocarbon profiles. Ecol. Evol. 2020, 11, 352–364. [Google Scholar] [CrossRef]
- Baker, J.E. Cuticular lipids of larvae of Attagenus megatoma. Insect Biochem. 1978, 8, 287–292. [Google Scholar] [CrossRef]
| Body Parts | Mass of Body Part (mg ± SD) | Extract Mass (mg ± SD) | Extract Mass per Total Insect Mass * (µg Extract/mg Body Weight ± SD) | Extract Mass per Mass of the Extracted Insect Body Part (µg Extract/mg Body Part ± SD) | |||
|---|---|---|---|---|---|---|---|
| Cuticicular Extracts | Internal Extracts | Cuticicular Extracts | Internal Extracts | Cuticular Extracts | Internal Extracts | ||
| Head | 10.03 ± 0.88 a | 4.46 ± 2.95 | 1.99 ± 0.95 | 32.84 ± 18.45 | 14.67 ± 6.54 c | 461.70 ± 328.99 | 207.75 ± 124.91 |
| Wings (one pair) | 3.10 ± 0.08 ab | 2.19 ± 0.48 A | 1.04 ± 0.13 A | 15.73 ± 2.64 B | 7.55 ± 1.06 dB | 704.75 ± 141.31 efghC | 337.53 ± 48.18 lC |
| Legs (three pairs) | 7.99 ± 1.06 b | 1.99 ± 0.68 | 1.51 ± 1.84 | 14.55 ± 4.75 | 10.75 ± 10.52 | 263.25 ± 133.20 eijk | 184.64 ± 212.35 |
| Thorax and abdomen | 115.61 ± 5.78 a | 2.31 ± 1.65 | 4.01 ± 0.29 | 16.22 ± 11.40 | 28.99 ± 2.90 cd | 19.48 ± 13.65 fi | 34.84 ± 4.24 l |
| Thorax and abdomen (dorsal side) ** | NA | 1.20 ± 0.82 | NA | 8.43 ± 4.54 | NA | 10.12 ± 6.69 gi | NA |
| Thorax and abdomen (ventral side) ** | NA | 1.10 ± 0.99 | NA | 7.78 ± 5.63 | NA | 9.35 ± 8.25 hk | NA |
| FFA | Concentration of FFA (ng/mg Body Part ± SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cuticular Lipids | Internal Lipids | |||||||
| Head | Wings | Legs | Thorax and Abdomen | Head | Wings | Legs | Thorax and Abdomen | |
| C7:0 | 192.79 ± 261.79 | 122.50 ± 59.80 | 57.28 ± 33.80 | 6.40 ± 3.56 | 4.68 ± 6.37 | 29.29 ± 12.87 | 19.75 ± 17.37 | 1.55 ± 1.35 |
| C8:0 | 263.32 ± 318.50 | 225.83 ± 88.10 | 110.30 ± 67.59 | 11.21 ± 6.25 | 44.80 ± 46.88 | 57.34 ± 22.30 | 38.83 ± 34.67 | 8.78 ± 4.75 |
| C9:0 | 491.46 ± 426.78 | 568.06 ± 143.54 | 255.11 ± 84.82 | 57.23 ± 38.45 | 92.60 ± 67.98 | 161.77 ± 27.75 | 116.90 ± 112.64 | 14.08 ± 2.66 |
| C10:0 | 189.13 ± 255.43 | 93.50 ± 8.62 | 67.06 ± 37.51 | 9.08 ± 6.65 | ND | 13.50 ± 13.01 | 16.86 ± 23.72 | 1.16 ± 1.01 |
| C11:0 | 108.42 ± 165.98 | 24.42 ± 27.73 | 11.50 ± 19.92 | 0.78 ± 0.57 | ND | ND | ND | ND |
| C12:0 | 165.40 ± 163.32 | 128.76 ± 54.61 | 76.24 ± 41.23 | 12.55 ± 9.27 | 4.12 ± 7.14 | 20.16 ± 20.20 | 19.37 ± 27.99 | 3.92 ± 1.00 |
| C13:0 | 96.32 ± 135.89 | 40.30 ± 18.83 | 19.85 ± 34.38 | 0.62 ± 1.08 | ND | ND | ND | ND |
| C14:0 | 376.85 ± 202.09 | 318.45 ± 95.29 | 287.05 ± 205.40 | 69.86 ± 44.23 | 36.24 ± 25.98 | 76.77 ± 22.35 | 58.20 ± 45.81 | 32.08 ± 9.60 |
| C15:0 | 192.64 ± 103.96 | 192.00 ± 112.85 | 222.23 ± 275.74 | 31.35 ± 16.68 | 3.87 ± 6.70 | 21.01 ± 20.49 | 22.06 ± 30.23 | 5.66 ± 3.04 |
| C16:1 | 2821.03 ± 2708.41 | 75.90 ± 67.80 | 263.78 ± 228.93 | 225.44 ± 27.09 | 256.12 ± 149.43 | 32.26 ± 31.44 | 217.91 ± 266.88 | 1351.70 ± 227.11 |
| C16:0 | 8561.72 ± 4167.84 | 5700.88 ± 2333.89 | 3852.48 ± 3535.31 | 1462.81 ± 774.09 | 844.95 ± 482.17 | 1399.51 ± 487.45 | 1499.76 ± 1544.96 | 1306.80 ± 195.63 |
| C17:0 | 505.07 ± 259.02 | 119.79 ± 129.57 | 192.87 ± 183.57 | 28.46 ± 17.36 | 27.99 ± 21.54 | 18.74 ± 18.21 | 43.02 ± 42.28 | 52.71 ± 45.91 |
