Reactivity of Z-3-Hexenal with Amino Groups Provides a Potential Mechanism for Its Direct Effects on Insect Herbivores
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Insect Herbivores
2.3. Analysis of Green Leaf Volatile Production in Damaged Leaf Tissue
2.4. Analyzing the Reaction of Individual Amino Acids with Z-3-Hexenal
2.5. Effects of Hexenylated Diet on Growth and Development of Beet Armyworm
2.6. Statistical Analysis
3. Results and Discussion
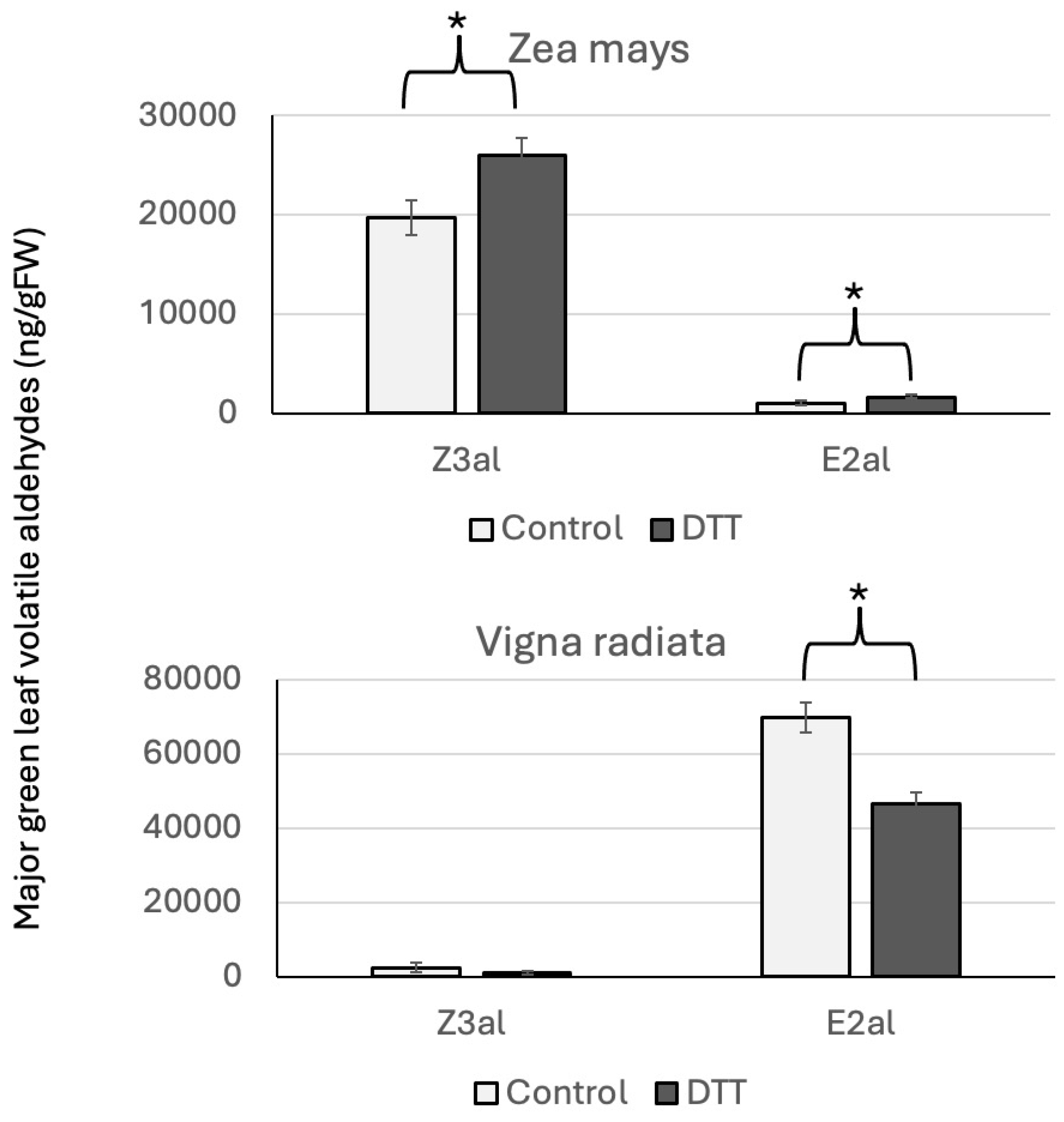
3.1. Amino Acids and GLV Production
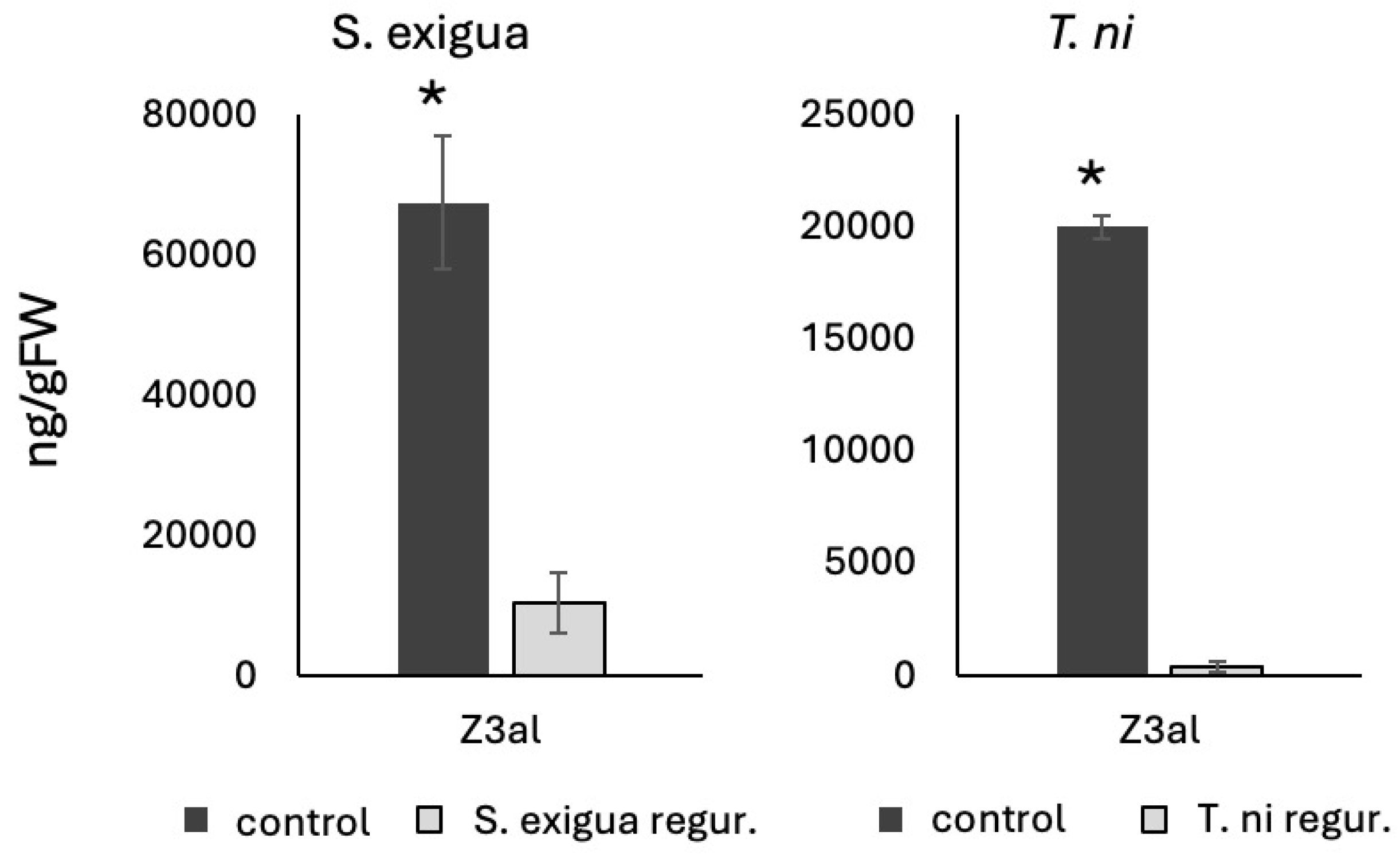
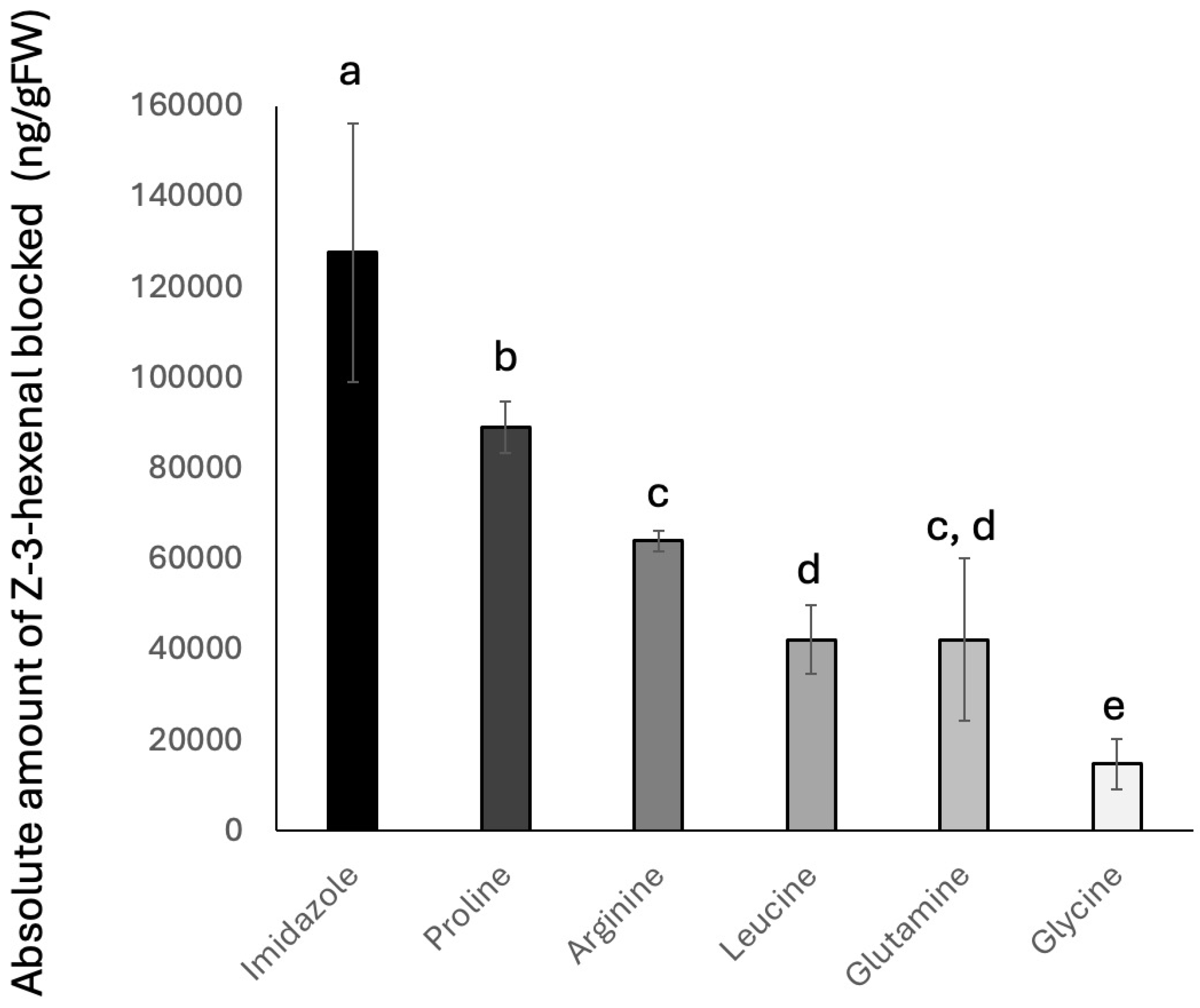
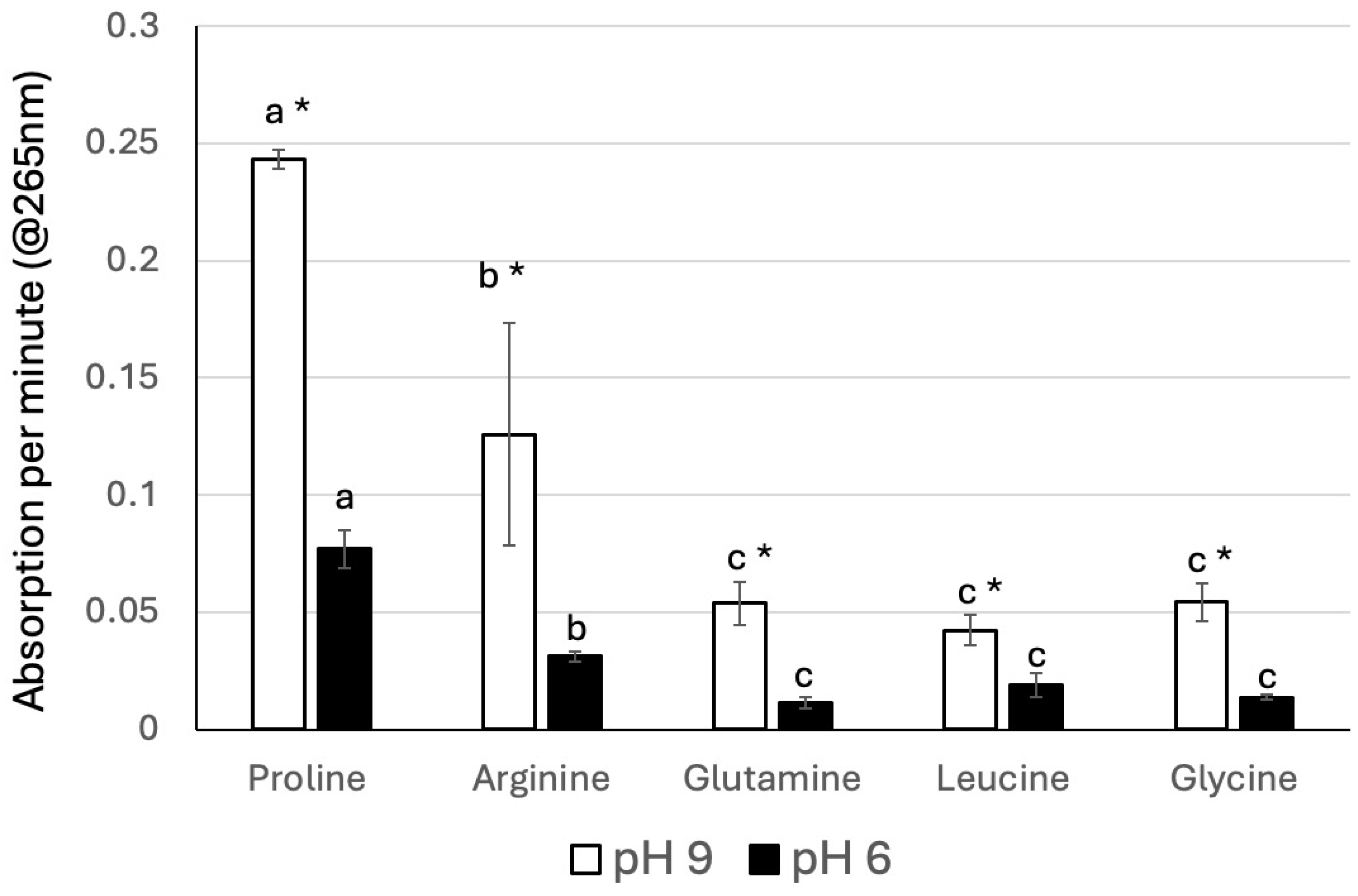
3.2. Inhibition of Z3al Production by Specific Amino Acids
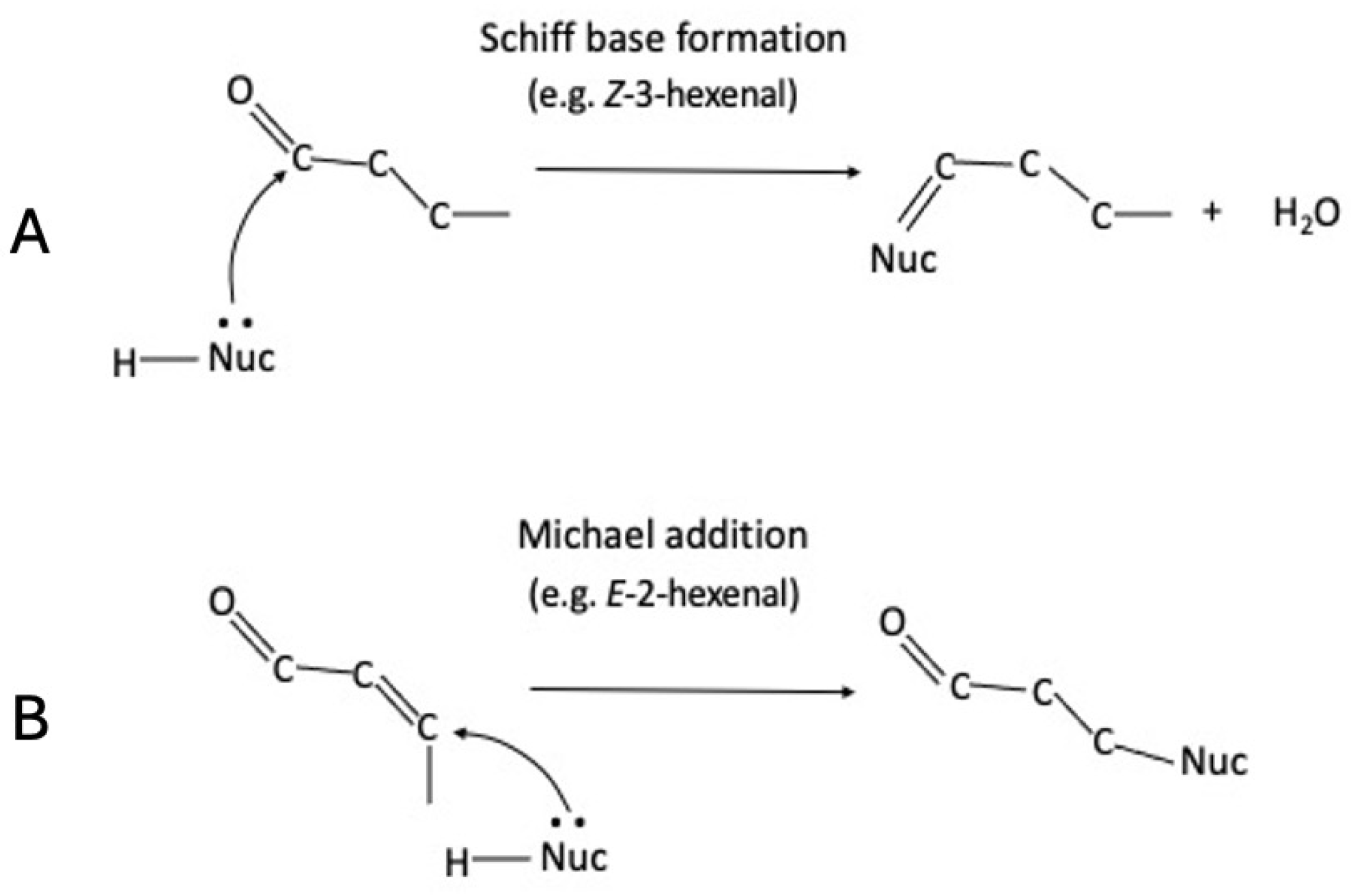
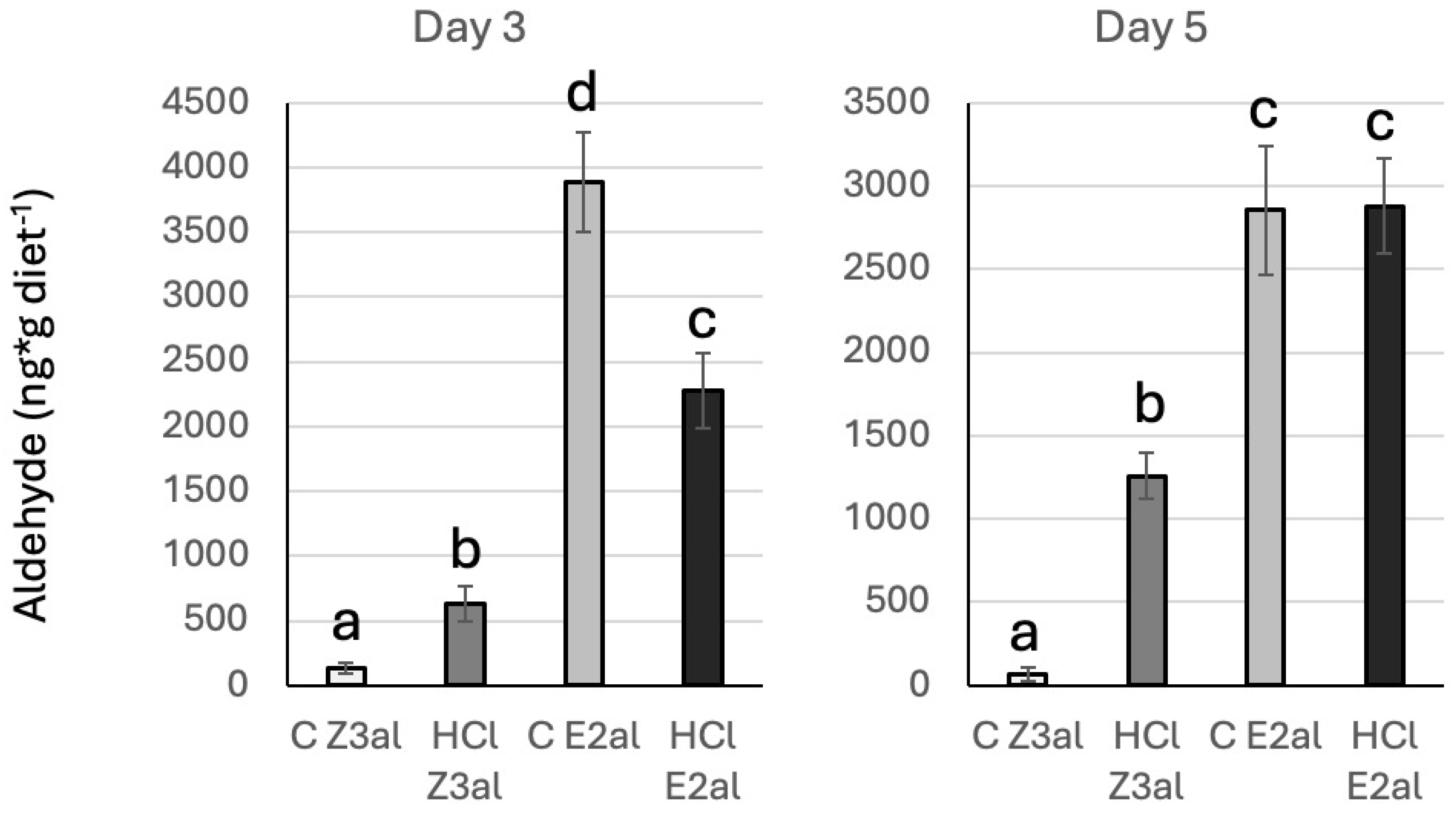
3.3. In Vitro Assays for Schiff Base Formation
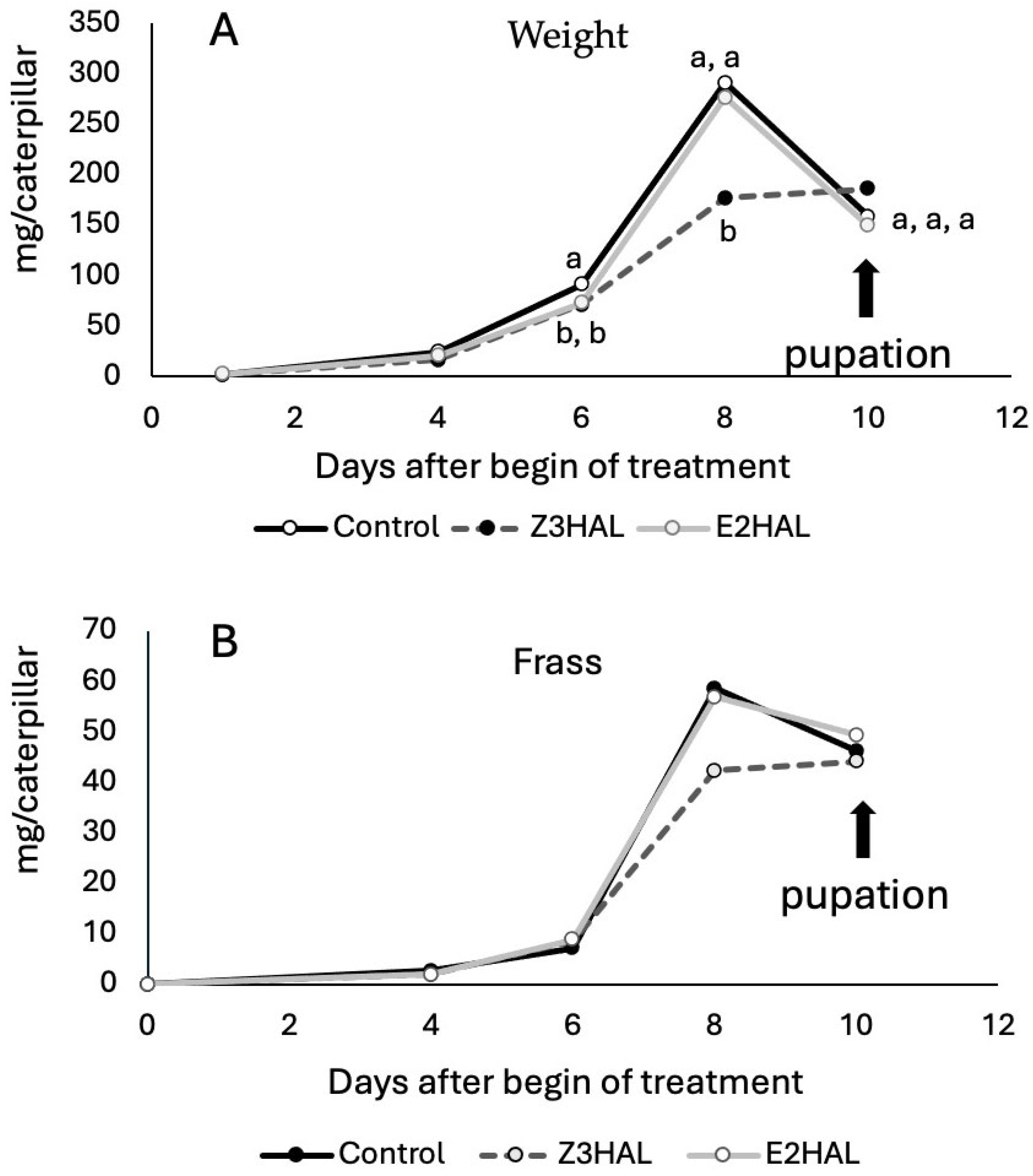
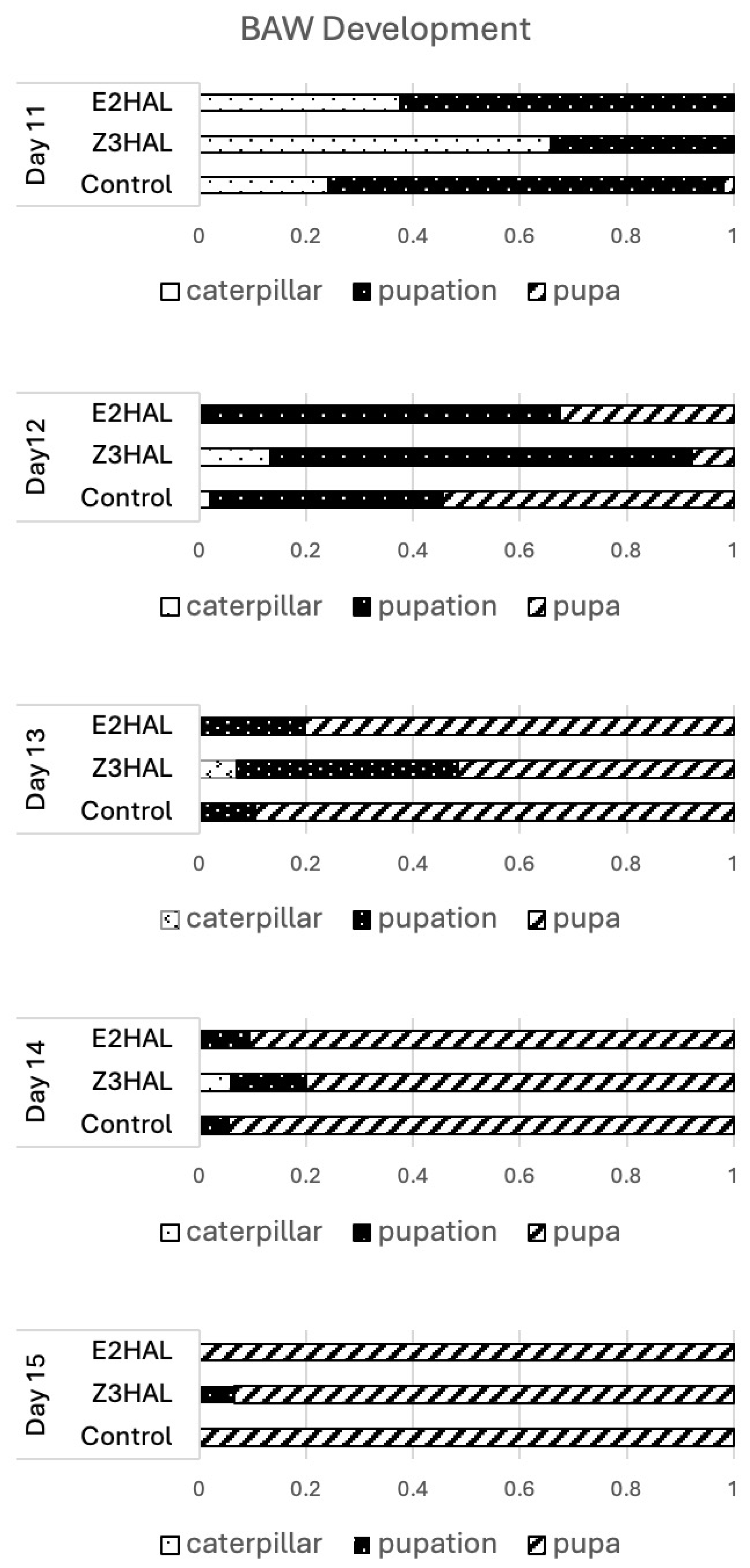
