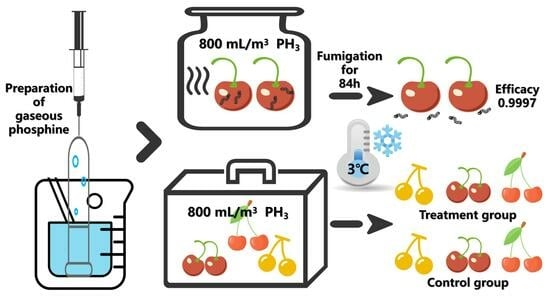
Low-Temperature Phosphine Fumigation Is Effective Against Drosophila suzukii in Sweet Cherry
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect, Fumigant, and Fruit
2.2. Developmental Duration Test
2.3. Tolerance Comparison Test
2.4. Toxicity Assay
2.5. Large-Scale Test
2.6. Postharvest Quality Evaluation
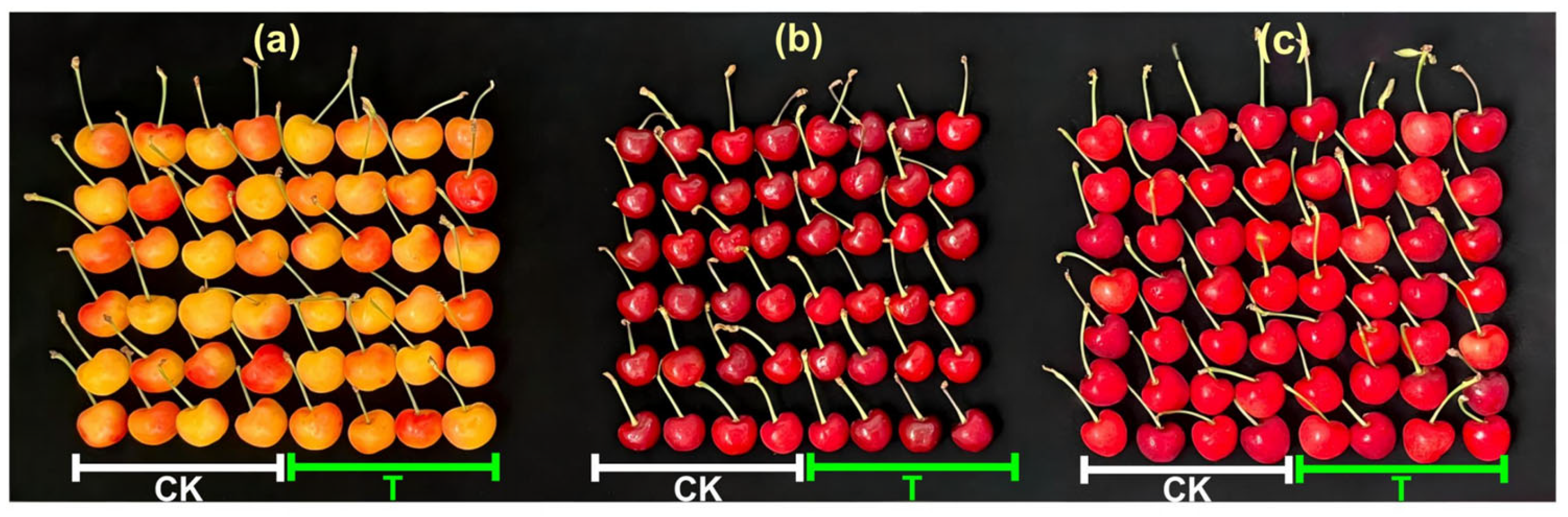
2.6.1. Visual Quality or Appearance
2.6.2. Respiration Measurement
2.6.3. Determination of Relative Conductivity
2.6.4. Fruit Firmness, SSC and TA
2.7. Data Analysis
3. Results
3.1. Development of D. suzukii in Cherry Fruit
3.2. Third Instar Larvae Exhibit Highest Tolerance to PH3 Fumigation in D. suzukii
3.3. Toxicity Test-Screening for Optimal Phosphine Treatment Concentration
3.4. Quality Evaluation of Cherries
3.4.1. Visual Quality
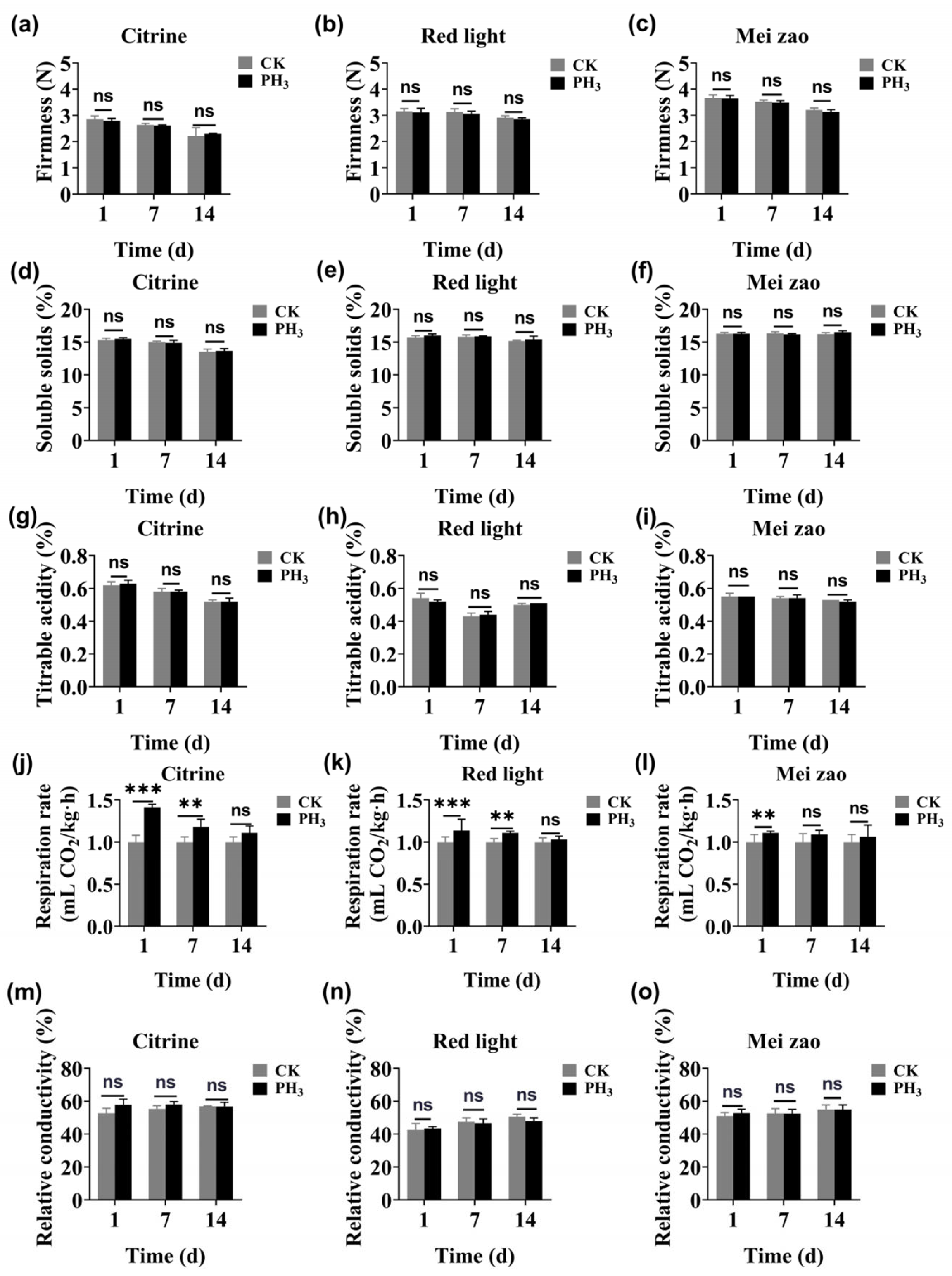
3.4.2. Firmness
3.4.3. Soluble Solids
3.4.4. Titrable Acidity
3.4.5. Respiration Rate
3.4.6. Relative Conductivity
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rednicka-Tober, D.; Ponder, A.; Hallmann, E.; Gowacka, A.; Rozpara, E. The Profile and Content of Polyphenols and Carotenoids in Local and Commercial Sweet Cherry Fruits (Prunus avium L.) and Their Antioxidant Activity In Vitro. Antioxidants 2019, 8, 534. [Google Scholar] [CrossRef] [PubMed]
- Zun-Ying, L.; Shi-Yuan, D.; Ming-Yong, Z.; Ya-Nan, D.U. Effects of 1-MCP on the post-harvested decay and quality of sweet cherry (Prunus avium L.) fruits. Food Sci. Technol. 2006. [Google Scholar] [CrossRef]
- Clayton-Cuch, D.; Yu, L.; Mcdougal, D.; Burbidge, C.A.; Bruning, J.B.; Bradley, D.; Bttcher, C.; Bulone, V. Biochemical and in silico characterization of glycosyltransferases from red sweet cherry (Prunus avium L.) reveals their broad specificity toward phenolic substrates. Food Chem. Mol. Sci. 2024, 8, 100193. [Google Scholar] [CrossRef]
- Murphy, K.A.; Unruh, T.R.; Zhou, L.M.; Zalom, F.G.; Shearer, P.W.; Beers, E.H.; Walton, V.M.; Miller, B.; Chiu, J.C. Using comparative genomics to develop a molecular diagnostic for the identification of an emerging pest Drosophila suzukii. Bull. Entomol. Res. 2015, 105, 364–372. [Google Scholar] [CrossRef]
