Impact of Salmonella enteritidis Infection and Mechanical Stress on Antimicrobial Peptide Expression in Hermetia illucens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Hermetia illucens Rearing
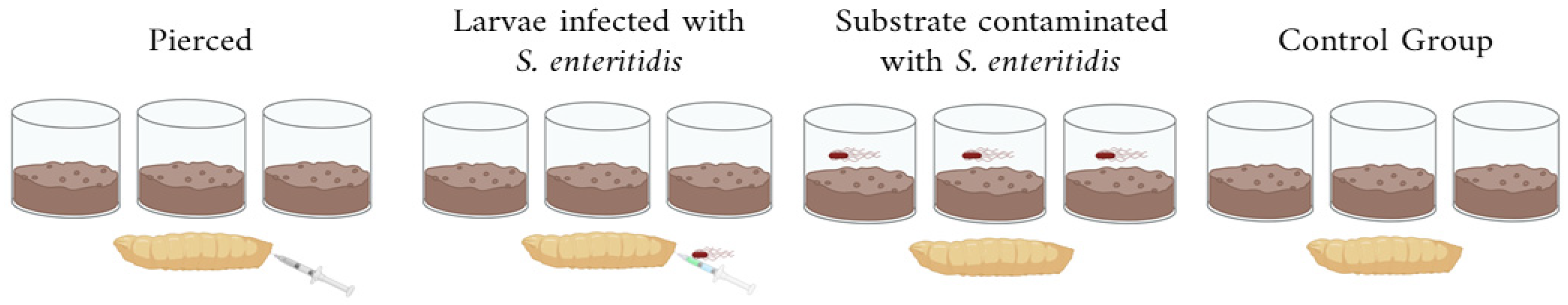
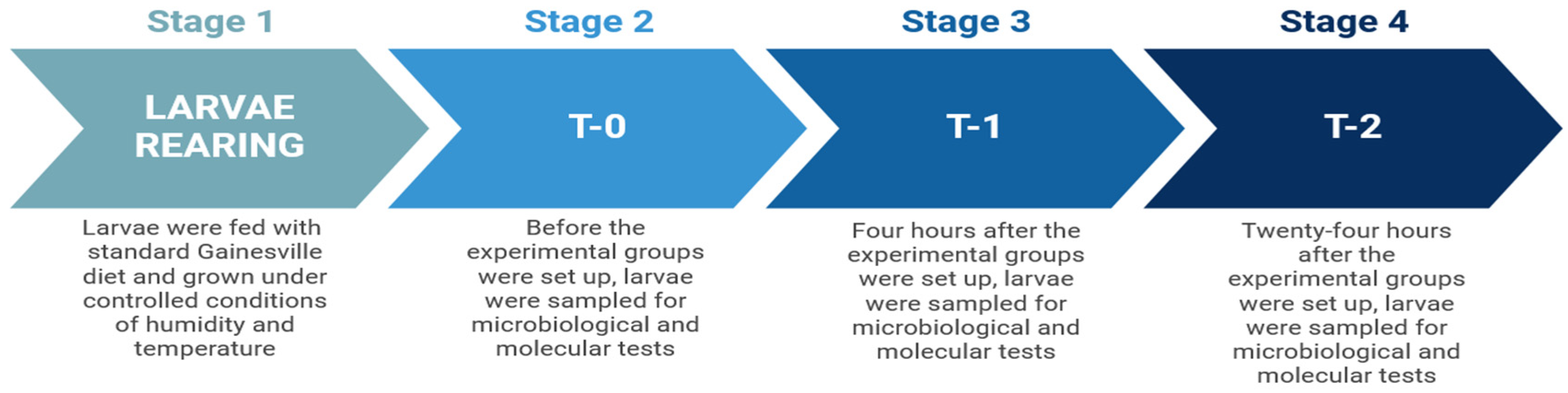
2.2. Experimental Setup
2.3. Salmonella enteritidis and Salmonella typhimurium Strains
2.4. Hemolymph Collection
2.5. Peptide Isolation and Quantification
2.6. Determination of the Minimum Inhibitory Concentration (MIC) of Hemolymph Against Salmonella enteritidis and Salmonella typhimurium
2.7. Evaluation of the Antibacterial Activity of Hemolymph via an Antibiogram Assay
2.8. Molecular Evaluation of Defensin and Cecropin Expression
2.9. Statistical Analysis
3. Results
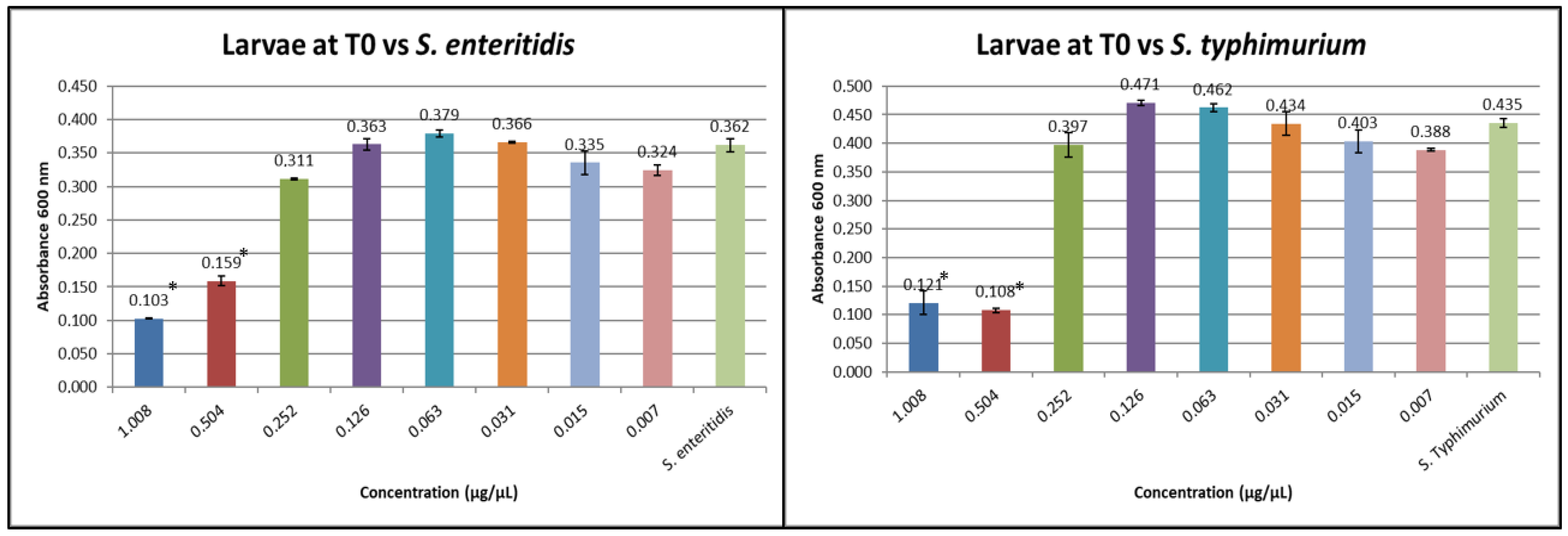
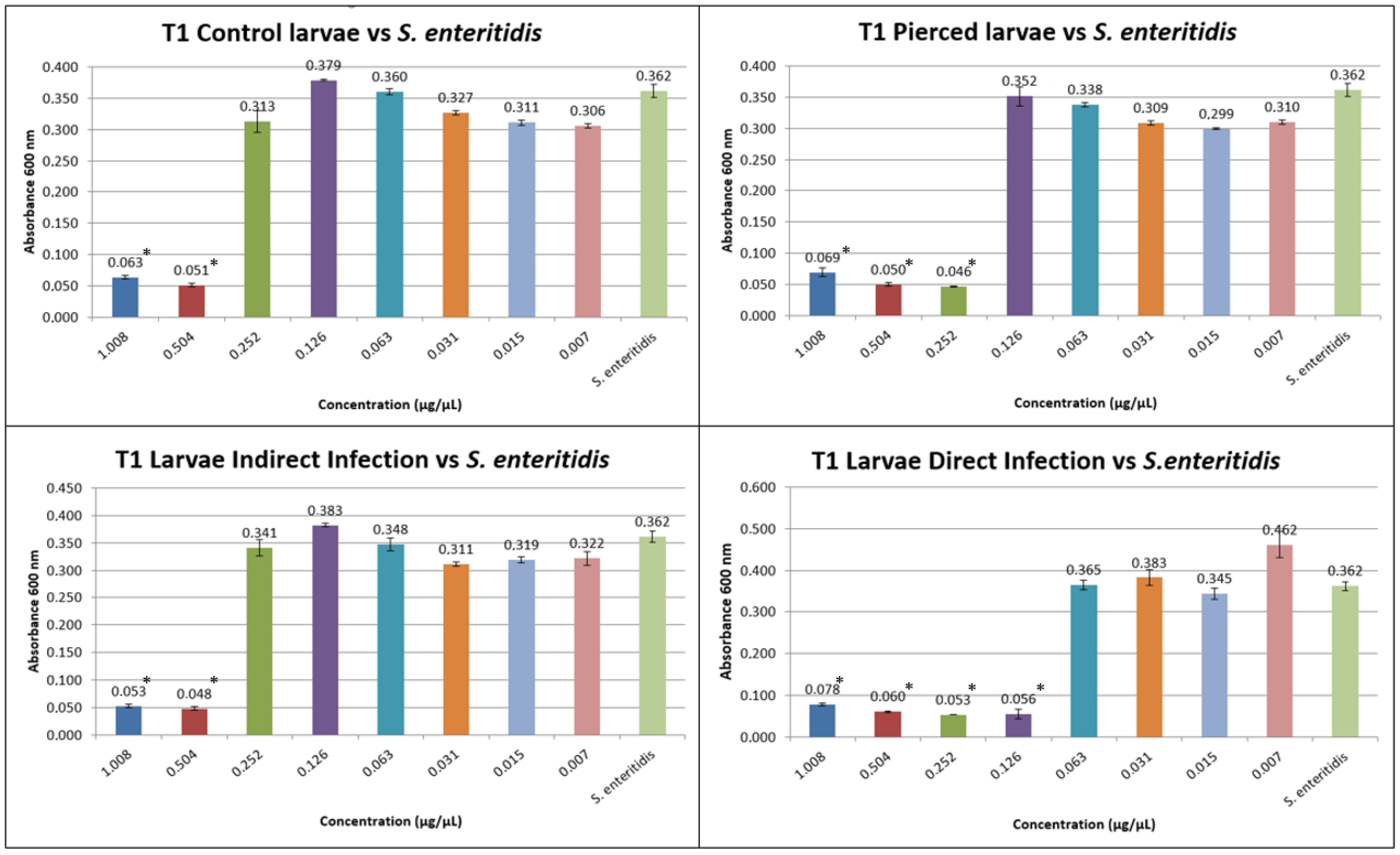
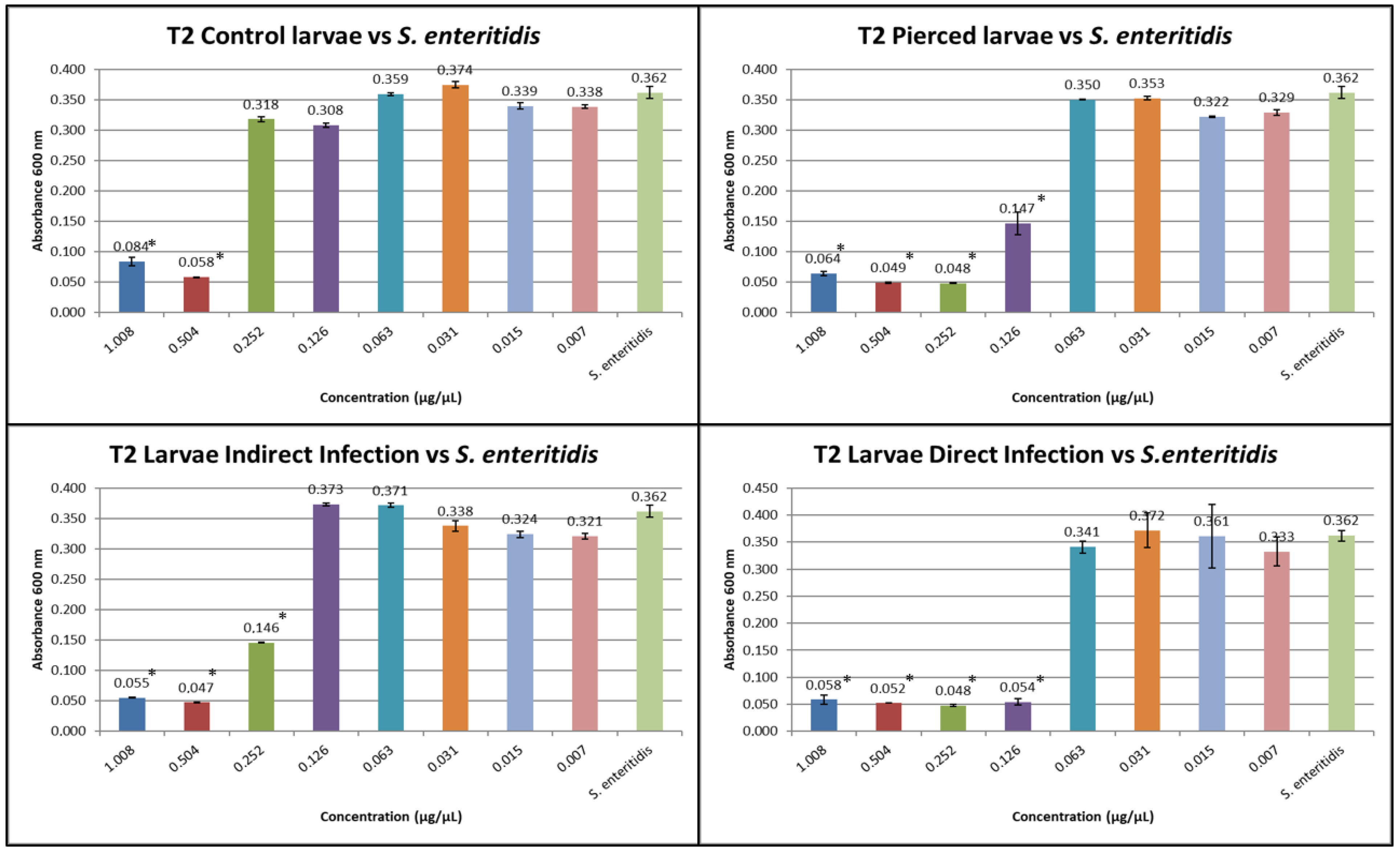
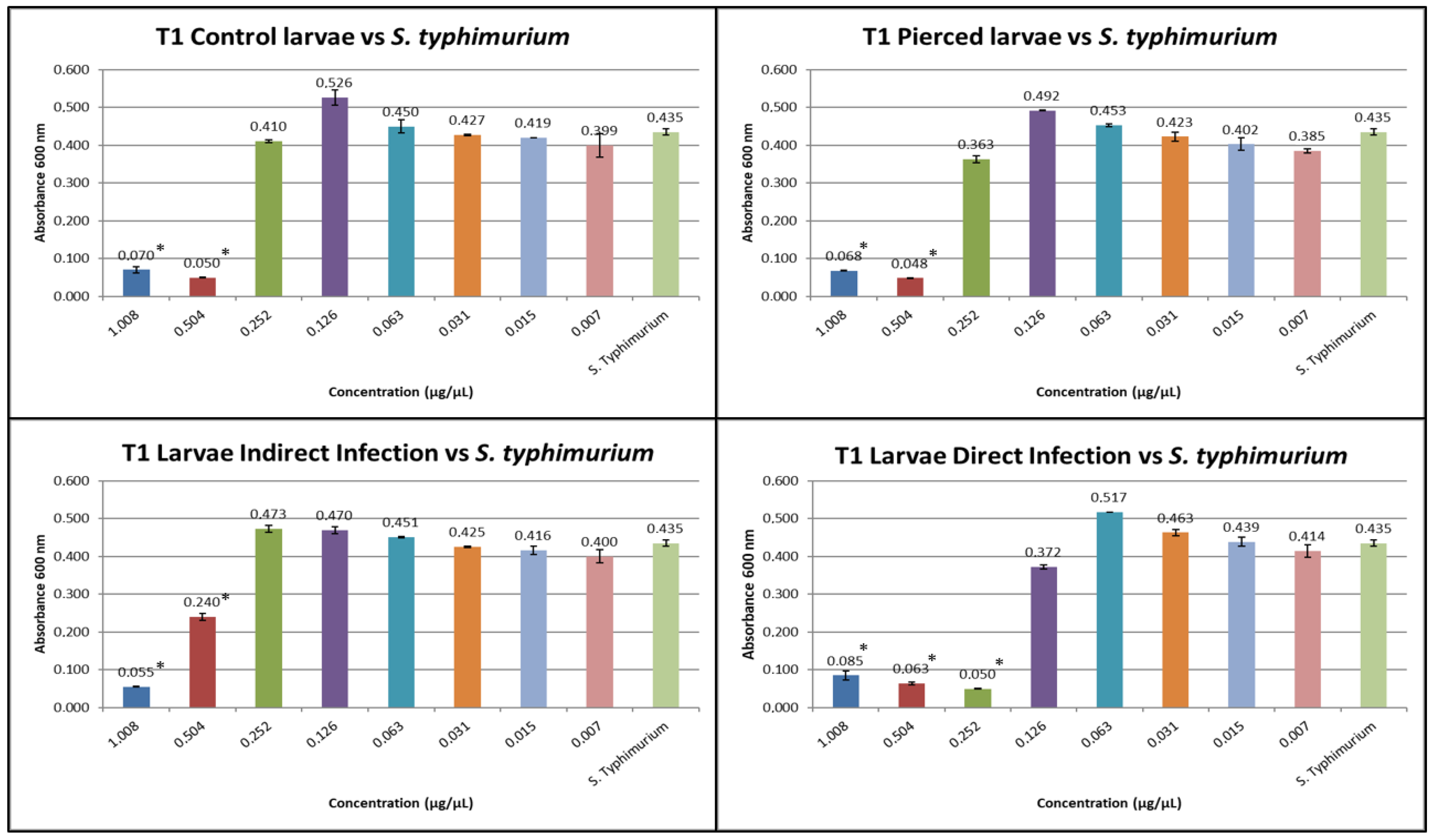
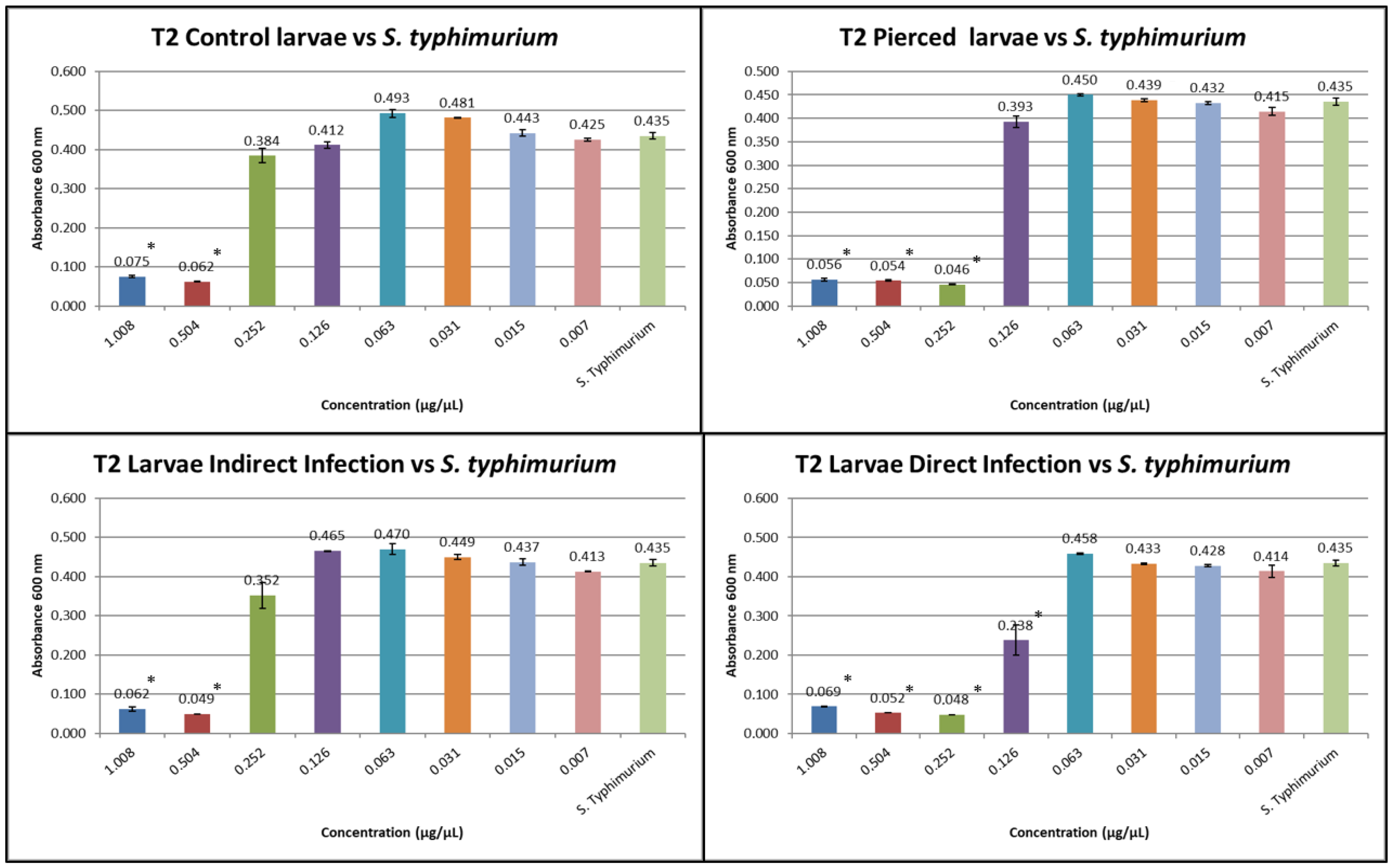
3.1. Results of Minimum Inhibitory Concentration (MIC)
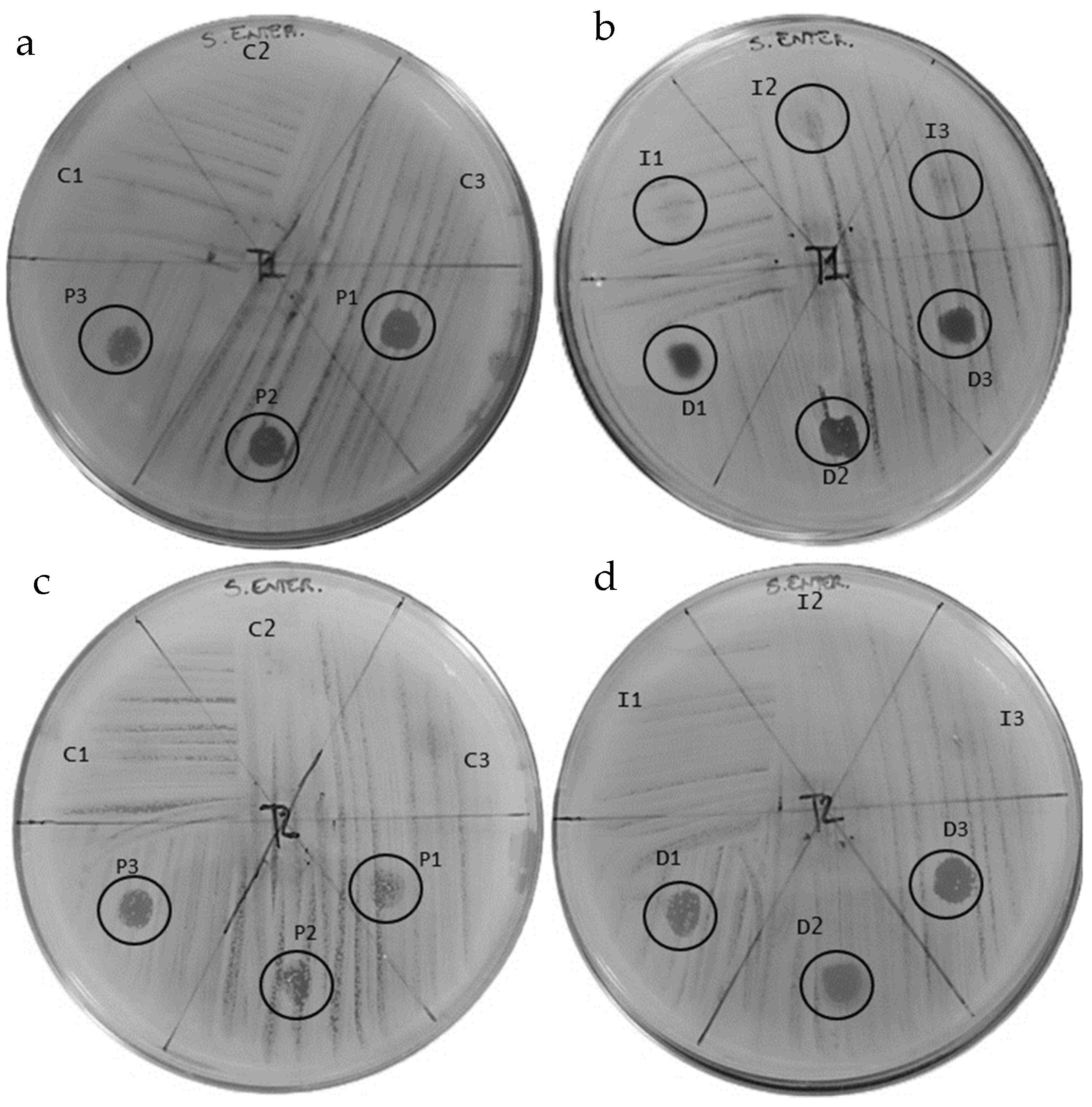
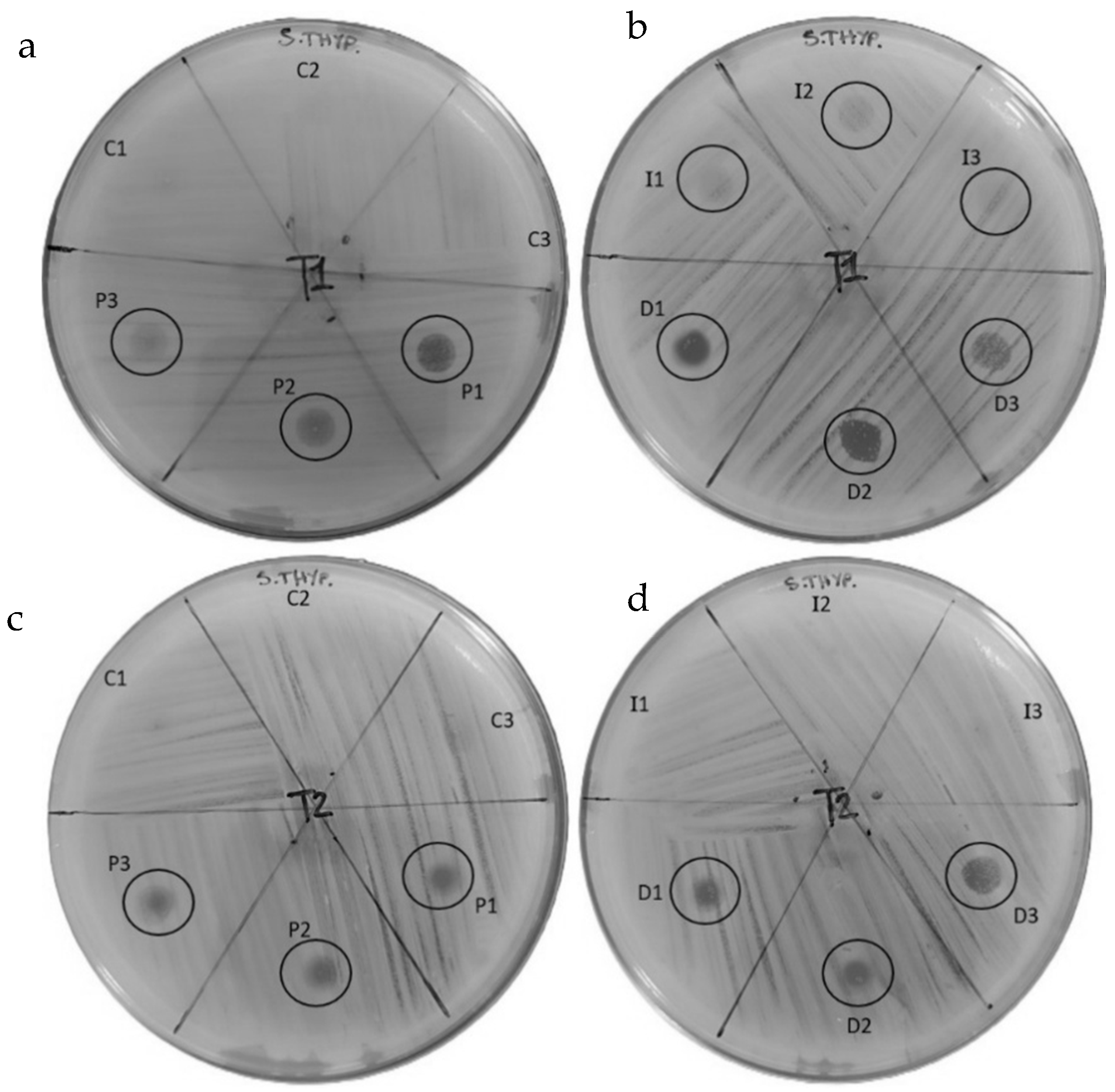
3.2. Results of the Antibiogram Assay
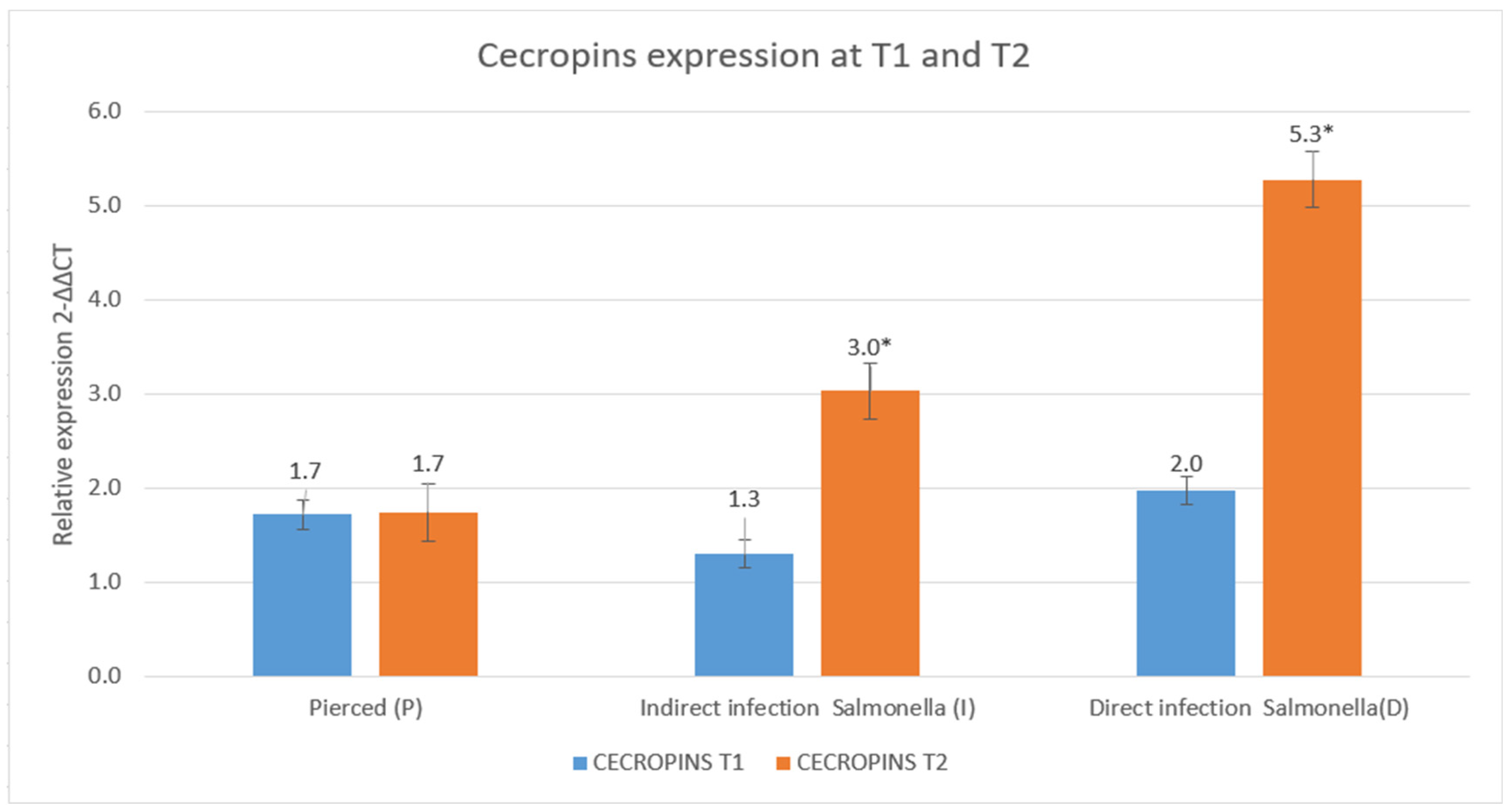
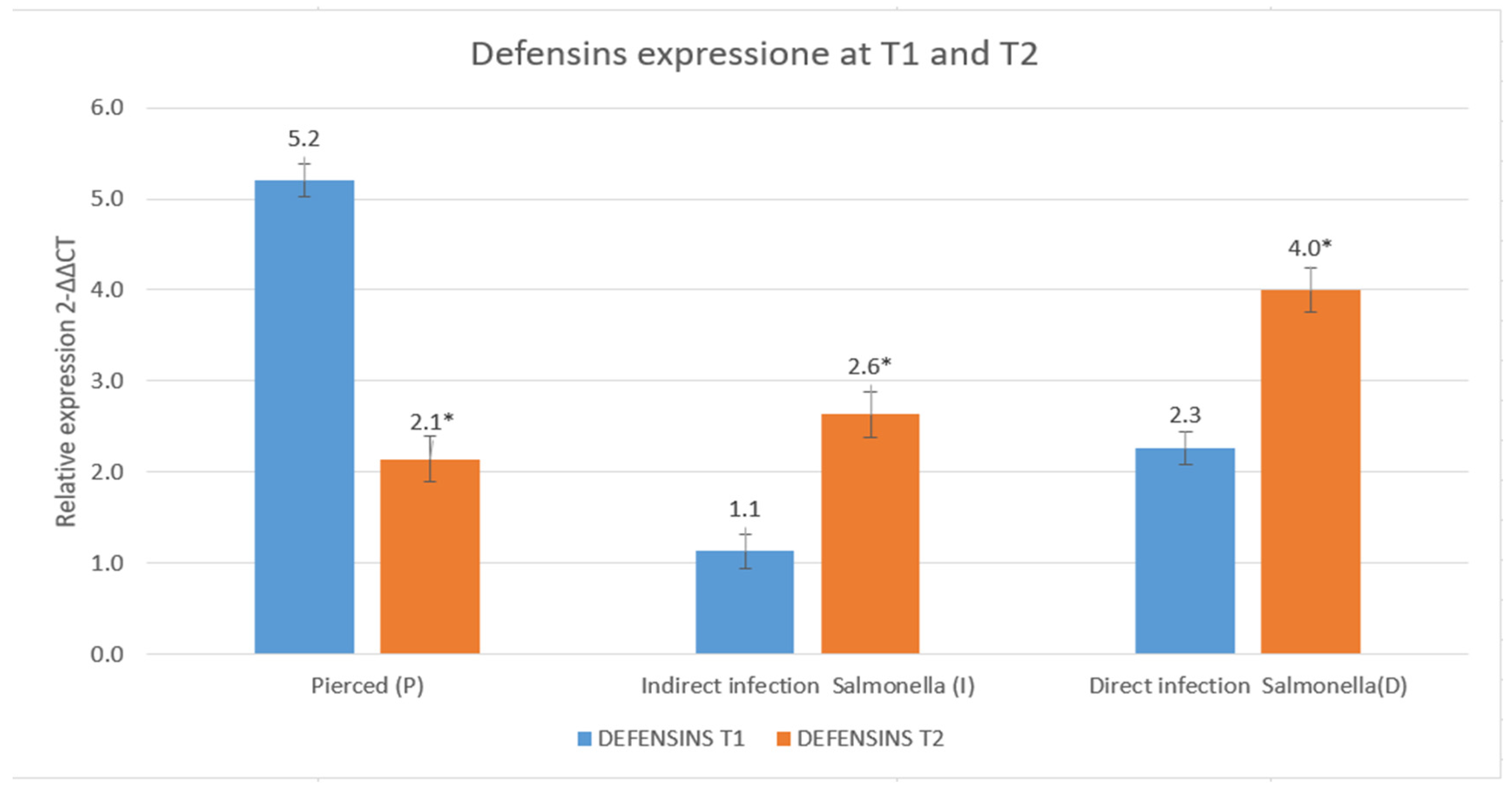
3.3. Results of the Expression of Cecropins and Defensins
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menkem, Z.E.; Ngangom, B.L.; Tamunjoh, S.S.A.; Boyom, F.F. Antibiotic Residues in Food Animals: Public Health Concern. Acta Ecol. Sin. 2019, 39, 411–415. [Google Scholar] [CrossRef]
- Bengtsson, B.; Greko, C. Antibiotic Resistance—Consequences for Animal Health, Welfare, and Food Production. Upsala J. Med. Sci. 2014, 119, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yuan, T.; Zhou, L.; Cheng, S.; Qu, X.; Lu, P.; Feng, Q. Impact Factors of the Accumulation, Migration and Spread of Antibiotic Resistance in the Environment. Environ. Geochem. Health 2021, 43, 1741–1758. [Google Scholar] [CrossRef] [PubMed]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial Peptides from Black Soldier Fly (Hermetia illucens) as Potential Antimicrobial Factors Representing an Alternative to Antibiotics in Livestock Farming. Animals 2021, 11, 1937. [Google Scholar] [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between Veterinary Antimicrobial Use and Antimicrobial Resistance in Food-Producing Animals: A Report on Seven Countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef]
