Simple Summary
This study investigated whether soil-applied silicon could enhance the resistance of biomass sorghum (BRS 716) (Sorghum bicolor) plants to aphid infestation (Melanaphis sorghi (Theobald, 1904) (Hemiptera: Aphididae)) while also boosting crop productivity. Sorghum plants were grown under controlled conditions and treated with varying silicon doses (0, 2, 4, and 6 tons per hectare). When exposed to aphid attacks, plants that received silicon displayed increased tolerance compared with untreated ones. Notably, the highest application rate (6 tons per hectare) was most effective, enabling plants to sustain healthy growth and generate greater biomass despite pest pressure. In addition to reinforcing resistance, silicon positively influenced key plant traits. It increased the amount of plant fiber (cellulose) and improved the uptake of essential nutrients such as phosphorus and calcium. However, it also led to a slight reduction in the concentration of certain other nutrients. These results highlight silicon’s multifaceted role in plant defense and development. Overall, this research supports the potential of silicon supplementation as part of an integrated pest and crop management strategy. Incorporating silicon into agricultural practices could contribute to more resilient and productive cropping systems, offering a promising alternative to conventional chemical controls and helping to promote sustainable farming methods.
Abstract
Silicon application shows potential for enhancing crop resistance to pests while improving productivity. This study evaluated silicon’s effects on agronomic traits and chemical composition of biomass sorghum (Sorghum bicolor) under aphid infestation (Melanaphis sorghi (Theobald, 1904) (Hemiptera: Aphididae)). Greenhouse-grown sorghum (hybrid BRS716) was treated with silicic acid (0, 2, 4, or 6 metric tons per hectare), applied at sowing and the five-leaf stage. Aphid-infested plants were monitored weekly for damage, alongside growth measurements (height, stem diameter, leaf retention). Post-harvest, fresh, and dry biomass were analyzed via near-infrared spectroscopy and chemical assays. Data were assessed using ANOVA and regression models. Results demonstrated that silicon reduced aphid infestation and damage at 6 metric tons per hectare. Silicon also increased cellulose content and improved phosphorus and calcium uptake, though nitrogen and potassium levels decreased. These findings suggest that silicon supplementation can strengthen sorghum’s natural defenses, enhance biomass production, and modify nutrient profiles. This approach offers a sustainable strategy to mitigate aphid damage while maintaining crop yield and quality, with potential applications in integrated pest management systems.
1. Introduction
The transition to renewable and sustainable energy sources has become a global priority to address climate change and reduce dependence on fossil fuels [1,2]. In this context, biomass sorghum (Sorghum bicolor) emerges as a promising feedstock for bioenergy due to its high lignocellulosic biomass yield, rapid growth, and adaptability to diverse climates [3,4,5]. Energy cogeneration from sorghum combines thermochemical and biochemical processes [4]. This integrated approach efficiently converts biomass into thermal and electrical energy while reducing greenhouse gas emissions [6,7].
Despite its potential, sorghum productivity is severely compromised by infestations of the sorghum aphid, Melanaphis sorghi (Theobald, 1904) (Hemiptera: Aphididae) [8]. This invasive pest was initially identified in the Americas in 2013 [9]. It infests various Sorghum species, notably Johnson grass (S. halepense) and grain sorghum (S. bicolor), resulting in substantial yield reductions [10,11,12]. In Brazil, M. sorghi has been documented in several agricultural regions, and infestations by this invasive pest have resulted in losses of up to 20% of areas cultivated with sorghum [13].
The aphid feeds on phloem sap, leading to nutrient depletion that induces chlorosis, wilting, and plant necrosis [14]. Additionally, the honeydew excreted by M. sorghi promotes sooty mold growth, which coats leaf surfaces and impairs photosynthesis [14]. Current management strategies, predominantly reliant on chemical insecticides, face challenges such as pest resistance and environmental concerns [15], underscoring the need for sustainable alternatives.
Silicon (Si) supplementation has emerged as an eco-friendly approach to enhance plant resistance to biotic stresses [16]. While not essential for plant development, Si accumulates in plant tissues, reinforcing cell walls and influencing physiological responses to pests and nutrient imbalances [17]. In sorghum, a species known for its high Si uptake, this element contributes to greater structural integrity and stress tolerance [18,19].
Notably, Si reduces susceptibility to aphids in crops. In sorghum, Si application decreased feeding by the greenbug (Schizaphis graminum (Rondani, 1852) (Hemiptera: Aphididae)) [20], while high Si doses (>4 t ha−1) suppressed M. sorghi’s biological parameters in grain sorghum [21]. Similar effects were observed in wheat, where Si activated jasmonic acid pathways [22] and enhanced resistance to Sitobion avenae (Fabricius, 1775) (Hemiptera: Aphididae) and Rhopalosiphum padi (Linnaeus, 1758) (Hemiptera: Aphididae) [23,24,25]. However, the mechanisms underlying Si-induced resistance in biomass sorghum under M. sorghi infestation remain underexplored, particularly its effects on agronomic traits and biomass quality.
This study evaluates the role of Si in enhancing the resistance of biomass sorghum (hybrid BRS716) to M. sorghi and its impact on crop productivity. We hypothesize that Si doses (2–6 t ha−1) will (1) reduce aphid infestation and damage and (2) improve agronomic performance and biomass composition. The null hypothesis posits no differential effects across Si doses (0–6 t ha−1) under infestation. Our findings aim to advance integrated pest management strategies for sustainable bioenergy production.
2. Materials and Methods
This experiment was conducted in a greenhouse at the Brazilian Agricultural Research Corporation (Embrapa Maize and Sorghum), located in Sete Lagoas, Minas Gerais, Brazil (19°28′ S, 44°15′08″ W). The biomass sorghum hybrid BRS716 was selected based on its high biomass production potential and suitability for energy cogeneration via biomass combustion, along with its broad adaptability to diverse regions of Brazil.
2.1. Melanaphis Sorghi Colony
A colony of M. sorghi was maintained on sorghum plants of the cultivar BRS Ponta Negra in 15 L pots, kept in a greenhouse with an average temperature of 26 ± 6 °C and relative humidity of 73%.
2.2. Soil Characterization, Preparation, and Fertilization
The soil used in this study was predominantly clayey, composed of 67% clay, 23% silt, and 10% sand. It had a pH of 6.1 (in water) and a silicon content of 12.12 mg kg−1. This soil, classified as dystrophic Red Oxisol, was collected from a ravine area at EMBRAPA (Sete Lagoas, Brazil). After collection, the soil was air-dried at an ambient temperature and subsequently sieved through a 2 mm mesh.