3.4. Effects of Z-3- and E-2-Hexenylated Diet on BAW Growth and Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsui, K.; Engelberth, J. Green leaf volatiles—the forefront of plant responses against biotic attack. Plant Cell Physiol. 2022, 63, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J. Green Leaf Volatiles: A New Player in the Protection against Abiotic Stresses? Int. J. Mol. Sci. 2024, 25, 9471. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef]
- Scala, A.; Allman, S.; Mirabella, R.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17911. [Google Scholar] [CrossRef]
- Ameye, M.; Allman, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2017, 220, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Murray, I.; Sobhy, I.S.; Bosak, L.; DeBlasio, S.; Erb, M.; Robert, C.A.M.; Vaughn, K.A.; et al. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013, 74, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential Metabolisms of Green Leaf Volatiles in Injured and Intact Parts of a Wounded Leaf Meet Distinct Ecophysiological Requirements. PLoS ONE 2012, 7, e36433. [Google Scholar] [CrossRef]
- Kunishima, M.; Yamauchi, Y.; Mizutani, M.; Kuse, M.; Takikawa, H.; Sugimoto, Y. Identification of (Z)-3:(E)-2-hexenal isomerases essential to the production of the leaf aldehyde in plants. J. Biol. Chem. 2016, 291, 14023–14033. [Google Scholar] [CrossRef]
- Spyropoulou, E.A.; Dekker, H.L.; Steemers, L.; van Maarseveen, J.H.; de Koster, C.G.; Haring, M.A.; Schuurink, R.C.; Allmann, S. Identification and characterization of (3 Z):(2 E)-hexenal isomerases from cucumber. Front. Plant Sci. 2017, 8, 1342. [Google Scholar] [CrossRef]
- Engelberth, J.; Engelberth, M. Variability in the capacity to produce damage-induced aldehyde green leaf volatiles among different plant species provides novel insights into biosynthetic diversity. Plants 2020, 9, 213. [Google Scholar] [CrossRef]
- Nakamura, S.; Hatanaka, A. Green-Leaf-Derived C6-Aroma Compounds with Potent Antibacterial Action That Act on Both Gram-Negative and Gram-Positive Bacteria. J. Agric. Food Chem. 2002, 50, 7639–7644. [Google Scholar] [CrossRef]