- Oliver, S.P.; Murinda, S.E.; Jayarao, B.M. Impact of Antibiotic Use in Adult Dairy Cows on Antimicrobial Resistance of Veterinary and Human Pathogens: A Comprehensive Review. Foodborne Pathog. Dis. 2011, 8, 337–355. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K. Use of Antibiotics as Feed Additives: A Burning Question. Front. Microbiol. 2014, 5, 334. [Google Scholar] [CrossRef]
- Regulation (EC) 1831/2003. Regulation (EC) 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Available online: https://eur-lex.europa.eu/eli/reg/2003/1831/oj/eng (accessed on 1 May 2025).
- Castanon, J.I.R. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/6. Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC. Available online: https://eur-lex.europa.eu/eli/reg/2019/6/oj/eng (accessed on 1 May 2025).
- Nogueira, R.; Baptista, C.J.; Gonçalves, L.; Coelho, A.C.; Faustino-Rocha, A.I.; Regueiro Purriños, M.; Gonzalo-Orden, J.M.; Oliveira, P.A. The Veterinary Medicinal Products Market Supply Gap: A Practical Insight Based on the Regulation (EU) 2019/6. Rev. Ciênc. Agrovet. 2024, 23, 153–158. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2022–2023. EFSA J. 2025, 23, e9237. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Vieira, A.R.; Karlsmose, S.; Lo Fo Wong, D.M.A.; Jensen, A.B.; Wegener, H.C.; Aarestrup, F.M. Global Monitoring of Salmonella Serovar Distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: Results of Quality Assured Laboratories from 2001 to 2007. Foodborne Pathog. Dis. 2011, 8, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Juniora, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-Analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same Species, Different Diseases: How and Why Typhoidal and Non-Typhoidal Salmonella Enterica Serovars Differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, N.K.S.; de Barros, V.B.; Jimenez, S.M.C.; Machado, E.d.C.L.; Dutra, R.A.F.; de Lima Filho, J.L. Salmonella Spp., Importante Agente Patogênico Veiculado Em Alimentos. Cienc. Saude Coletiva 2008, 13, 1675–1683. [Google Scholar] [CrossRef]