The prepared soil was placed in 20 L containers arranged inside a greenhouse. Nine BRS716 sorghum seeds were sown in each container, and after germination, the plants were thinned, maintaining three plants per pot.
Silicon (Si) was manually incorporated into the soil, previously dissolved in water to ensure complete integration. The Si source used was precipitated silicic acid (SiO2·xH2O, molar mass = 60.08 g mol−1; assay ≥ 99% SiO2) (Merck KGaA, Darmstadt, Germany) [26]. Sorghum plants were treated with increasing doses of silicic acid (0, 2, 4, or 6 t ha−1). Half of each dose was applied at sowing (calculated for a 20 cm soil depth, equivalent to 0.5, 1, or 1.5 g L−1, respectively), and the remaining half was applied at the fully expanded five-leaf stage.
The higher doses were selected because sorghum efficiently absorbs Si, allowing us to evaluate the effects of elevated silicic acid concentrations. Additionally, the application timing was synchronized with standard sorghum fertilization practices to ensure agronomic relevance.
For initial fertilization, an 8-28-16 NPK fertilizer (Fertilizantes Heringer, Iguatama, Brazil) was applied at a rate of 80 kg ha−1, supplemented with 40 kg ha−1 of urea (Fertilizantes Heringer, Iguatama, Brazil), also applied at the fully expanded five-leaf stage.
2.3. Assessment of Agronomic Parameters
This experiment was conducted in a greenhouse measuring 12 m (length) × 4 m (width) × 3.20 m (height), covered with light-diffusing polyethylene film, between April and June 2022. Controlled conditions included temperature (26 ± 6 °C, regulated by an automatic exhaust system activated above 30 °C and cooling walls), relative humidity (73 ± 5%, maintained by ventilation and an evaporative system), and natural photoperiod (11.34 ± 0.2 h). Temperature and humidity were monitored using a digital thermometer. A completely randomized design was adopted, with three replicates, each consisting of three plants.
To establish M. sorghi colonies on plants (with two-to-three fully expanded leaves), a pot containing M. sorghi-infested sorghum plants (cv. BRS Ponta Negra—a cultivar routinely used for rearing aphid colonies under laboratory conditions) was placed equidistantly among every four experimental pots. This method simulated the natural infestation pattern observed in sorghum fields.
Infestation and damage assessments were conducted weekly using a visual observation scale [21,27], adapted from the M. sacchari protocol [28] (Table 1 and Table 2; Figure 1 and Figure 2).

Table 1.
Infestation scale of Melanaphis sorghi in sorghum.

Table 2.
Damage severity scale of Melanaphis sorghi in sorghum.
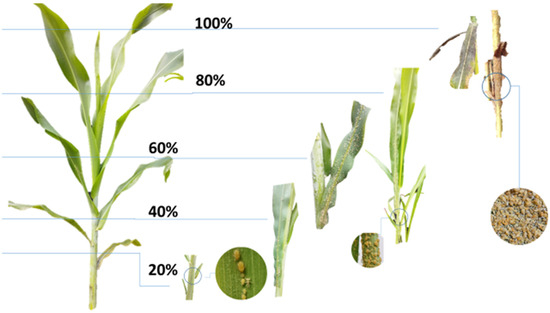
Figure 1.
Infestation scale for evaluation of sorghum aphid (Melanaphis sorghi).

Figure 2.
Damage scale for evaluation of sorghum aphid (Melanaphis sorghi). Score 1: Few visible lesions; Score 2: Initial reddish spots on the midrib and presence of exuviae; Score 3: Scattered reddish spots, leaf mar-gin yellowing, and moderate exuviae; Score 4: Leaves with reddish and yellowish spots, bronzed appearance, and abundant exuviae; Score 5: Leaves exhibiting reddish-yellow discoloration, initial leaf tissue desiccation, and reduced exuviae; Score 6: Complete plant death.
Additionally, plant growth characteristics were measured, including height (from soil level to the youngest folded leaf), stem diameter (measured with a digital caliper), and the number of green leaves (not completely dried) per plant.
After harvest, fresh weight was recorded using a digital scale with an accuracy of ±2 g. For dry weight determination, plants were dried in a forced-air oven at 65 °C for 96 h and then weighed on a digital scale (±2 g accuracy).
2.4. Chemical and Bromatological Composition Analysis
After drying and weighing, samples were ground in a knife mill and sent to the laboratory for bromatological analysis using near-infrared spectroscopy (NIRS). Samples were placed in glass plates with an internal diameter of 90 mm, used as sample holders for NIR spectra recording. Spectra were collected using a Büchi NIRFlex N-500 FT-NIR spectrometer (Flawil, Switzerland) equipped with a diffuse reflectance accessory. Data were acquired using NIRWare Operator software (version 1.5) and processed in MATLAB (version 7.13) using the PLS Toolbox (version 6.5) PLS routine to determine lignin, cellulose, and hemicellulose contents [29].
Chemical analyses were conducted by the Brazilian Laboratory of Environmental and Agricultural Analyses—LABRAS (Monte Carmelo, Brazil) on dried and ground samples, following the plant nutritional status assessment protocol [30]. Nitrogen and silicon levels were determined after sulfuric acid digestion; phosphorus, potassium, calcium, magnesium, sulfur, copper, iron, manganese, and zinc were quantified after nitroperchloric digestion; and boron was analyzed after dry digestion. These procedures provided a comprehensive characterization of the elements present in plant tissue.
2.5. Statistical Analysis
The data were analyzed using analysis of variance (ANOVA) at a significance level of p < 0.05, employing the SISVAR statistical software (version 5.8) [31]. When significant effects were detected, linear and quadratic regression analyses were conducted, followed by the Scott–Knott test to compare treatments with and without infestation. Graphs were generated using JAMOVI (version 2.4.11) [32] and Minitab (version 21.1.0) [33].
3. Results
3.1. Interaction Between Silicon and Melanaphis Sorghi Infestation
Analysis (Supplementary Materials) showed that, at the infestation level, both infestation scores (μ = 37.4%) and damage scores (μ = 1.40) caused by M. sorghi increased over days after emergence (DAE) across all silicon doses evaluated (p < 0.01). However, as silicon doses (0, 2, 4, and 6 t ha−1) increased, infestation and damage levels in sorghum plants progressively decreased. Plants without silicon application (0 t ha−1) exhibited the highest infestation (μFinal Score = 98.4%) and damage scores (μFinal Score = 4.50), while those treated with the highest dose (6 t ha−1) showed the lowest values (μFinal Score = 70% and 3.17) (Figure 3).