- Mackey, B.; Swedman, A.; Follett, P.A. Effect of Low-Oxygen Conditions Created by Modified Atmosphere Packaging on Radiation Tolerance in Drosophila suzukii (Diptera: Drosophilidae) in Sweet Cherries. J. Econ. Entomol. 2018, 11, 141–145. [Google Scholar]
- Kim, G.H. Synergistic Effect of Cold Treatment Combined with Ethyl Formate Fumigation against Drosophila suzukii (Diptera: Drosophilidae). Insects 2022, 13, 664. [Google Scholar] [CrossRef]
- Kwon, T.H.; Park, C.G.; Lee, B.H.; Zarders, D.R.; Cha, D.H. Ethyl formate fumigation and ethyl formate plus cold treatment combination as potential phytosanitary quarantine treatments of Drosophila suzukii in blueberries. J. Asia-Pac. Entomol. 2021, 24, 129–135. [Google Scholar] [CrossRef]
- Bing, X.L.; Gerlach, J.; Loeb, G.; Buchon, N. Nutrient-Dependent Impact of Microbes on Drosophila suzukii Development. mBio 2018, 9, mbio.02199-17. [Google Scholar] [CrossRef]
- Biondi, A.; Traugott, M.; Desneux, N. Special issue on Drosophila suzukii: From global invasion to sustainable control. J. Pest Sci. 2016, 89, 603–604. [Google Scholar] [CrossRef]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef]
- Deans, C.; Hutchison, W. The Protein Paradox: Elucidating the Complex Nutritional Ecology of the Invasive Berry Pest, Spotted-Wing Drosophila (Diptera: Drosophila suzukii). Front. Insect Sci. 2021, 1, 787169. [Google Scholar] [CrossRef] [PubMed]
- Follett, P.A.; Swedman, A.; Prices, D.K. Postharvest irradiation treatment for quarantine control of Drosophila suzukii (Diptera: Drosophilidae) in fresh commodities. J. Econ. Entomol. 2014, 107, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, M.J.; Choi, D.S.; Kim, I. Complete mitochondrial genome of the spotted-wing drosophila, Drosophila suzukii (Diptera: Drosophilidae). Mitochondrial DNA Part B 2016, 1, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.-B. Nitric Oxide Fumigation for Control of Spotted Wing Drosophila (Diptera: Drosophilidae) in Strawberries. J. Econ. Entomol. 2018, 111, 1180–1184. [Google Scholar] [CrossRef]
