The Rheb-mTORC1 Coordinates Cell Cycle Progression and Endoreplication in Bombyx mori
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Cells
2.2. Rapamycin Treatment
2.3. CCK-8 Cell Proliferation Viability Assay
2.4. RNA Extraction and Quantitative Real-Time PCR (qPCR) Analysis
2.5. Western Blot (WB)
2.6. Cell Cycle Analysis
2.7. Ethynyl-2-Deoxyuridine (EdU) Assay
2.8. DNA Content Analysis
2.9. Statistical Analysis
3. Results
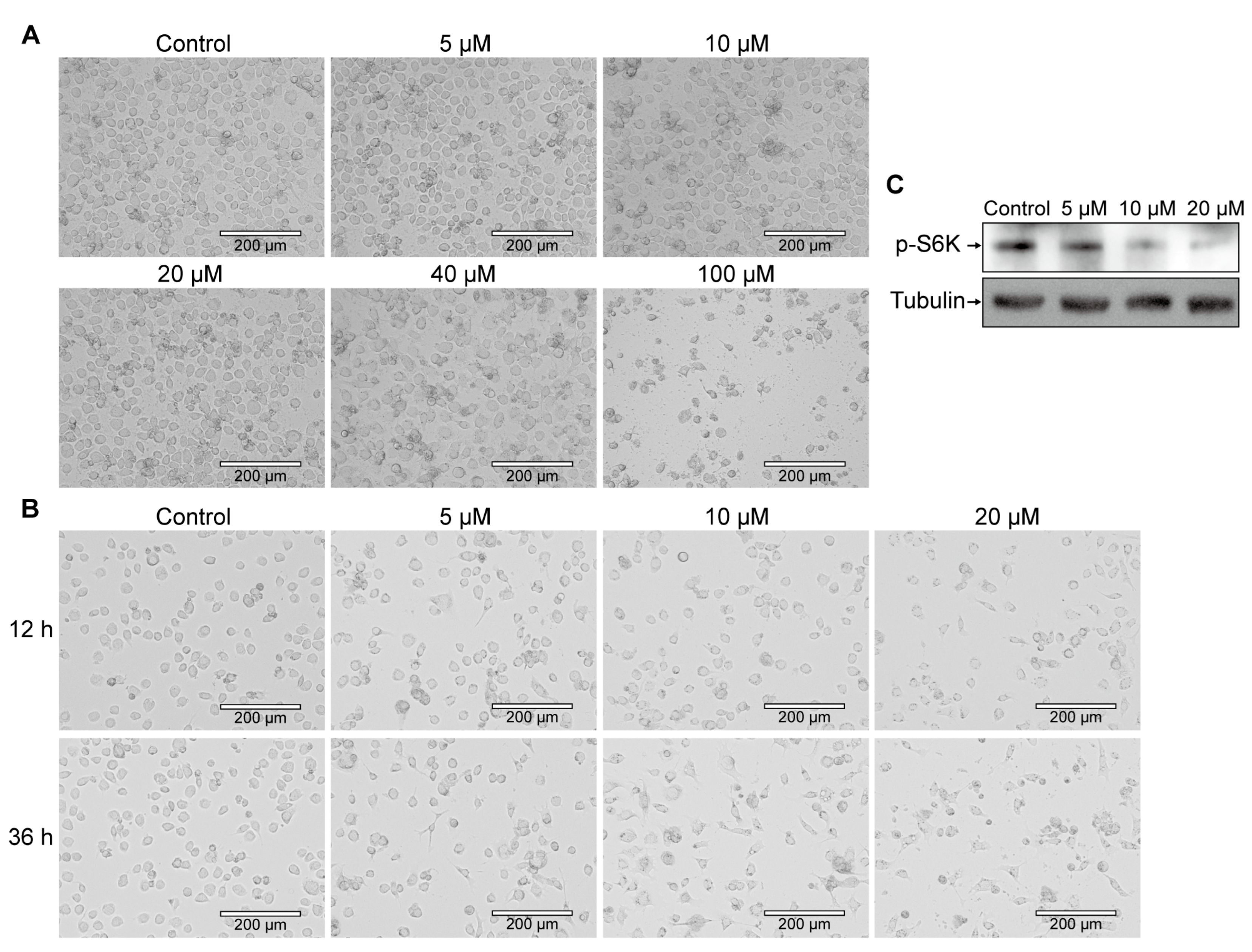
3.1. Exploration of the mTORC1 Signaling Pathway Inhibitor Rapamycin
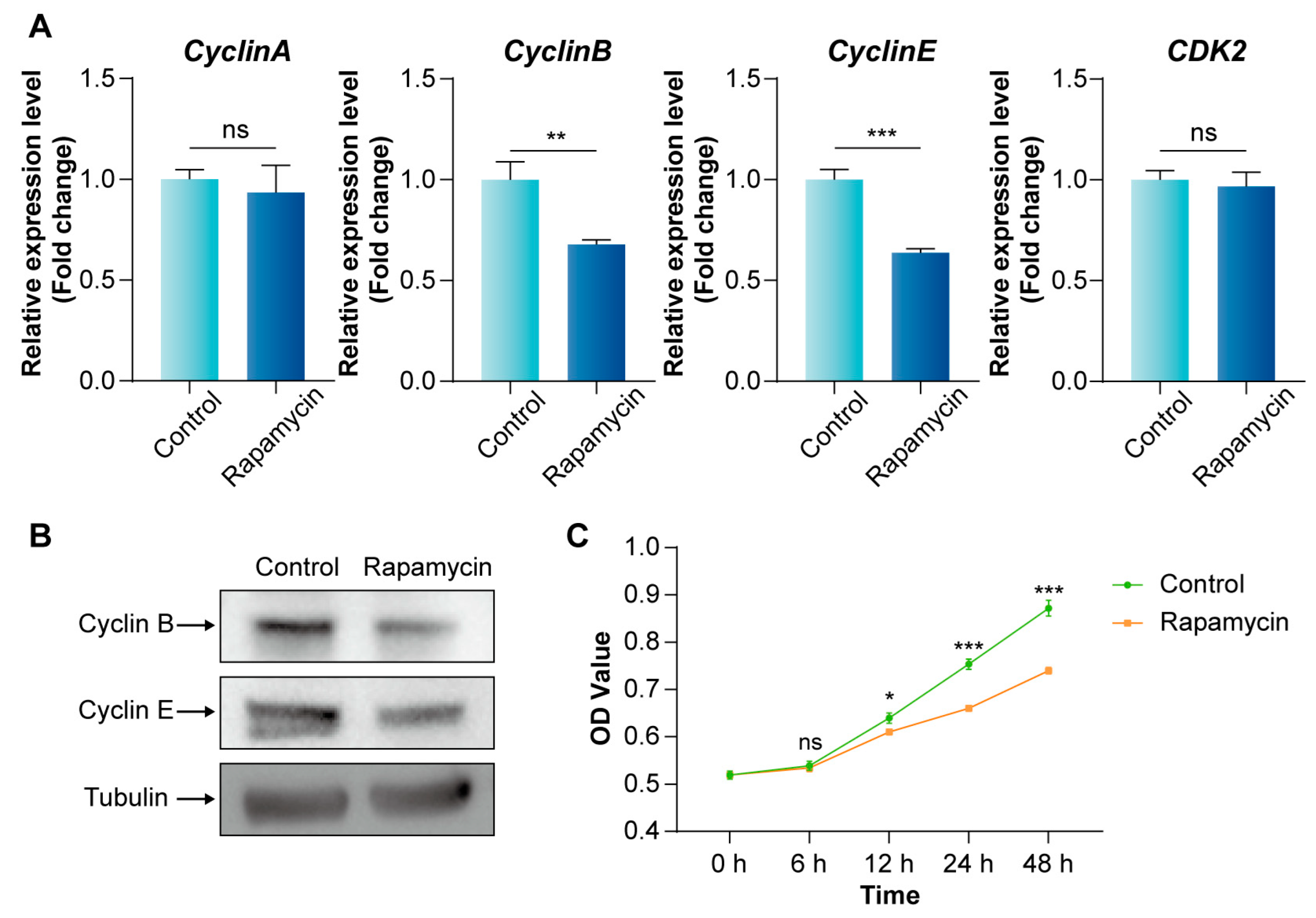
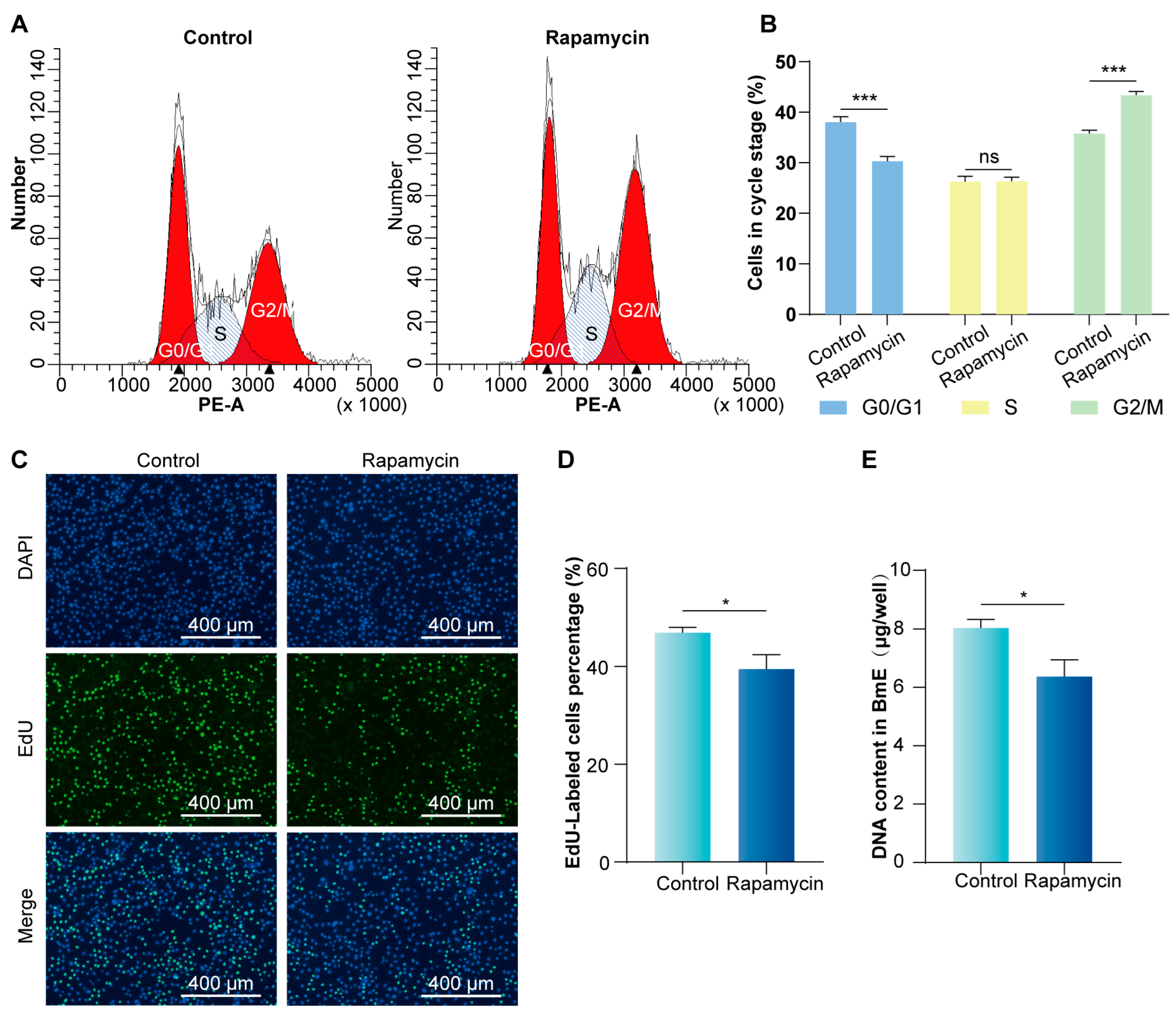
3.2. Inhibition of mTORC1 Signaling Pathway Arrests the BmE Cell Cycle at the G2/M Phase and Reduces Proliferation
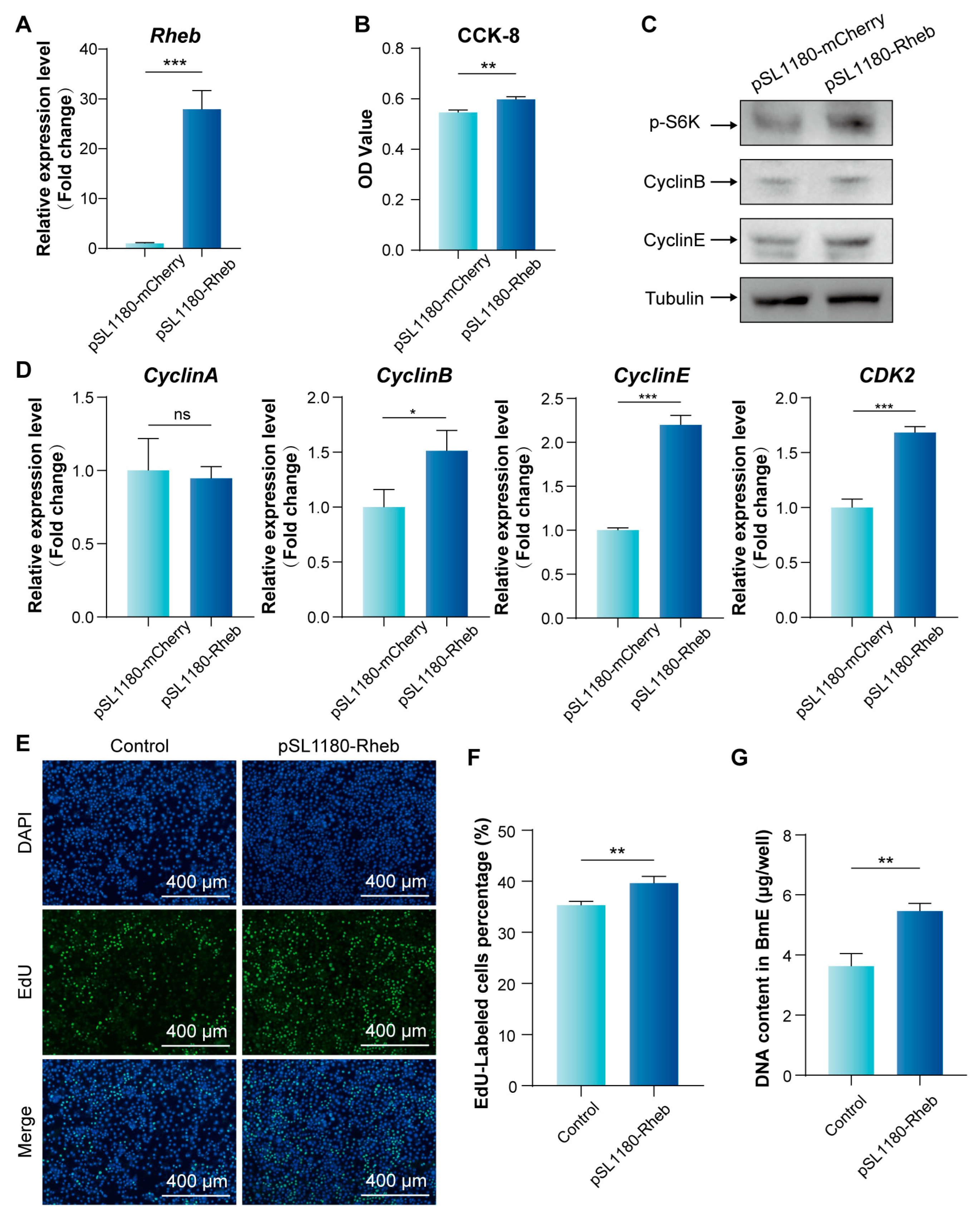
3.3. Enhanced mTORC1 Signaling Pathway Activity via Rheb Overexpression Promotes BmE Cell Proliferation and S Phase Progression
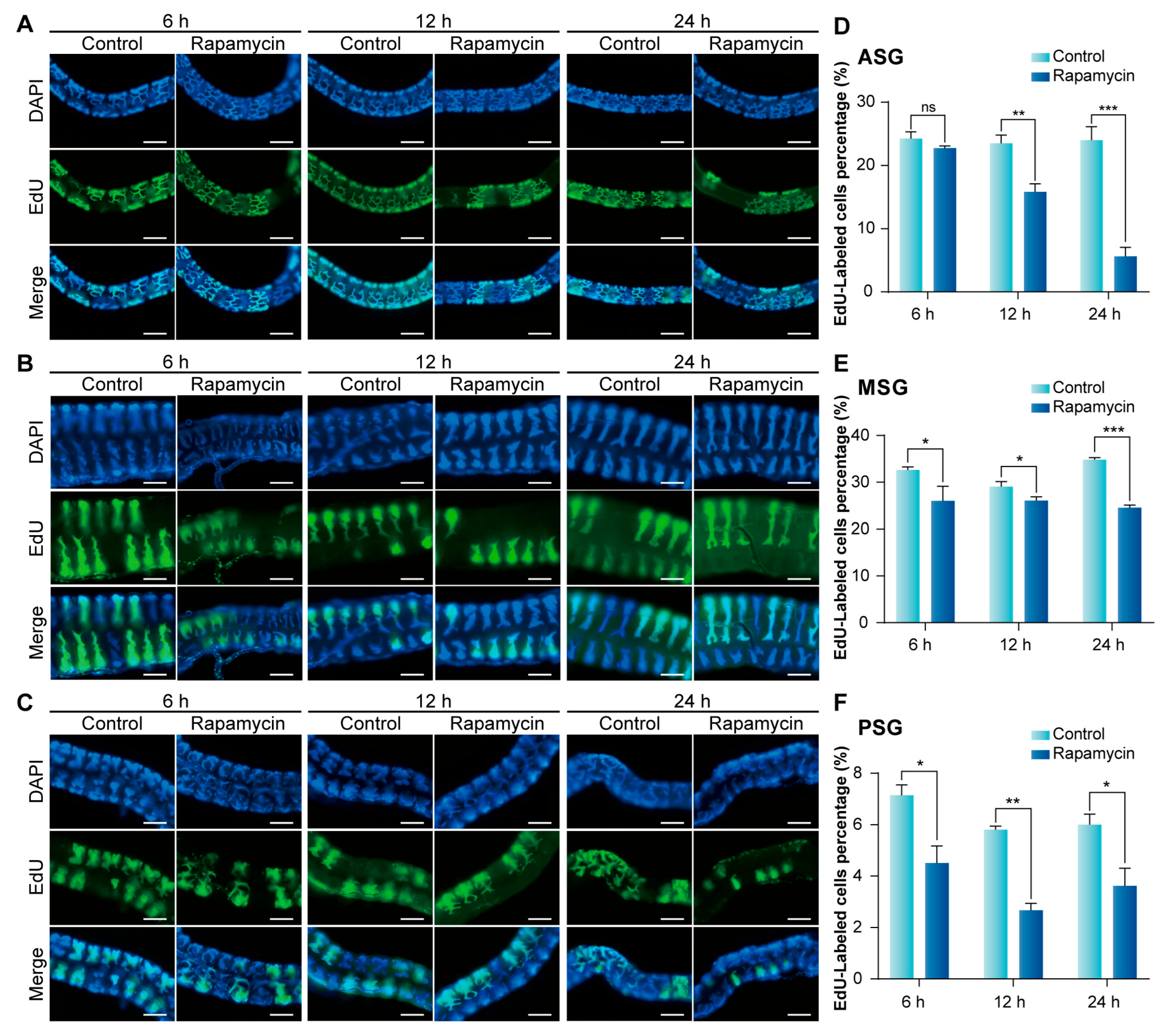
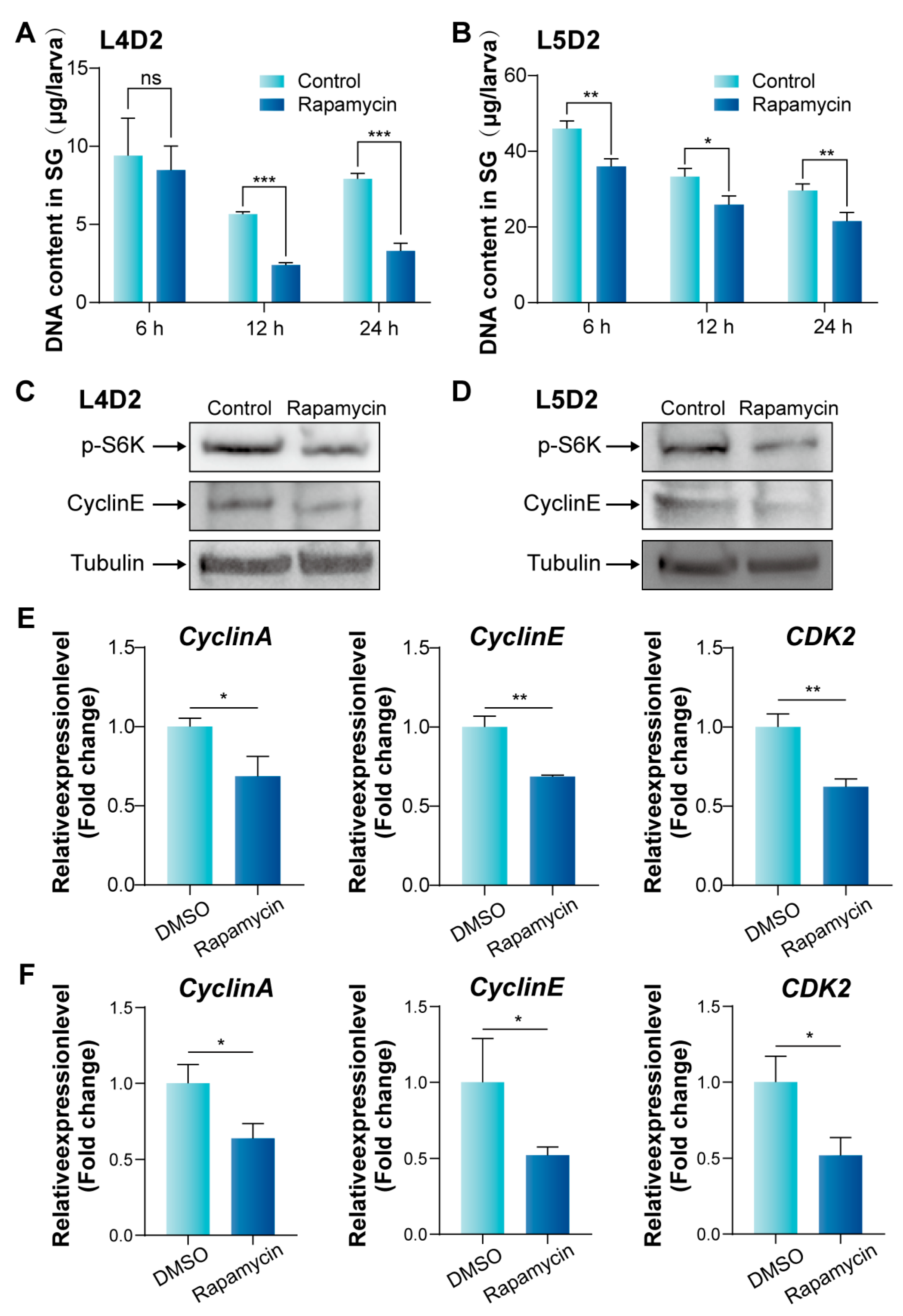
3.4. Inhibition of mTORC1 Signaling Pathway Suppresses Endoreplication in Silk Gland
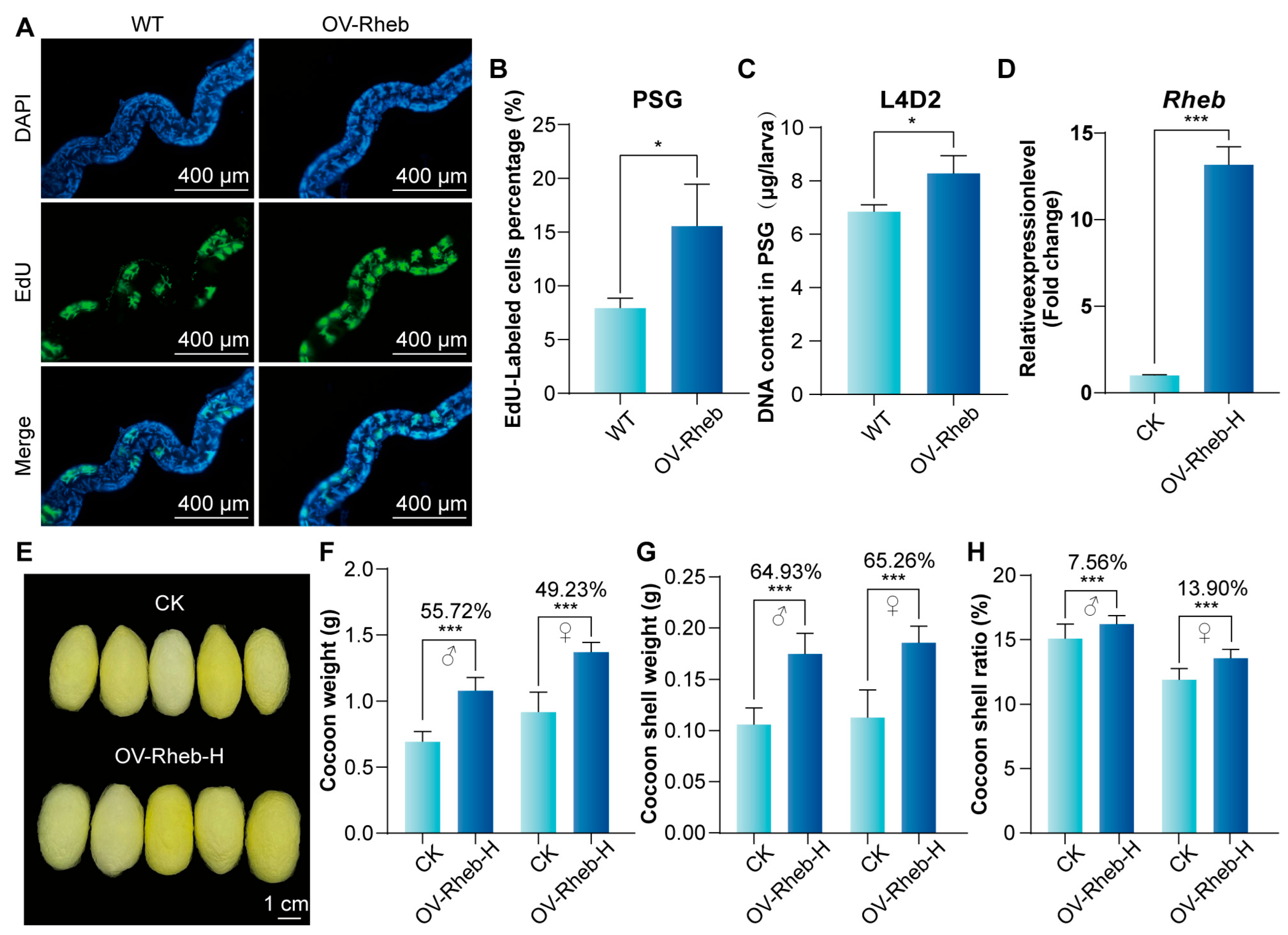