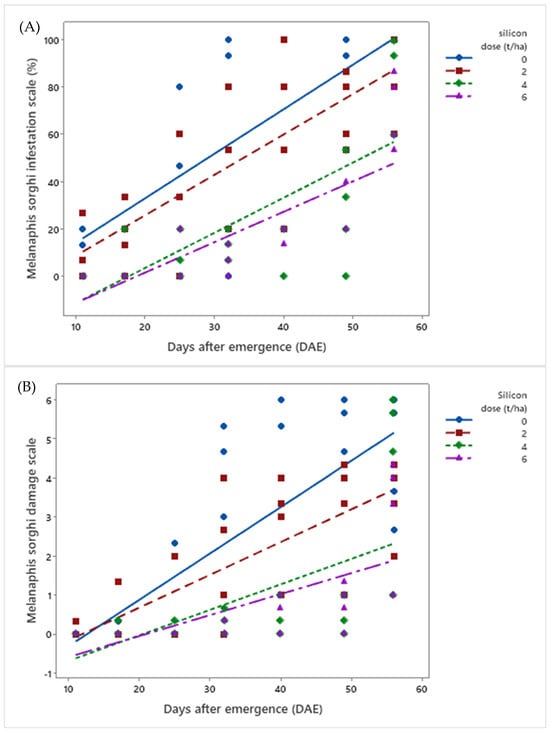
Figure 3.
Linear regressions for (A) infestation score and (B) damage score of Melanaphis sorghi in biomass sorghum subjected to four silicic acid doses over 56 days after emergence. Regression equations for infestation: Dose 0: y = 0.106x − 0.433 (R2 = 93.58%); Dose 2: y = 0.086x − 0.428 (R2 = 95.66%); Dose 4: y = 0.080x − 1.46 (R2 = 63.16%); Dose 6: y = 0.060x − 1.22 (R2 = 73.42%). Regression equations for damage: Dose 0: y = 0.119x − 1.500 (R2 = 88.42%); Dose 2: y = 0.084x − 1.011 (R2 = 93.87%); Dose 4: y = 0.065x − 1.351 (R2 = 47.50%); Dose 6: y = 0.053x − 1.139 (R2 = 74.63%). Location: Sete Lagoas, Minas Gerais, Brazil, 2025.
Plant dry weight (μ = 13.15 g) also increased with higher silicon doses (p = 0.0036), both in infested (μDose6 = 14.00 g) and non-infested plants (μDose6 = 17.06 g) (Figure 4).
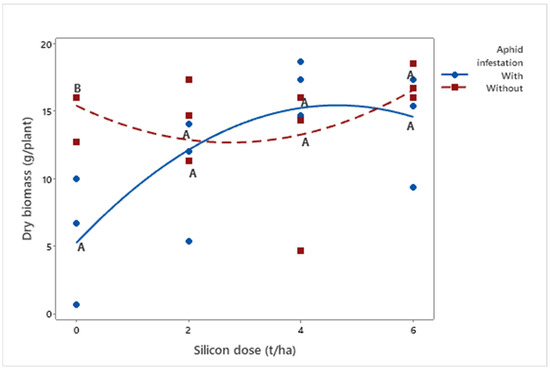
Figure 4.
Quadratic regressions of dry biomass (g/plant) as a function of silicon dose (t/ha) in biomass sorghum plants with and without Melanaphis sorghi infestation. Different letters denote significant differences (p < 0.05) between with and without-infested treatments. The regression equations were as follows: infested plants: y = −0.472x2 + 4.388x + 5.224 (R2 = 91.02%); non-infested plants: y = 0.364x2 − 2.001x + 15.414 (R2 = 62.59%). Location: Sete Lagoas, Minas Gerais, Brazil, 2025.
Additionally, cellulose concentration (μ = 33.64%) was influenced by silicon application (p = 0.018). While cellulose levels in non-infested plants remained stable (μDose6 = 33.47%) with increasing silicon doses, infested plants showed a progressive increase, exceeding 40% at the highest dose (6 t ha−1). These findings suggest that silicon may stimulate changes in cell wall composition in response to pest attack (Figure 5).
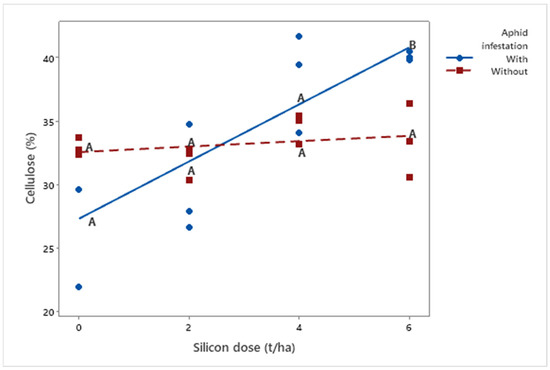
Figure 5.
Regression analysis of cellulose percentage as a function of silicon dose (t/ha) in biomass sorghum plants with and without Melanaphis sorghi infestation. Different letters denote significant differences (p < 0.05) between with and without -infested treatments. The regression equations were as follows: infested plants: y = −2.246x + 27.33 (R2 = 91.29%); non-infested plants: y = 0.212x + 32.574 (R2 = 24.51%). Location: Sete Lagoas, Minas Gerais, Brazil, 2025.
Manganese (Mn) (μ = 71.19 mg/kg) and zinc (Zn) (μ = 18.29 mg/kg) concentrations responded differently to silicon doses depending on infestation status. Mn increased in infested plants (μDose6 = 82.81 mg/kg) with higher silicon doses but decreased in non-infested plants (μDose6 = 56.14 mg/kg; p = 0.001). Conversely, Zn levels decreased in infested plants (μDose6 = 15.25 mg/kg) with increasing silicon doses but increased in non-infested plants (μDose6 = 22.17 mg/kg; p = 0.008) (Figure 6).
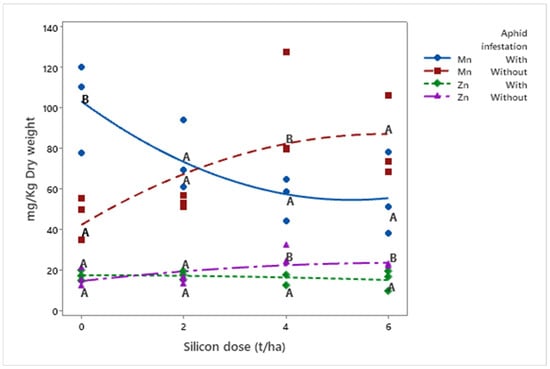
Figure 6.