- Buonocore, F.; Fausto, A.M.; Della Pelle, G.; Roncevic, T.; Gerdol, M.; Picchietti, S. Attacins: A Promising Class of Insect Antimicrobial Peptides. Antibiotics 2021, 10, 212. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, P.; Ma, Y.; Jin, N.; Sun, S.; Dong, X.; Li, X.; Xu, J.; Zhou, H.; Xu, W. Transformation of Food Waste to Source of Antimicrobial Proteins by Black Soldier Fly Larvae for Defense against Marine Vibrio Parahaemolyticus. Sci. Total Environ. 2022, 826, 154163. [Google Scholar] [CrossRef]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect Antimicrobial Peptides: Potential Weapons to Counteract the Antibiotic Resistance. Cell. Mol. Life Sci. 2021, 78, 4259–4282. [Google Scholar] [CrossRef]
- Harlystiarini, H.; Mutia, R.; Wibawan, I.W.T.; Astuti, D.A. In Vitro Antibacterial Activity of Black Soldier Fly (Hermetia illucens) Larva Extracts Against Gram-Negative Bacteria. Bul. Peternak. 2019, 43, 125–129. [Google Scholar] [CrossRef]
- Li, Z.; Mao, R.; Teng, D.; Hao, Y.; Chen, H.; Wang, X.; Wang, X.; Yang, N.; Wang, J. Antibacterial and Immunomodulatory Activities of Insect Defensins-DLP2 and DLP4 against Multidrug-Resistant Staphylococcus Aureus. Sci. Rep. 2017, 7, 12124. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Antibacterial Peptides: Basic Facts and Emerging Concepts. J. Intern. Med. 2003, 254, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Mcdermott, A.M.; Zasloff, M. Antimicrobial Peptides and Wound Healing: Biological and Therapeutic Considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F. Insect Immunology and Hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef]
- Vallet-Gely, I.; Lemaitre, B.; Boccard, F. Bacterial Strategies to Overcome Insect Defences. Nat. Rev. Microbiol. 2008, 6, 302–313. [Google Scholar] [CrossRef]
- Szczepanik, K.; Świątkiewicz, M. Hermetia Illucens as a Source of Antimicrobial Peptides—A Review of in vitro and in vivo Studies. Ann. Anim. Sci. 2024, 24, 77–88. [Google Scholar] [CrossRef]
- Fratini, F.; Pecorini, C.; Resci, I.; Copelotti, E.; Nocera, F.P.; Najar, B.; Mancini, S. Evaluation of the Synergistic Antimicrobial Activity of Essential Oils and Cecropin A Natural Peptide on Gram-Negative Bacteria. Animals 2025, 15, 282. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Yi, H.-Y.; Chowdhury, M.; Huang, Y.-D.; Yu, X.-Q. Insect Antimicrobial Peptides and Their Applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef]