| C18:2 | 1822.09 ± 2065.72 | 66.34 ± 78.10 | 310.73 ± 211.23 | 139.64 ± 32.38 | 205.30 ± 165.42 | 58.39 ± 31.54 | 341.89 ± 372.72 | 1241.33 ± 199.41 |
| C18:1 | 8824.73 ± 9740.00 | 880.82 ± 614.15 | 1690.09 ± 1168.86 | 554.00 ± 73.15 | 1112.93 ± 866.09 | 815.14 ± 302.08 | 1935.62 ± 1998.02 | 3245.52 ± 349.59 |
| C18:0 | 7630.55 ± 3329.36 | 5179.97 ± 2973.85 | 3353.72 ± 3325.72 | 1157.58 ± 745.98 | 727.07 ± 503.24 | 946.88 ± 610.97 | 1384.60 ± 1623.10 | 562.69 ± 99.04 |
| C19:0 | 310.39 ± 482.22 | 9.03 ± 15.64 | 14.91 ± 25.83 | 43.65 ± 15.81 | ND | ND | ND | 8.63 ± 5.62 |
| C20:5 | 2135.89 ± 2770.37 | ND | 142.50 ± 85.46 | 16.34 ± 11.57 | 256.91 ± 249.18 | ND | 272.47 ± 327.16 | 583.30 ± 135.54 |
| C20:4 | 886.83 ± 1111.07 | ND | 119. 06 ± 79.37 | 46.96 ± 8.44 | 89.79 ± 83.03 | ND | 197.77 ± 234.67 | 474.75 ± 165.56 |
| C20:0 | 72.40 ± 63.07 | 76.12 ± 66.72 | 54.00 ± 76.13 | 38.63 ± 33.93 | 7.47 ± 11.21 | 18.69 ± 19.05 | 20.81 ± 27.58 | 11.06 ± 2.01 |
| C22:0 | 57.88 ± 62.75 | 75.57 ± 73.98 | 42.05 ± 56.65 | 6.29 ± 5.45 | 13.73 ± 21.30 | 58.37 ± 56.42 | 44.49 ± 59.46 | 5.53 ± 0.90 |
| C24:0 | 48.89 ± 47.41 | 65.58 ± 58.98 | 39.06 ± 61.74 | 9.94 ± 11.33 | 15.34 ± 24.74 | 50.77 ± 46.60 | 31.22 ± 36.53 | 2.95 ± 2.57 |
| Sum of FFAs | 35753.80 ± 23741.37 abcA | 13964.79 ± 6581.49 adB | 11181.88 ± 9582.56 b | 4147.93 ± 1290.02 cd | 3743.91 ± 2528.51 A | 3778.59 ± 1651.56 B | 6281.54 ± 6812.38 | 8917.00 ± 1426.60 |
| FFA | Concentration of FFA (ng/mg of Whole Thorax and Abdomen ± SD) | |
|---|---|---|
| Thorax and Abdomen Dorsal Side | Thorax and Abdomen Ventral Side | |
| C7:0 | 3.22 ± 3.00 | 3.17 ± 0.56 |
| C8:0 | 5.10 ± 3.54 | 6.11 ± 2.72 |
| C9:0 | 27.93 ± 34.31 | 29.30 ± 4.18 |
| C10:0 | 5.04 ± 5.91 | 4.03 ± 0.75 |
| C11:0 | 0.48 ± 0.83 | 0.30 ± 0.36 |
| C12:0 | 6.78 ± 6.93 | 5.77 ± 2.34 |
| C13:0 | 0.62 ± 1.08 | ND |
| C14:0 | 38.87 ± 35.60 | 30.99 ± 8.65 |
| C15:0 | 17.23 ± 13.30 | 14.12 ± 3.39 |
| C16:1 | 129.25 ± 30.04 | 96.19 ± 3.31 |
| C16:0 | 807.11 ± 554.77 | 655.70 ± 219.98 |
| C17:0 | 15.39 ± 13.23 | 13.07 ± 4.13 |
| C18:2 | 92.11 ± 47.97 | 47.53 ± 15.62 |
| C18:1 | 337.76 ± 216.25 | 216.25 ± 44.19 |
| C18:0 | 633.63 ± 516.18 | 523.96 ± 230.15 |
| C19:0 | 30.11 ± 18.57 | ND |
| C20:5 | 9.17 ± 9.40 | 15.91 ± 5.60 |
| C20:4 | 31.05 ± 14.04 | 13.54 ± 2.76 |
| C20:0 | 19.71 ± 21.51 | 7.17 ± 2.17 |
| C22:0 | 1.99 ± 1.73 | 18.92 ± 12.42 |
| C24:0 | 7.00 ± 7.57 | 4.30 ± 3.73 |
| Sum of FFAs | 2212.54 ± 1445.57 | 1706.35 ± 417.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozdowski, M.; Boguś, M.I. Compartmentalization of Free Fatty Acids in Blood-Feeding Tabanus bovinus Females. Insects 2025, 16, 696. https://doi.org/10.3390/insects16070696
Drozdowski M, Boguś MI. Compartmentalization of Free Fatty Acids in Blood-Feeding Tabanus bovinus Females. Insects. 2025; 16(7):696. https://doi.org/10.3390/insects16070696
Chicago/Turabian StyleDrozdowski, Mikołaj, and Mieczysława Irena Boguś. 2025. "Compartmentalization of Free Fatty Acids in Blood-Feeding Tabanus bovinus Females" Insects 16, no. 7: 696. https://doi.org/10.3390/insects16070696
APA StyleDrozdowski, M., & Boguś, M. I. (2025). Compartmentalization of Free Fatty Acids in Blood-Feeding Tabanus bovinus Females. Insects, 16(7), 696. https://doi.org/10.3390/insects16070696