Concentrations of Mn and Zn (mg kg−1 dry weight) in biomass sorghum plants subjected to different silicon doses (t ha−1), with and without Melanaphis sorghi infestation. Solid lines represent trends for Mn under infestation and Zn under infestation, while dashed lines represent trends without infestation. Different letters denote significant differences (p < 0.05) between with and without -infested treatments. Regression equations for manganese: with infestation: y = 1.748x2 − 18.433x + 103.330 (R2 = 99.66%); without infestation: y = −1.260x2 + 15.053x + 12.361 (R2 = 75.10%). Regression equations for zinc: with infestation: y = −0.062x2 − 0.032x + 17.536 (R2 = 91.63%); without infestation: y = −0.222x2 + 2.838x + 14.600 (R2 = 75.10%). Location: Sete Lagoas, Minas Gerais, Brazil, 2025.
No significant differences were observed in stem diameter (μ = 9.60 mm; p = 0.134) or the concentrations of sulfur (S) (μ = 1.11 g/kg; p = 0.269), boron (B) (μ = 14.32 mg/kg; p = 0.107), copper (Cu) (μ = 3.32 mg/kg; p = 0.749), or iron (Fe) (μ = 143.40 mg/kg; p = 0.277) across silicon doses and infestation treatments.
3.2. Effect of Silicon on Plant Growth and Composition
Silicon application positively impacted fresh plant weight (μ = 94.53 g; p = 0.006). A quadratic relationship was observed between silicon doses and final fresh weight (μDose6 = 109.47 g), with increments up to a saturation point (μDose4 = 115.72 g), suggesting an optimal dose for maximizing growth (Figure 7).
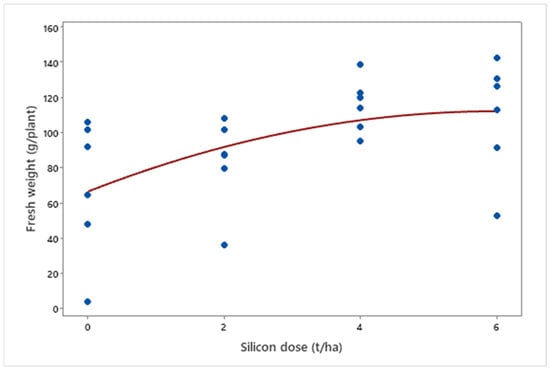
Figure 7.
Fresh weight of biomass sorghum plants treated with different Si doses (0, 2, 4, and 6 t ha−1) at 56 days after emergence. Regression equation: y = −1.262x2 + 15.183x + 66.657 (R2 = 88.63%). Location: Sete Lagoas, Minas Gerais, Brazil, 2025.
Regarding cell wall composition, silicon application induced distinct changes in lignin (μ = 3.98%; p = 0.008) and hemicellulose (μ = 28.98%; p < 0.001) levels. While lignin decreased (μDose6 = 3.66%), hemicellulose (μDose6 = 30.23%) slightly increased, indicating that silicon modulates the biosynthesis and deposition of structural components (Figure 8).
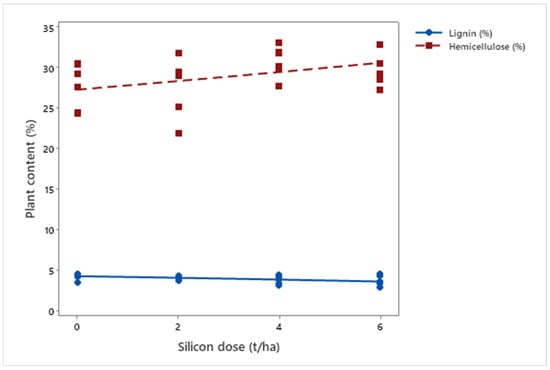
Figure 8.
Lignin and hemicellulose concentrations in biomass sorghum plants subjected to different silicon doses (t ha−1). Regression equation: hemicellulose: y = 0.553x + 27.318 (R2 = 62.30%).
In mineral nutrition, silicon affected macronutrient levels. A 36% reduction in nitrogen (p < 0.001) and a 48% reduction in potassium (p < 0.001) were observed, while phosphorus (p = 0.003) and calcium (p < 0.001) increased by 46% and 57%, respectively (Figure 9). Finally, silicon accumulation in plants increased exponentially with applied doses (p = 0.012) (Figure 10).
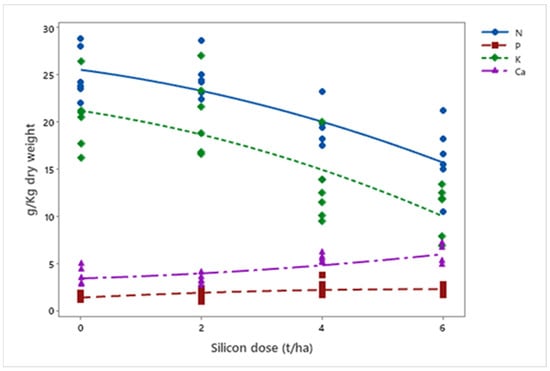
Figure 9.
Concentrations of N, P, K, and Ca (g kg−1 dry weight) in biomass sorghum plants subjected to different silicon doses (t ha−1). Regression equations: N: y = −0.130x2 − 0.852x + 25.500 (R2 = 93.08%); P: y = −0.027x2 + 0.311x + 1.347 (R2 = 69.52%); K: y = −0.149x2 − 0.965x + 21.188 (R2 = 88.43%); Ca: y = 0.040x2 + 0.185x + 3.390 (R2 = 77.18%). Location: Sete Lagoas, MG, Brazil, 2025.
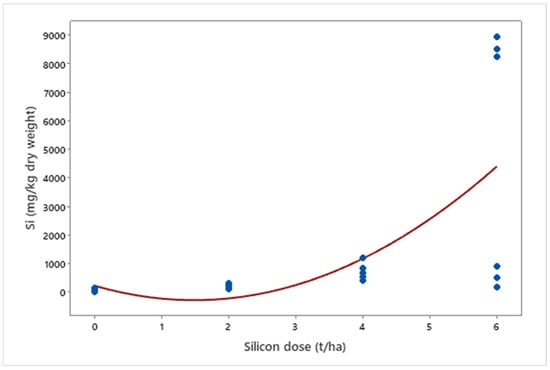
Figure 10.