3.5. Rheb Promotes Endoreplication in PSG and Improves Silk Yield by Enhancing mTORC1 Signaling Pathway Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Q.; Li, S.; Feng, Q. Advances in silkworm studies accelerated by the genome sequencing of Bombyx mori. Annu. Rev. Entomol. 2014, 59, 513–536. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, A.; Nakato, H.; Izumi, S.; Tomino, S. Biosynthesis of major plasma proteins in the primary culture of fat body cells from the silkworm, Bombyx mori. Cell Tissue Res. 1999, 297, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Cai, X.J.; Liu, M.; Lv, J.; Tang, H.; Tan, J.; Lu, C. Establishment and characterization of an ovarian cell line of the silkworm, Bombyx mori. Tissue Cell 2010, 42, 42–46. [Google Scholar] [CrossRef]
- Dhawan, S.; Gopinathan, K.P. Cell cycle events during the development of the silk glands in the mulberry silkworm Bombyx mori. Dev. Genes. Evol. 2003, 213, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, M.R.; Shimada, T.; Abe, H. The genetics and genomics of the silkworm, Bombyx mori. Annu. Rev. Entomol. 2005, 50, 71–100. [Google Scholar] [CrossRef]
- Sudhakar, B.; Gopinathan, K.P. Expression of cyclin E in endomitotic silk-gland cells from mulberry silkworm. Gene 2000, 257, 77–85. [Google Scholar] [CrossRef]