- Zou, H.; Li, L.; Li, B.; Ren, Y.; Liu, T. Phosphine and phosphine plus ethyl formate for controlling papaya mealybug (Hemiptera: Pseudococcidae) on succulents. J. Econ. Entomol. 2024, 118, 152–159. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Li, B.; Zhang, F.; Dong, S.; Wang, Y. Toxicity of Phosphine Fumigation Against Bactrocera tau at Low Temperature. J. Econ. Entomol. 2014, 107, 601–605. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Zhang, F.; Gong, S.; Li, T.; Zhan, G.; Wang, Y. Effect of Low-Temperature Phosphine Fumigation on the Survival ofBactrocera correcta(Diptera: Tephritidae). J. Econ. Entomol. 2015, 108, 1624–1629. [Google Scholar] [CrossRef]
- Liu, Y.B. Low-temperature phosphine fumigation of chilled lettuce under insulated cover for postharvest control of western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera:Thripidae). J. Asia-Pac Entomol. 2011, 14, 323–325. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Liu, B.; Ren, L.; Gong, S.; Liu, T. Low temperature phosphine fumigation for postharvest control of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae) on oriental lily. Postharvest Biol. Technol. 2015, 100, 136–141. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Li, B.; Zhan, G.; Wang, Y. Evaluation of Low-Temperature Phosphine Fumigation for Control of Oriental Fruit Fly in Loquat Fruit. J. Econ. Entomol. 2018, 111, 1165–1170. [Google Scholar] [CrossRef]
- Liu, Y.-B. Low-Temperature Fumigation of Harvested Lettuce Using a Phosphine Generator. J. Econ. Entomol. 2018, 111, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-B. Low Temperature Phosphine Fumigation for Postharvest Control of Western Flower Thrips (Thysanoptera: Thripidae) on Lettuce, Broccoli, Asparagus, and Strawberry. J. Econ. Entomol. 2008, 101, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Y.; Liu, T.; Li, L.; Li, T. Effects of low temperature phosphine fumigation on postharvest quality of white chrysanthemum ‘Dabaiju’. Sci. Hortic. 2012, 142, 92–97. [Google Scholar] [CrossRef]
- Cai, C.; Chen, K.S.; Xu, W.P.; Zhang, W.S.; Li, X.; Ferguson, I. Effect of 1-MCP on postharvest quality of loquat fruit. Postharvest Biol. Technol. 2006, 40, 155–162. [Google Scholar] [CrossRef]
- Couey, M.H.; Victor, C. Confidence Limits and Sample Size in Quarantine Research. J. Econ. Entomol. 1986, 79, 887–890. [Google Scholar] [CrossRef]