- Allmann, S.; Baldwin, I.T. Insects Betray Themselves in Nature to Predators by Rapid Isomerization of Green Leaf Volatiles. Science 2010, 329, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Allmann, S.; Spaethe, A.; Bisch-Knaden, S.; Kallenbach, M.; Reinecke, A.; Sachse, S.; Baldwin, I.T.; Hansen, B.S. Feeding-induced rearrangement of green leaf volatiles reduces moth oviposition. eLife 2013, 2, e00421. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Pearse, I.S.; Ignatia, L.; Karban, R.; and Dehesh, K. Insect herbivores selectively suppress the HPL branch of the oxylipin pathway in host plants. Plant J. 2013, 73, 653–662. [Google Scholar] [CrossRef]
- Takai, H.; Ozawa, R.; Takabayashi, J.; Fujii, S.; Arai, K.; Ichiki, R.T.; Koeduka, T.; Dohra, H.; Ohnishi, T.; Taketazu, S.; et al. Silkworms suppress the release of green leaf volatiles by mulberry leaves with an enzyme from their spinnerets. Sci. Rep. 2018, 8, 11942. [Google Scholar] [CrossRef]
- Jones, A.C.; Seidl-Adams, I.; Engelberth, J.; Hunter, C.T.; Alborn, H.; Tumlinson, J.H. Herbivorous Caterpillars Can Utilize Three Mechanisms to Alter Green Leaf Volatile Emission. Environ. Entomol. 2019, 48, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Cofer, T.M.; Engelberth, J.; Tumlinson, J.H. Herbivorous caterpillars and the green leaf volatile (GLV) quandary. J. Chem. Ecol. 2022, 48, 337–345. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malonyldialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Sonowal, H.; Ramana, K.V. 4-Hydroxy-trans-2-nonenal in the regulation of anti-oxidative and pro-inflammatory signaling pathways. Oxid. Med. Cell. Longev. 2019, 2019, 5937326. [Google Scholar] [CrossRef]
- Campos-Pinto, I.; Mendez, L.; Schouten, J.; Wilkins, J.; Federova, M.; Pitt, A.R.; Davis, P.; Spickett, C.M. Epitope mapping and characterization of 4-hydroxy-2-nonenal modified human serum albumin using two different polyclonal antibodies. Free Radic. Biol. Med. 2019, 144, 234–244. [Google Scholar] [CrossRef]