- Edgar, B.A.; Orr-Weaver, T.L. Endoreplication cell cycles: More for less. Cell 2001, 105, 297–306. [Google Scholar] [CrossRef]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Ma, Y.; Zeng, W.; Ba, Y.; Luo, Q.; Ou, Y.; Liu, R.; Ma, J.; Tang, Y.; Hu, J.; Wang, H.; et al. A single-cell transcriptomic atlas characterizes the silk-producing organ in the silkworm. Nat. Commun. 2022, 13, 3316. [Google Scholar] [CrossRef]
- Teleman, A.A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 2009, 425, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, H.; Zhu, J.; Ma, S.; Liu, Y.; Jiang, R.J.; Xia, Q.; Li, S. Ras1(CA) overexpression in the posterior silk gland improves silk yield. Cell Res. 2011, 21, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.L.; Yang, Y.; Li, Z.; Wu, Y.T.; He, X.C.; Li, H.; Cheng, D.J. Enhanced Expression in Silkworm Silk Gland Promotes DNA Replication and Silk Production. Insects 2021, 12, 361. [Google Scholar] [CrossRef]
- Qian, W.; Zhang, X.; Yuan, D.; Wu, Y.; Li, H.; Wei, L.; Li, Z.; Dai, Z.; Song, P.; Sun, Q.; et al. USP8 and Hsp70 regulate endoreplication by synergistically promoting Fzr deubiquitination and stabilization. Sci. Adv. 2025, 11, eadq9111. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, X.; Wang, L.X.; Liu, Z.Y.; Yang, W.Y.; Jing, C.; Xiao, Y.; Zhu, Y.; Dong, Z.Q.; Lu, C.; et al. Bombyx mori Suppressor of Hairless is involved in the regulation of the silkworm cell cycle and endoreplication of the silk glands. Int. J. Biol. Macromol. 