Silicon (Si) concentrations (mg kg−1 dry weight) in biomass sorghum plants subjected to different silicon doses (t ha−1). Regression equation: y = 229.605x2 − 680.835x + 223.529 (R2 = 97.11%). Location: Sete Lagoas, MG, Brazil, 2025.
3.3. Effect of Melanaphis Sorghi Infestation on Plant Growth and Nutrition
Infestation by M. sorghi significantly impaired plant growth and nutritional parameters. In vegetative growth, infested plants exhibited a 17% reduction in average height (p = 0.006) compared with non-infested plants (Figure 11A). Consistent results were observed in a 12% reduction in leaf number per plant (p = 0.022) (Figure 11B) and a 29% decrease in fresh weight (p = 0.002) (Figure 11C).
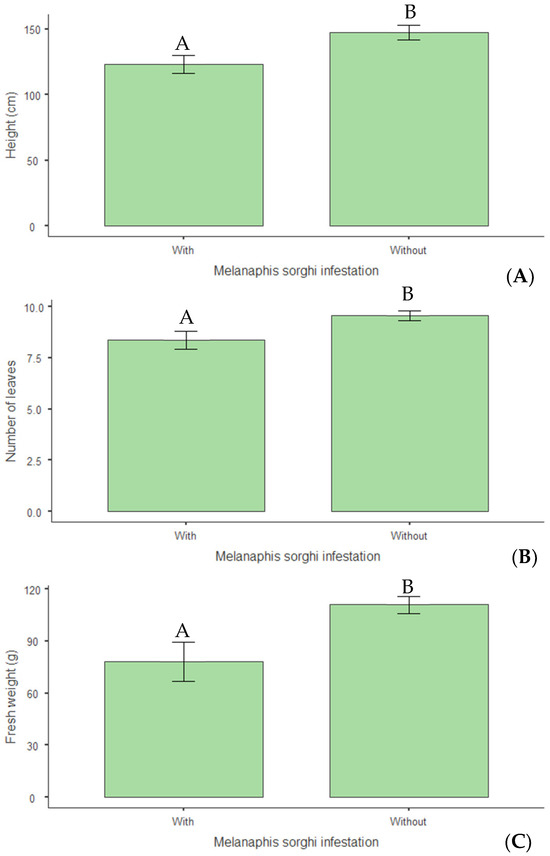
Figure 11.
(A) Plant height (cm); (B) number of leaves; (C) fresh weight (g) per plant of biomass sorghum subjected to different silicon doses (t ha−1), with and without Melanaphis sorghi infestation. Different letters denote significant differences (p < 0.05) between with and without -infested treatments. Location: Sete Lagoas, MG, Brazil, 2025.
Concerning structural composition, a 14% reduction in hemicellulose levels (p < 0.01) was observed in infested plants (Figure 12A), indicating that infestation negatively altered this cell wall constituent. Infestation also reduced the plants’ calorific value by 3% (p = 0.015) (Figure 12B). Regarding mineral nutrition, reductions of 14% in nitrogen (p = 0.009) (Figure 13A), 19% in phosphorus (p = 0.046) (Figure 13B), and 20% in magnesium (p = 0.015) (Figure 13C) were observed in infested plants.
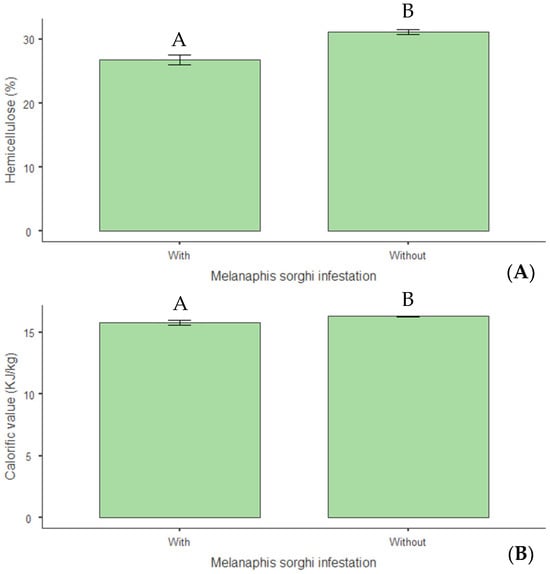
Figure 12.
(A) Hemicellulose (%) and (B) calorific value (kJ kg−1) in biomass sorghum plants subjected to different silicon doses (t ha−1), with and without Melanaphis sorghi infestation. Different letters denote significant differences (p < 0.05) between with and without -infested treatments. Location: Sete Lagoas, MG, Brazil, 2024.
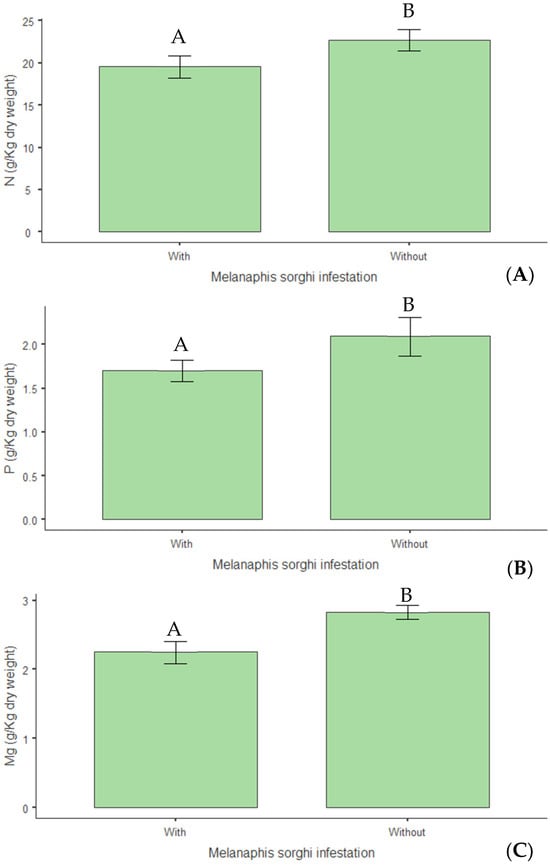
Figure 13.