- Shah, P.N.; Maistrou, S.; Willemsen, I.; van Loon, J.J.A.; Dicke, M. Transcriptomic Response of Hermetia illucens L. (Diptera: Stratiomyidae) to Wounding and Gram-Negative Bacterial Infection. J. Insects Food Feed 2024, 1, 1–21. [Google Scholar] [CrossRef]
- Herman, N.; Vitenberg, T.; Hayouka, Z.; Opatovsky, I. Regulation of Antimicrobial Peptides in Hermetia illucens in Response to Fungal Exposure. Sci. Rep. 2024, 14, 29561. [Google Scholar] [CrossRef] [PubMed]
- Scieuzo, C.; Giglio, F.; Rinaldi, R.; Lekka, M.E.; Cozzolino, F.; Monaco, V.; Monti, M.; Salvia, R.; Falabella, P. In Vitro Evaluation of the Antibacterial Activity of the Peptide Fractions Extracted from the Hemolymph of Hermetia illucens (Diptera: Stratiomyidae). Insects 2023, 14, 464. [Google Scholar] [CrossRef]
- Candian, V.; Savio, C.; Meneguz, M.; Gasco, L.; Tedeschi, R. Effect of the Rearing Diet on Gene Expression of Antimicrobial Peptides in Hermetia illucens (Diptera: Stratiomyidae). Insect Sci. 2023, 30, 933–946. [Google Scholar] [CrossRef]
- Azmiera, N.; Al-Talib, H.; Sahlan, N.; Krasilnikova, A.; Sahudin, S.; Heo, C.C. Antimicrobial Activity of Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae) Larval Hemolymph against Various Pathogenic Bacteria. J. Pure Appl. Microbiol. 2023, 17, 2493–2501. [Google Scholar] [CrossRef]
- Lee, K.S.; Yun, E.Y.; Goo, T.W. Antimicrobial Activity of an Extract of Hermetia illucens Larvae Immunized with Lactobacillus Casei against Salmonella Species. Insects 2020, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xiao, T.; Wu, L.; Li, Y.; Duan, X.; Liu, W.; Liu, K.; Jin, W.; Ren, H.; Sun, J.; et al. Comprehensive Profiling of Serotypes, Antimicrobial Resistance and Virulence of Salmonella Isolates from Food Animals in China, 2015–2021. Front. Microbiol. 2023, 14, 1133241. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Sogari, G.; Bernini, V.; Camorali, C.; Rossi, F.; Neviani, E.; Lazzi, C. A Critical Review of Intrinsic and Extrinsic Antimicrobial Properties of Insects. Trends Food Sci. Technol. 2022, 122, 40–48. [Google Scholar] [CrossRef]