- Alzahrani, S.M.; Ebert, P.R. Pesticidal Toxicity of Phosphine and Its Interaction with Other Pest Control Treatments. Curr. Issues Mol. Biol. 2023, 45, 2461–2473. [Google Scholar] [CrossRef]
- Bolonhezi, S.; Athié, I.; Gomes, R.A.; Valentini, S.R.; De Castro, M.P.M. Effects of carbon dioxide and phosphine mixtures on resistant populations of stored-grain insects. J. Stored Prod. Res. 1998, 34, 27–32. [Google Scholar]
- Constantin, M.; Jagadeesan, R.; Chandra, K.; Ebert, P.; Nayak, M.K. Synergism Between Phosphine (PH3) and Carbon Dioxide (CO2): Implications for Managing PH3 Resistance in Rusty Grain Beetle (Laemophloeidae: Coleoptera). J. Econ. Entomol. 2020, 113, 1999–2006. [Google Scholar] [CrossRef]
- Andreadis, S.S.; Athanassiou, C.G. A review of insect cold hardiness and its potential in stored product insect control. Crop Prot. 2017, 91, 93–99. [Google Scholar] [CrossRef]
- Chaudhry, M.Q. Phosphine resistance. Pestic. Outlook 2000, 11, 88–91. [Google Scholar] [CrossRef]
- De Lima, C.P.; Jessup, A.J.; Mansfield, E.R.; Daniels, D. Cold treatment of table grapes infested with Mediterranean fruit fly Ceratitis capitata (Wiedemann) and Queensland fruit fly Bactrocera tryoni (Froggatt) Diptera: Tephritidae. N. Z. J. Crop. Hortic. Sci. 2011, 39, 95–105. [Google Scholar] [CrossRef]
- Benschoter, C.A. Low-Temperature Storage as a Quarantine Treatment for the Caribbean Fruit Fly (Diptera: Tephritidae) in Florida Citrus. J. Econ. Entomol. 1984, 77, 1233–1235. [Google Scholar] [CrossRef]
- Saeed, N.; Tonina, L.; Battisti, A.; Mori, N. Postharvest short cold temperature treatment to preserve fruit quality after Drosophila suzukii damage. Int. J. Pest Manag. 2020, 66, 23–30. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Domokos-Szabolcsy, V.; Abdalla, N.A.; Taha, H.S.; Fári, M. Postharvest Management of Fruits and Vegetables Storage; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Manivannan, S. Toxicity of phosphine on the developmental stages of rust-red flour beetle, Tribolium castaneum Herbst over a range of concentrations and exposures. J. Food Sci. Technol. 2015, 52, 6810–6815. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Li, B.; Liu, Q.; Ren, Y.L.; Wang, P.; Liu, T. Phosphine fumigation followed by forced hot-air treatment for postharvest control of Bactrocera dorsalis in dragon fruit. J. Pest Sci. 2024, 98, 799–810. [Google Scholar] [CrossRef]