(A) Nitrogen (N); (B) phosphorus (P); (C) magnesium (Mg) content (g kg−1 dry weight) in biomass sorghum subjected to different silicon doses (t ha−1), with and without Melanaphis sorghi infestation. Different letters denote significant differences (p < 0.05) between with and without -infested treatments. Location: Sete Lagoas, MG, Brazil, 2025.
4. Discussion
The results of this study demonstrated the impact of silicon (Si) and M. sorghi infestation on the growth, mineral nutrition, and structural composition of hybrid biomass sorghum plants (BRS716), providing relevant insights for integrated pest management and nutrient application in agriculture.
Silicon application exhibited a significant protective effect against M. sorghi-induced damage, with a marked reduction in infestation and damage levels in plants treated with increasing Si doses. The direct relationship between Si dose and decreased infestation and damage underscores Si’s role in enhancing plant structural integrity. The observed modulation of cellulose concentration supports this hypothesis, as elevated levels of this structural polysaccharide in Si-treated infested plants suggest cell wall reinforcement in response to biotic stress [34,35].
Other changes in cell wall composition included reduced lignin content. Studies indicate that higher Si concentrations may interfere with lignin oligomer aggregation, inhibiting its formation [36]. In tobacco, for instance, Si treatment reduced lignin accumulation even in leaves not directly exposed to mechanical stress, highlighting Si’s modulatory role in stress responses [37]. Additionally, Si stimulates the production of secondary metabolites, such as phenolics, which may influence lignin biosynthesis under stress conditions [38]. This reduction in lignin is particularly advantageous for industrial applications like bioenergy production, as lignin is a major limiting factor in biomass digestibility during biofuel conversion [39].
This selective cell wall reinforcement suggests an adaptive mechanism to biotic stress [40]. Alterations in structural polysaccharides (e.g., cellulose and hemicellulose) increase cell wall rigidity and hinder insect access to cellular contents [41,42]. These structural adaptations not only protect against pest attacks but also enhance the plant’s suitability for industrial applications, such as bioenergy production, underscoring its dual relevance for agriculture and industry [43,44,45,46].
Another contributing factor, widely cited in other studies, is silica deposition in cell walls [47,48]. This physical barrier impedes insect stylet penetration, inhibiting feeding, as described by recent studies [49,50]. Additionally, the Si-induced modulation of chemical compounds may reduce tissue attractiveness to aphids, reinforcing plant defense mechanisms [23].
For instance, Si enhances the activity of defense-related enzymes like peroxidase and polyphenol oxidase, both crucial for plant responses to herbivory [51]. Additionally, Si accumulation in plant tissues has been linked to the increased production of phenolic compounds—natural deterrents against herbivores [52].
Silicon supplementation can also reshape resource allocation in plants, potentially affecting the balance between structural defenses and the biosynthesis of defensive metabolites [53]. Furthermore, Si interacts with plant physiology in complex ways, influencing nutrient uptake and hormonal regulation, which in turn modulate the production of secondary metabolites involved in pest resistance [35]. The Si-driven changes in phenolic content are especially relevant for aphid resistance. These secondary metabolites can disrupt aphid feeding behavior and reproduction [53].
While chewing insects may be more directly affected by structural and chemical barriers, phloem-feeding aphids might experience a less immediate impact [52]. This suggests that Si contributes to a broader and more multi-layered defense strategy, complementing, rather than replacing, the plant’s innate physiological responses to herbivory.
These findings align with prior studies on sap-sucking pest management. Different Si forms (e.g., nano-silica, tetraethyl orthosilicate, Na2SiO3, and K2SiO3) reduced aphid density in wheat, with tetraethyl orthosilicate yielding the best results [54]. Higher soil Si levels decreased aphid numbers in both resistant and susceptible wheat varieties, reinforcing Si’s role as a resistance inducer that strengthens cell walls and creates physical barriers against insects [25].
Beyond reducing pest populations, Si also influences aphid life cycle and feeding behavior. A concentration of 2 g·L−1 of Si prolonged nymphal duration, reduced longevity and fertility, and decreased feeding preference in S. avenae [24]. These behavioral effects stem from Si’s interference with insect feeding and reproduction. Up to 80% mortality in first-instar S. graminum nymphs occurred on wheat treated with 100 mL·L−1 of Si [55].
Other studies corroborate Si’s benefits across crops. Silicic acid treatment reduced Lipaphis erysimi (Kalt.) (Hemiptera: Aphididae) populations in rapeseed while improving photosynthesis and stomatal conductance [56].
Beyond structural improvements, Si application significantly increases fresh weight and dry biomass, even under M. sorghi infestation. This benefit may stem from Si’s ability to enhance photosynthetic efficiency, such as by promoting higher chlorophyll levels (crucial for photosynthesis, as demonstrated in maize under saline stress [57]) and by improving stomatal conductance and transpiration rates, which facilitate gas exchange and CO2 uptake [58]. Additionally, Si helps mitigate aphid-induced oxidative stress [59,60]. However, the lower biomass in infested plants indicates that insect damage partially limits growth potential, likely due to continuous sap extraction, which compromises water and nutrient uptake [8,61,62]. These results emphasize the need for complementary strategies to maximize Si’s benefits.
From a nutritional perspective, Si application altered macronutrient levels, reducing nitrogen and potassium concentrations. This may reflect competition between Si and these nutrients for specific transporters [63,64,65]. Conversely, increased phosphorus and calcium levels suggest Si selectively modulates nutrient absorption and redistribution, preserving those critical for energy metabolism and structural integrity [65,66,67,68].
The modulation of manganese and zinc concentrations in this study may reflect complex interactions among Si, nutrients, and biotic stress [69]. Future research should explore how these interactions influence plant resistance to pests and fertilizer-use efficiency. While Si offers clear benefits, its management must be optimized to avoid adverse effects on plant nutrition.
Stem diameter and sulfur (S), iron (Fe), copper (Cu), and boron (B) concentrations were unaffected by Si or M. sorghi infestation. The lack of significant changes in stem diameter suggests this structural trait is less sensitive to Si or aphid presence compared with other growth and compositional parameters. For micronutrients (Fe, Cu, B) and S, stable concentrations may indicate a limited interaction with Si or a minimal impact of infestation on their uptake and redistribution. This stability likely reflects the plant’s ability to maintain the homeostasis of these elements under biotic stress, given their roles in essential metabolic processes like photosynthesis and protein synthesis [70].