- Connor, R.E.; Marnett, L.J.; Liebler, D.C. Protein-selective capture to analyze electrophile adduction of Hsp90 by 4-hydroxynonenal. Chem Res Toxicol 2011, 24, 1275–1282. [Google Scholar] [CrossRef]
- Zarkovic, K.; Jakovcevic, A.; Zarkovic, N. Contribution of the HNE-immunohistochemistry to modern pathological concepts of major human diseases. Free Radic. Biol. Med. 2017, 111, 110–126. [Google Scholar] [CrossRef]
- Almeras, E.; Stolz, S.; Vollenweider, S.; Reymond, P.; Mene-Saffrane, L.; Farmer, E.E. Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 2003, 34, 205–216. [Google Scholar] [CrossRef]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Selman, S.; Engelberth, M.; Engelberth, J. Organizing the Chaos: Novel Insights into the Regulation of Z-3-Hexenal Production in Damaged Maize Leaves. Plants 2024, 13, 2772. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Engelberth, M. Developmental stages affect the capacity to produce aldehyde green leaf volatiles in Zea mays and Vigna radiata. Plants 2022, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Cofer, T.M.; Tumlinson, J.H. The carboxylesterase AtCXE12 converts volatile (Z)-3-hexenyl acetate to (Z)-3-hexenol in Arabidopsis leaves. Plant Physiol. 2025, 197, kiaf119. [Google Scholar] [CrossRef]
- Engelberth, J.; Contreras, C.F.; Dalvi, C.; Li, T.; Engelberth, M. Early transcriptome analyses of Z-3-Hexenol-treated Zea mays revealed distinct transcriptional networks and anti-herbivore defense potential of green leaf volatiles. PLoS ONE 2013, 8, e77465. [Google Scholar] [CrossRef]
- Lin, Y.H.; Silven, J.J.M.; Wybouw, N.; Fandino, R.A.; Dekker, H.L.; Vogel, H.; Wu, Y.L.; de Koster, C.; Große-Wilde, E.; Haring, M.A.; et al. A salivary GMC oxidoreductase of Manduca sexta re-arranges the green leaf volatile profile of its host plant. Nat. Commun. 2023, 14, 3666. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Adolph, S.; Jung, V.; Rattke, J.; Pohnert, G. Wound closure in the invasive green alga Caulerpa taxifolia by enzymatic activation of a protein cross-linker. Angew. Chem. Int. Ed. 2005, 44, 2806–2808. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engelberth, J.; Engelberth, M. Reactivity of Z-3-Hexenal with Amino Groups Provides a Potential Mechanism for Its Direct Effects on Insect Herbivores. Insects 2025, 16, 582. https://doi.org/10.3390/insects16060582
Engelberth J, Engelberth M. Reactivity of Z-3-Hexenal with Amino Groups Provides a Potential Mechanism for Its Direct Effects on Insect Herbivores. Insects. 2025; 16(6):582. https://doi.org/10.3390/insects16060582
Chicago/Turabian StyleEngelberth, Jurgen, and Marie Engelberth. 2025. "Reactivity of Z-3-Hexenal with Amino Groups Provides a Potential Mechanism for Its Direct Effects on Insect Herbivores" Insects 16, no. 6: 582. https://doi.org/10.3390/insects16060582
APA StyleEngelberth, J., & Engelberth, M. (2025). Reactivity of Z-3-Hexenal with Amino Groups Provides a Potential Mechanism for Its Direct Effects on Insect Herbivores. Insects, 16(6), 582. https://doi.org/10.3390/insects16060582