- Austin, C.J.; Moehring, A.J. Local thermal adaptation detected during multiple life stages across populations of Drosophila melanogaster. J. Evol. Biol. 2019, 32, 1342–1351. [Google Scholar] [CrossRef]
- Pedersen, K.E.; Pedersen, N.N.; Meyling, N.V.; Fredensborg, B.L.; Cedergreen, N. Differences in life stage sensitivity of the beetle Tenebrio molitor towards a pyrethroid insecticide explained by stage-specific variations in uptake, elimination and activity of detoxifying enzymes—ScienceDirect. Pestic. Biochem. Physiol. 2020, 162, 113–121. [Google Scholar] [CrossRef]
- Lampiri, E.; Agrafioti, P.; Athanassiou, C.G. Delayed mortality, resistance and the sweet spot, as the good, the bad and the ugly in phosphine use. Sci. Rep. 2021, 11, 3933. [Google Scholar] [CrossRef]
- Mckenzie, J.A. The Influence of Low Temperature on Survival and Reproduction in Populations of Drosophila Melanogaster. Aust. J. Zool. 1975, 23, 237–247. [Google Scholar] [CrossRef]
- Štětina, T.; Poupardin, R.; Moos, M.; Šimek, P.; Šmilauer, P.; Koštál, V. Larvae of Drosophila melanogaster exhibit transcriptional activation of immune response pathways and antimicrobial peptides during recovery from supercooling stress. Insect Biochem. Mol. Biol. 2019, 105, 60–68. [Google Scholar] [CrossRef]
- Liu, B. Advances in Postharvest Pest Control on Perishable Commodities Using Ultralow Oxygen Treatment and Low Temperature Phosphine Fumigation. In Proceedings of the Controlled Atmosphere & Fumigation in Stored Products International Conference, Chengdu, China, 21–26 September 2008. [Google Scholar]

| Instar | 4 h | |||
|---|---|---|---|---|
| No. of Insects | Mortality ± SE (%) | No. of Insects | Corrected Mortality ± SE (%) | |
| Egg | 436 | 10.43% a | 533 | 90.77% a |
| 1st instar Larva (1 L) | 329 | 8.31% ab | 372 | 62.41% b |
| 2nd instar Larva (2 L) | 322 | 6.53% ab | 357 | 71.36% b |
| 3rd instar Larva (3 L) | 359 | 1.69% b | 278 | 20.09% c |
| Pupa | 130 | 1.70% b | 520 | 90.19% a |
| ANOVA | F = 6.83; df = 4, 10; p < 0.01 | F = 181.22; df = 4, 10; p < 0.001 | ||