- Makarova, O.; Rodriguez-Rojas, A.; Eravci, M.; Weise, C.; Dobson, A.; Johnston, P.; Rolff, J. Antimicrobial Defence and Persistent Infection in Insects Revisited. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150296. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R.; Menin, L. Anti-microbial Peptides: From Invertebrates to Vertebrates. Immunol. Rev. 2004, 198, 169–184. [Google Scholar] [CrossRef]
- Ali Mohammadie Kojour, M.; Han, Y.S.; Jo, Y.H. An Overview of Insect Innate Immunity. Entomol. Res. 2020, 50, 282–291. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.-P.; Smagghe, G.; Liu, D.-D.; Dai, R.-H.; Yang, H. Antimicrobial Peptides Play Important Roles in Innate Immunity and Recovery from Chill Coma in Lasioderma serricorne. Entomol. Gen. 2024, 44, 695–704. [Google Scholar] [CrossRef]
- Little, T.J.; Kraaijeveld, A.R. Ecological and Evolutionary Implications of Immunological Priming in Invertebrates. Trends Ecol. Evol. 2004, 19, 58–60. [Google Scholar] [CrossRef]
- Nakagawa, A.; Sakamoto, T.; Kanost, M.R.; Tabunoki, H. The Development of New Methods to Stimulate the Production of Antimicrobial Peptides in the Larvae of the Black Soldier Fly Hermetia illucens. Int. J. Mol. Sci. 2023, 24, 15765. [Google Scholar] [CrossRef]
- Auza, F.A.; Purwanti, S.; Syamsu, J.A.; Natsir, A. Antibacterial Activities of Black Soldier Flies (Hermetia illucens. l) Extract towards the Growth of Salmonella typhimurium, E. coli and Pseudomonas aeruginosa. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012024. [Google Scholar] [CrossRef]
- Romoli, O.; Saviane, A.; Bozzato, A.; D’Antona, P.; Tettamanti, G.; Squartini, A.; Cappellozza, S.; Sandrelli, F. Differential Sensitivity to Infections and Antimicrobial Peptide-Mediated Immune Response in Four Silkworm Strains with Different Geographical Origin. Sci. Rep. 2017, 7, 1048. [Google Scholar] [CrossRef] [PubMed]
- Critchlow, J.T.; Norris, A.; Tate, A.T. The Legacy of Larval Infection on Immunological Dynamics over Metamorphosis. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190066. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial Activity and Resistance: Influencing Factors. Front. Pharmacol. 2017, 8, 36. [Google Scholar] [CrossRef]