2024, 268, 131819. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, S.; Li, C.; Zhou, W.; Dai, F.; Tong, X. BmE2F1 regulates endoreplication of silk gland cells in silkworm, Bombyx mori. Int. J. Biol. Macromol. 2024, 291, 138916. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Li, H.; Zhang, X.; Tang, Y.; Yuan, D.; Huang, Z.; Cheng, D. Fzr regulates silk gland growth by promoting endoreplication and protein synthesis in the silkworm. PLoS Genet. 2023, 19, e1010602. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Brown, E.J.; Albers, M.W.; Shin, T.B.; Ichikawa, K.; Keith, C.T.; Lane, W.S.; Schreiber, S.L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 1994, 369, 756–758. [Google Scholar] [CrossRef]
- Dibble, C.C.; Manning, B.D. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat. Cell Biol. 2013, 15, 555–564. [Google Scholar] [CrossRef]
- Holz, M.K.; Ballif, B.A.; Gygi, S.P.; Blenis, J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 2005, 123, 569–580. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Howell, J.J.; Asara, J.M.; Manning, B.D. Stimulation of de Novo Pyrimidine Synthesis by Growth Signaling Through mTOR and S6K1. Science 2013, 339, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Li, Y.F.; Chen, X.Y.; Tang, X.F.; Zhang, C.D.; Wang, L.; Chen, P.; Pan, M.H.; Lu, C. DNA Synthesis during Endomitosis Is Stimulated by Insulin via the PI3K/Akt and TOR Signaling Pathways in the Silk Gland Cells of. Int. J. Mol. Sci. 2015, 16, 6266–6280. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, H.; Wang, X.; Chang, L.; Liu, Q.; Xia, Q.; Zhao, P. BmSLC7A5 is essential for silk protein synthesis and larval development in Bombyx mori. Insect Sci. 2024. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Chang, L.; Wang, X.; Liu, Q.; Tang, Z.; Xia, Q.; Zhao, P. A strategy for improving silk yield and organ size in silk-producing insects. FEBS J. 2024, 291, 4286–4300. [Google Scholar] [CrossRef] [PubMed]
- Montagne, J.; Stewart, M.J.; Stocker, H.; Hafen, E.; Kozma, S.C.; Thomas, G. Drosophila S6 kinase: A regulator of cell size. Science 1999, 285, 2126–2129. [Google Scholar] [CrossRef]
- Zhang, H.; Stallock, J.P.; Ng, J.C.; Reinhard, C.; Neufeld, T.P. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Gene Dev. 2000, 14, 2712–2724. [Google Scholar] [CrossRef]