| PH3 (mL/m3) | 4 h | ||
|---|---|---|---|
| No. of Insects | Mortality ± SE (%) | Corrected Mortality ± SE (%) | |
| 0 | 160 | 0.61 ± 0.86 d | 0.00 ± 0.00 f |
| 50 | 419 | 23.16 ± 3.08 e | 22.70 ± 2.67 e |
| 100 | 318 | 33.61 ± 4.03 d | 31.75 ± 1.95 d |
| 200 | 195 | 41.90 ± 1.47 c | 41.71 ± 1.47 c |
| 400 | 234 | 72.56 ± 4.52 b | 73.22 ± 4.3 b |
| 800 | 267 | 82.70 ± 1.93 a | 82.58 ± 2.1 a |
| 1600 | 182 | 85.83 ± 2.84 a | 85.72 ± 2.97 a |
| ANOVA | F = 243.16; df = 6, 14; p < 0.001 | F = 339.08; df = 6, 14; p < 0.001 | |
| Treatment | n | Slope ± SE | Hetero. a | LT50 (95% CI) (Lower–Upper) | LT90 (95% CI) (Lower–Upper) | LT99 (95% CI) (Lower–Upper) | LT99.9968 (95% CI) (Lower–Upper) |
|---|---|---|---|---|---|---|---|
| Low Temperature PH3 fumigation | 2732 | 2.513 ± 0.097 | 0.94 | 2.33 (2.11~2.56) | 10.51 (9.49~11.77) | 19.63 (17.18~22.29) | 90.98 (72.38~118.44) |
| Time | Temperature | Concentration of PH3 | Variety of Cherry | Number of Larvae | Phytosanitory Treatment Efficacy |
|---|---|---|---|---|---|
| 84 h | 3 °C | 800 mL/m3 | Citrine | 31,186 | 0.9997 |
| Red light | 30,166 | 0.9997 | |||
| Mei zao | 30,635 | 0.9997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, H.; Li, L.; Zhang, J.; Li, B.; Xiao, Y.; Ren, Y.; Huang, J.; Chen, W.; Liu, T. Low-Temperature Phosphine Fumigation Is Effective Against Drosophila suzukii in Sweet Cherry. Insects 2025, 16, 635. https://doi.org/10.3390/insects16060635
Zou H, Li L, Zhang J, Li B, Xiao Y, Ren Y, Huang J, Chen W, Liu T. Low-Temperature Phosphine Fumigation Is Effective Against Drosophila suzukii in Sweet Cherry. Insects. 2025; 16(6):635. https://doi.org/10.3390/insects16060635
Chicago/Turabian StyleZou, Hang, Li Li, Jun Zhang, Baishu Li, Yu Xiao, Yonglin Ren, Ju Huang, Wei Chen, and Tao Liu. 2025. "Low-Temperature Phosphine Fumigation Is Effective Against Drosophila suzukii in Sweet Cherry" Insects 16, no. 6: 635. https://doi.org/10.3390/insects16060635
APA StyleZou, H., Li, L., Zhang, J., Li, B., Xiao, Y., Ren, Y., Huang, J., Chen, W., & Liu, T. (2025). Low-Temperature Phosphine Fumigation Is Effective Against Drosophila suzukii in Sweet Cherry. Insects, 16(6), 635. https://doi.org/10.3390/insects16060635