- Andrejko, M.; Mizerska-Dudka, M.; Jakubowicz, T. Antibacterial Activity in Vivo and in Vitro in the Hemolymph of Galleria mellonella Infected with Pseudomonas Aeruginosa. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 152, 118–123. [Google Scholar] [CrossRef]
- Luo, Y.H.; Ye, B.P.; Gao, S.Q.; Tang, G.J.; Li, D.P.; Huang, Y.H.; Huang, Z.Z.; Yang, X.; Naman, A.M.; Qian, Y.X.; et al. Evaluation of Antibacterial Activities of Hemolymph and Methanol Extracts from Black Soldier Fly (Hermetia illucens). J. Insects Food Feed. 2024, 4. [Google Scholar] [CrossRef]
- Mastore, M.; Binda Rossetti, S.; Giovannardi, S.; Scarì, G.; Brivio, M.F. Inducible Factors with Antimicrobial Activity after Immune Challenge in the Haemolymph of Red Palm Weevil (Insecta). Innate Immun. 2015, 21, 392–405. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and Host-Defense Peptides as New Anti-Infective Therapeutic Strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Martynowycz, M.W.; Rice, A.; Andreev, K.; Nobre, T.M.; Kuzmenko, I.; Wereszczynski, J.; Gidalevitz, D. Salmonella Membrane Structural Remodeling Increases Resistance to Antimicrobial Peptide LL-37. ACS Infect. Dis. 2019, 5, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Liu, Y.; Zhang, S.; Sun, S.; Wu, M.; Dong, X.; Tong, H.; Xu, J.; Zhou, H.; Guan, S.; et al. C/N-Dependent Element Bioconversion Efficiency and Antimicrobial Protein Expression in Food Waste Treatment by Black Soldier Fly Larvae. Int. J. Mol. Sci. 2022, 23, 5036. [Google Scholar] [CrossRef] [PubMed]
- Siva-Jothy, M.T.; Thompson, J.J.W. Short-Term Nutrient Deprivation Affects Immune Function. Physiol. Entomol. 2002, 27, 206–212. [Google Scholar] [CrossRef]
- Oppenheim, J.J.; Biragyn, A.; Kwak, L.W.; Yang, D. Roles of Antimicrobial Peptides Such as Defensins in Innate and Adaptive Immunity. Ann. Rheum. Dis. 2003, 62, ii17–ii21. [Google Scholar] [CrossRef]
- Fu, J.; Zong, X.; Jin, M.; Min, J.; Wang, F.; Wang, Y. Mechanisms and Regulation of Defensins in Host Defense. Signal Transduct. Target. Ther. 2023, 8, 300. [Google Scholar] [CrossRef]
- Tettamanti, G. IL Sistema Immunitario Della Mosca Soldato, Hermetia illucens. In Atti della Accademia Nazionale Italiana di Entomologia, Rendiconti Anno LXX; Accademia Entomologia: Florence, Italy, 2022; pp. 95–99. [Google Scholar]