Infestation by M. sorghi negatively impacted growth, structural composition, and nutrient levels, reducing nitrogen, phosphorus, and magnesium concentrations. Declines in height, leaf number, and fresh weight highlight the deleterious effects of aphid sap consumption, which impairs nutrient transport and photosynthesis. Similar studies reported substantial damage in M. sorghi-infested crops, including reduced vegetative vigor and nutrient-use efficiency [14,15]. Aphid-induced stress disrupts nutrient absorption and redistribution [71]. Moreover, reduced hemicellulose and nitrogen levels in infested plants support the hypothesis that aphid attacks compromise both metabolic functionality and mechanical resistance, adversely affecting agricultural productivity [72,73].
Infestation also lowered the plants’ calorific value, likely due to reduced hemicellulose content and biomass loss from aphid feeding. This decline may impair the biomass’s energy efficiency for cogeneration, underscoring the need for management strategies to mitigate aphid damage.
The interaction between Si and M. sorghi in this study revealed Si’s potential to partially offset infestation-related damage, positioning it as a tool for integrated pest management. The 6 t ha−1 Si dose was most effective, minimizing infestation severity and promoting the highest dry weight increase under biotic stress. However, the agronomic viability of this dose requires careful evaluation, considering application costs, Si source availability, and potential impacts on nutrient uptake.
Because Si is non-essential and does not fully eliminate M. sorghi damage, its economic justification for large-scale use may be limited. Nevertheless, in some contexts, Si application offers measurable financial benefits. For example, foliar Si spraying (1.50 mL/L) in Indian sorghum fields increased yields by 2850 kg/ha, achieving a favorable benefit/cost ratio of 1.61 [74]. Economic returns, however, depend on stress severity, yield potential, and crop variety. While Si can mitigate losses under moderate-to-high stress, its benefits may not outweigh costs in low-yielding systems or less responsive cultivars [75,76].
Therefore, integrated approaches—combining reduced Si doses with complementary management practices—could offer a more sustainable and cost-effective pest control strategy. Similar to the use of natural enemies [77], silicon supplementation may improve the suppression of parasitoids on M. sorghi, highlighting its potential for integrated pest management (IPM) in sorghum [78]. These synergistic effects could enhance agricultural resilience and ecosystem health. Future studies should investigate such combinatorial strategies to optimize Si’s role in crop protection and bioenergy production.
5. Conclusions
Infestation by M. sorghi negatively impacts plant growth, nutrient uptake, and structural integrity. However, Si application mitigates these adverse effects by enhancing plant metabolism and resistance.
The addition of Si increases the resistance of biomass sorghum BRS716 to M. sorghi, significantly reducing infestation levels and damage. Increasing Si doses improves both fresh and dry biomass accumulation and modifies cell wall composition by elevating cellulose content in infested plants.
Plant-accumulated Si levels rise exponentially with applied doses, confirming Si as a promising tool for the sustainable management of biomass sorghum BRS716. This enhances biotic stress tolerance and agronomic performance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects16060566/s1, Table S1: Summary of analysis of variance (ANOVA) and probability values (P) for Infestation and Damage scores under different silicon doses; Table S2: ANOVA results for growth and biomass traits in sorghum plants treated with silicon and exposed to Melanaphis sorghi; Table S3: Summary of analysis of variance (ANOVA) and probability values (P) for lignin, cellulose, hemicellulose, and calorific value of biomass sorghum plants subjected to different silicon doses with and without Melanaphis sorghi infestation; Table S4: Summary of analysis of variance (ANOVA) and probability values (P) for nitrogen, phosphorus, potassium, calcium, magnesium, and sulfur in biomass sorghum plants subjected to different silicon doses with and without Melanaphis sorghi infestation; Table S5: Summary of analysis of variance (ANOVA) and probability values (P) for boron, copper, iron, manganese, zinc, and silicon in biomass sorghum plants subjected to different silicon doses with and without Melanaphis sorghi infestation.
Author Contributions
Conceptualization, D.G.S. and S.M.M.; methodology, D.G.S., G.S.A., N.M.S., T.F.S. and A.A.N.; software, D.G.S. and M.L.F.S.; validation, M.L.F.S. and S.M.M.; formal analysis, D.G.S.; investigation, D.G.S.; resources, S.M.M. and M.L.F.S.; data curation, D.G.S. and S.M.M.; writing—original draft preparation, D.G.S.; writing—review and editing, L.L.C.D., M.L.F.S., R.A.C.P. and S.M.M.; visualization, S.M.M.; supervision, S.M.M.; project administration, S.M.M.; funding acquisition, S.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPEMIG, number APQ-01180-23 and within the scope of the doctoral scholarship; CAPES/PROEX number 32018010009P4 and FINEP, number 01.13.0295.00 Ref. 0468/12.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the Postgraduate Program in Agricultural Sciences (PPGCA) and Postgraduate Program in Bioengineering (PPBE) at the Federal University of São João del-Rei (UFSJ) for their scientific support. We thank Embrapa Maize and Sorghum for the structure made available for the bioassay. FAPEMIG, CAPES/PROEX and FINEP for financial support.