- Patel, P.H.; Thapar, N.; Guo, L.; Martinez, M.; Maris, J.; Gau, C.L.; Lengyel, J.A.; Tamanoi, F. Rheb GTPase is required for cell cycle progression and cell growth. J. Cell Sci. 2003, 116, 3601–3610. [Google Scholar] [CrossRef]
- Yamada, H.; Nakao, H.; Takasu, Y.; Tsubouchi, K. Preparation of undegraded native molecular fibroin solution from silkworm cocoons. Mat. Sci. Eng. C-Bio S 2001, 14, 41–46. [Google Scholar] [CrossRef]
- De Veylder, L.; Larkin, J.C.; Schnittger, A. Molecular control and function of endoreplication in development and physiology. Trends Plant Sci. 2011, 16, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guo, Y.; Wang, J.; Tao, C.; Cao, J.; Cheng, T.; Liu, C. Bmgsb is involved in the determination of cell fate by affecting the cell cycle genes in the silk gland of Bombyx mori. Int. J. Biol. Macromol. 2024, 283, 136914. [Google Scholar] [CrossRef]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef]
- Chantranupong, L.; Scaria, S.M.; Saxton, R.A.; Gygi, M.P.; Shen, K.; Wyant, G.A.; Wang, T.; Harper, J.W.; Gygi, S.P.; Sabatini, D.M. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell 2016, 165, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Guan, K.L. Nutrient signaling to mTOR and cell growth. Trends Biochem. Sci. 2013, 38, 233–242. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Qin, S.; Zhang, T.; Yao, J.; Yi, Y.; Deng, L. Mechanistic Target of Rapamycin Complex 1: From a Nutrient Sensor to a Key Regulator of Metabolism and Health. Adv. Nutr. 2022, 13, 1882–1900. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X. Research progress of mTOR inhibitors. Eur. J. Med. Chem. 2020, 208, 112820. [Google Scholar] [CrossRef]
- Ballou, L.M.; Lin, R.Z. Rapamycin and mTOR kinase inhibitors. J. Chem. Biol. 2008, 1, 27–36. [Google Scholar] [CrossRef]
- Oldham, S.; Montagne, J.; Radimerski, T.; Thomas, G.; Hafen, E. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes. Dev. 2000, 14, 2689–2694. [Google Scholar] [CrossRef]
- Wu, M.Y.W.; Cully, M.; Andersen, D.; Leevers, S.J. Insulin delays the progression of cells through G2/M by activating the dTOR/dRaptor complex. Embo J. 2007, 26, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Saucedo, L.J.; Gao, X.S.; Chiarelli, D.A.; Li, L.; Pan, D.; Edgar, B.A. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 2003, 5, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Chern, J.J.; Cai, Y.; Liu, M.Y.; Choi, K.W. TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature 2007, 445, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Layalle, S.; Arquier, N.; Léopold, P. The TOR Pathway Couples Nutrition and Developmental Timing in Drosophila. Dev. Cell 2008, 15, 568–577. [Google Scholar] [CrossRef]