- Bruno, D.; Montali, A.; Mastore, M.; Brivio, M.F.; Mohamed, A.; Tian, L.; Grimaldi, A.; Casartelli, M.; Tettamanti, G. Insights Into the Immune Response of the Black Soldier Fly Larvae to Bacteria. Front. Immunol. 2021, 12, 745160. [Google Scholar] [CrossRef]
- Imamura, M.; Nakahara, Y.; Kanda, T.; Tamura, T.; Taniai, K. A Transgenic Silkworm Expressing the Immune-Inducible Cecropin B-GFP Reporter Gene. Insect Biochem. Mol. Biol. 2006, 36, 429–434. [Google Scholar] [CrossRef]
- Liu, S.H.; Wei, D.; Yuan, G.R.; Jiang, H.B.; Dou, W.; Wang, J.J. Antimicrobial Peptide Gene Cecropin-2 and Defensin Respond to Peptidoglycan Infection in the Female Adult of Oriental Fruit Fly, Bactrocera dorsalis (Hendel). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 206, 1–7. [Google Scholar] [CrossRef]
- Brady, D.; Grapputo, A.; Romoli, O.; Sandrelli, F. Insect Cecropins, Antimicrobial Peptides with Potential Therapeutic Applications. Int. J. Mol. Sci. 2019, 20, 5862. [Google Scholar] [CrossRef]
- Raj, P. Current Status of Defensins and Their Role in Innate and Adaptive Immunity. FEMS Microbiol. Lett. 2002, 206, 9–18. [Google Scholar] [CrossRef]
- Viljakainen, L. Evolutionary Genetics of Insect Innate Immunity. Brief. Funct. Genom. 2015, 14, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.; Montali, A.; Gariboldi, M.; Wrońska, A.K.; Kaczmarek, A.; Mohamed, A.; Tian, L.; Casartelli, M.; Tettamanti, G. Morphofunctional characterization of hemocytes in black soldier fly larvae. Insect Sci. 2023, 30, 912–932. [Google Scholar] [CrossRef]
| Name | Sequence 5′–3′ |
|---|---|
| TOT-CECROPINS FW | CGGTCAAAGCGAAGCTGGTT |
| TOT-CECROPINS RW | TGCCAGAACATTGGCTCCTTG |
| TOT-DEFENSINS FW | TAGTGGAGCAGCATTATCGGG |
| TOT-DEFENSINS RW | GTCGTCCCATGGCAATACAATG |
| Bacterial Strain: Salmonella enteritidis | ||||
|---|---|---|---|---|
| Sample | Inhibition Halo (mm) | Colonies | Inhibition Halo (mm) | Colonies |
| T1 | T2 | |||
| C-1 | Absence | Presence | Absence | Presence |
| C-2 | Absence | Presence | Absence | Presence |
| C-3 | Absence | Presence | Absence | Presence |
| P-1 | 4.1 | Absence | 2.1 | +++ |
| P-2 | 3.5 | + | 1.7 | +++ |
| P-3 | 3.2 | + | 2.5 | ++ |
| I-1 | 2.1 | ++++ | Absence | Presence |
| I-2 | 2.0 | ++++ | Absence | Presence |
| I-3 | 1.9 | ++++ | Absence | Presence |
| D-1 | 3.5 | + | 4.0 | ++ |
| D-2 | 4.0 | + | 3.6 | + |
| D-3 | 3.2 | +++ | 3.4 | + |
| Bacterial Strain: Salmonella typhimurium | ||||
|---|---|---|---|---|
| Sample | Halo of Inhibition (mm) | Colonies | Halo of Inhibition (mm) | Colonies |
| T1 | T2 | |||
| C-1 | Absence | Presence | Absence | Presence |
| C-2 | Absence | Presence | Absence | Presence |
| C-3 | Absence | Presence | Absence | Presence |
| P-1 | 4.0 | ++ | 2.1 | +++ |
| P-2 | 4.1 | +++ | 2.0 | +++ |
| P-3 | 3.6 | ++++ | 2.7 | +++ |
| I-1 | 3.4 | Presence | Absence | Presence |
| I-2 | 3.2 | Presence | Absence | Presence |
| I-3 | 3.1 | Presence | Absence | Presence |
| D-1 | 4.1 | + | 2.5 | ++ |
| D-2 | 4.3 | + | 2.2 | +++ |
| D-3 | 3.5 | +++ | 2.7 | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santori, D.; Fausto, A.M.; Gelli, A.; Pifferi, A.R.; Dottarelli, S.; Cucci, S.; Di Donato, F.; Grifoni, G.; Sezzi, E. Impact of Salmonella enteritidis Infection and Mechanical Stress on Antimicrobial Peptide Expression in Hermetia illucens. Insects 2025, 16, 692. https://doi.org/10.3390/insects16070692
Santori D, Fausto AM, Gelli A, Pifferi AR, Dottarelli S, Cucci S, Di Donato F, Grifoni G, Sezzi E. Impact of Salmonella enteritidis Infection and Mechanical Stress on Antimicrobial Peptide Expression in Hermetia illucens. Insects. 2025; 16(7):692. https://doi.org/10.3390/insects16070692
Chicago/Turabian StyleSantori, Davide, Anna Maria Fausto, Alessio Gelli, Anna Rita Pifferi, Samuele Dottarelli, Sofia Cucci, Francesca Di Donato, Goffredo Grifoni, and Erminia Sezzi. 2025. "Impact of Salmonella enteritidis Infection and Mechanical Stress on Antimicrobial Peptide Expression in Hermetia illucens" Insects 16, no. 7: 692. https://doi.org/10.3390/insects16070692
APA StyleSantori, D., Fausto, A. M., Gelli, A., Pifferi, A. R., Dottarelli, S., Cucci, S., Di Donato, F., Grifoni, G., & Sezzi, E. (2025). Impact of Salmonella enteritidis Infection and Mechanical Stress on Antimicrobial Peptide Expression in Hermetia illucens. Insects, 16(7), 692. https://doi.org/10.3390/insects16070692