- Patel, P.H.; Tamanoi, F. Using Drosophila and yeast genetics to investigate a role for the Rheb GTPase in cell growth. Methods Enzym. 2006, 407, 443–454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Liu, H.; Liu, Q.; Tang, X.; Xu, J.; Luo, G.; Xia, Q.; Zhao, P. The Rheb-mTORC1 Coordinates Cell Cycle Progression and Endoreplication in Bombyx mori. Insects 2025, 16, 647. https://doi.org/10.3390/insects16070647
Tang Z, Liu H, Liu Q, Tang X, Xu J, Luo G, Xia Q, Zhao P. The Rheb-mTORC1 Coordinates Cell Cycle Progression and Endoreplication in Bombyx mori. Insects. 2025; 16(7):647. https://doi.org/10.3390/insects16070647
Chicago/Turabian StyleTang, Zhangchen, Huawei Liu, Qingsong Liu, Xin Tang, Jiahui Xu, Gan Luo, Qingyou Xia, and Ping Zhao. 2025. "The Rheb-mTORC1 Coordinates Cell Cycle Progression and Endoreplication in Bombyx mori" Insects 16, no. 7: 647. https://doi.org/10.3390/insects16070647
APA StyleTang, Z., Liu, H., Liu, Q., Tang, X., Xu, J., Luo, G., Xia, Q., & Zhao, P. (2025). The Rheb-mTORC1 Coordinates Cell Cycle Progression and Endoreplication in Bombyx mori. Insects, 16(7), 647. https://doi.org/10.3390/insects16070647