Conflicts of Interest
Maria Lúcia F. Simeone, Rafael A. Parrella and Simone M. Mendes were employed by the company Brazilian Agricultural Research Corporation (Embrapa). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EMBRAPA | Brazilian Agricultural Research Corporation |
| FAPEMIG | Minas Gerais State Research Support Foundation |
| FINEP | Funding Agency for Studies and Projects |
| NIRS | Near-Infrared Spectroscopy |
| PPBE | Postgraduate Program in Bioengineering |
| PPGCA | Postgraduate Program in Agricultural Sciences |
| UFSJ | Federal University of São João del-Rei |
References
- Mammadov, N.; Akbarova, S. Research Areas on Transition to Renewable Energy Sources in Bibliographic Publications. BIO Web Conf. 2025, 151, 02007. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, R. Advancing Global Sustainability: The Role of the Sharing Economy, Environmental Patents, and Energy Efficiency in the Group of Seven’s Path to Sustainable Development. Sustainability 2025, 17, 322. [Google Scholar] [CrossRef]
- Bernardeli, A.; Damasceno, C.M.B.; de Magalhães, J.V.; de Souza, V.F.; de Oliveira Melo, J.; de Oliveira, A.A.; Simeone, M.L.F.; Borém, A.; Schaffert, R.E.; da Costa Parrella, R.A.; et al. Population Genomics and Molecular Breeding of Sorghum. In Population Genomics: Crop Plants; Springer: Cham, Switzerland, 2022; pp. 289–340. [Google Scholar] [CrossRef]
- Bakari, H.; Djomdi; Ruben, Z.F.; Roger, D.D.; Cedric, D.; Guillaume, P.; Pascal, D.; Philippe, M.; Gwendoline, C. Sorghum (Sorghum bicolor L. Moench) and Its Main Parts (By-Products) as Promising Sustainable Sources of Value-Added Ingredients. Waste Biomass Valor. 2023, 14, 1023–1044. [Google Scholar] [CrossRef]
- Castro, F.M.R.; Lombardi, G.M.R.; Nunes, J.A.R.; da Costa Parrella, R.A.; Bruzi, A.T. Accumulation of Biomass and Lignocellulosic Compounds in Photoperiod-Sensitive Biomass Sorghum Genotypes. Biomass Bioenerg. 2022, 158, 106344. [Google Scholar] [CrossRef]
- Adeoye, A.O.; Lawal, O.S.; Quadri, R.O.; Malomo, D.; Aliyu, M.T.; Dang, G.E.; Emojevu, E.O.; Maikato, M.J.; Yahaya, M.G.; Omonije, O.O.; et al. Sustainable Energy via Thermochemical and Biochemical Conversion of Biomass Wastes for Biofuel Production. In Transportation Energy and Dynamics; Springer: Singapore, 2023; pp. 245–306. [Google Scholar] [CrossRef]
- Ameen, M.; Mahmood, A.; Shehzad, A.N.; Zia, M.A.; Javaid, M.M. Sorghum’s Potential Unleashed: A Comprehensive Exploration of Bio-Energy Production Strategies and Innovations. Bioresour. Technol. Rep. 2024, 27, 101906. [Google Scholar] [CrossRef]
- Thudi, M.; Reddy, M.S.; Naik, Y.D.; Cheruku, V.K.R.; Sangireddy, M.K.R.; Cuevas, H.E.; Knoll, J.E.; Louis, J.; Kousik, C.S.; Toews, M.D.; et al. Invasive Sorghum Aphid: A Decade of Research on Deciphering Plant Resistance Mechanisms and Novel Approaches in Breeding for Sorghum Resistance to Aphids. Crop Sci. 2024, 64, 2436–2458. [Google Scholar] [CrossRef]
- Armstrong, J.S.; Mbülwe, L.; Sekula-Ortiz, D.; Villanueva, R.T.; Rooney, W.L. Resistance to Melanaphis sacchari (Hemiptera: Aphididae) in Forage and Grain Sorghums. J. Econ. Entomol. 2017, 110, 259–265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Armstrong, J.S.; Harris-Shultz, K.R.; Ni, X.Z.; Wang, H.L.; Knoll, J.E.; Anderson, W.F. Utilizing Biodemographic Indices to Identify Perennial Bioenergy Grasses as Sugarcane Aphid (Hemiptera: Aphididae) Host Plants. Trends Entomol. 2019, 15, 1–14. [Google Scholar]
- Armstrong, J.S.; Rooney, W.L.; Peterson, G.C.; Villenueva, R.T.; Brewer, M.J.; Sekula-Ortiz, D. Sugarcane Aphid (Hemiptera: Aphididae): Host Range and Sorghum Resistance Including Cross-Resistance from Greenbug Sources. J. Econ. Entomol. 2015, 108, 576–582. [Google Scholar] [CrossRef]
- Paudyal, S.; Armstrong, J.S.; Harris-Shultz, K.; Wang, H.; Giles, K.; Rott, P.; Payton, M. Evidence of Host Plant Specialization Among the US Sugarcane Aphid (Hemiptera: Aphididae) Genotypes. Trends Entomol. 2019, 15, 47–58. [Google Scholar]
- Avellar, G.S.; Marriel, I.E.; Menezes, C.B.; Santos, D.G.; Santos, N.M.; Mendes, S.M. Pulgão Melanaphis sorghi (Theobald, 1904) (Hemiptera: Aphididae) na cultura do sorgo: Análise do cenário brasileiro. Entomol. Commun. 2023, 5, ec05042. [Google Scholar] [CrossRef]
- Uyi, O.; Toews, M.D. Melanaphis sorghi: A Review and Synthesis of Its Control Options 10 Years Post Detection of a New Invasive Haplotype in the United States of America. CABI Rev. 2024, 19, 1–15. [Google Scholar] [CrossRef]
- Vasquez, A.; Belsky, J.; Khanal, N.; Puri, H.; Balakrishnan, D.; Joshi, N.K.; Louis, J.; Studebaker, G.; Kariyat, R. Melanaphis sacchari/sorghi Complex: Current Status, Challenges and Integrated Strategies for Managing the Invasive Sap-Feeding Insect Pest of Sorghum. Pest Manag. Sci. 2024, 80, 2427–2441. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.K.; Song, X.P.; Tian, D.D.; Guo, D.J.; Chen, Z.L.; Zhong, C.S.; Nikpay, A.; Singh, M.; Rajput, V.D.; Singh, R.K.; et al. Influence of Silicon on Biocontrol Strategies to Manage Biotic Stress for Crop Protection, Performance, and Improvement. Plants 2021, 10, 2163. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, A.; Tandon, A.; Sharma, V. Insights into the Molecular Mechanisms of Uptake, Phytohormone Interactions and Stress Alleviation by Silicon: A Beneficial but Non-Essential Nutrient for Plants. Plant Growth Regul. 2023, 101, 1–13. [Google Scholar] [CrossRef]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of Silicon Uptake, Transport, and Deposition in Plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef]
- Carvalho, J.S.; Frazão, J.J.; de Mello Prado, R.; de Souza Júnior, J.P.; Costa, M.G. Silicon Modifies C:N:P Stoichiometry and Improves the Physiological Efficiency and Dry Matter Mass Production of Sorghum Grown Under Nutritional Sufficiency. Sci. Rep. 2022, 12, 16082. [Google Scholar] [CrossRef]
- Carvalho, S.P.; Moraes, J.C.; Carvalho, J.G. Efeito do Silício na Resistência do Sorgo (Sorghum bicolor) ao Pulgão-Verde Schizaphis graminum (Rond.) (Homoptera: Aphididae). An. Soc. Entomol. Bras. 1999, 28, 505–510. [Google Scholar] [CrossRef]
- Santos, D.G.D.; Dias, L.L.C.; Avellar, G.S.D.; Simeone, M.L.F.; Oliveira, I.R.D.; Menezes, C.B.D.; Santos, N.M.D.; Mendes, S.M. Effect of Silicon on Melanaphis sorghi (Theobald, 1904) (Hemiptera: Aphididae) Infesting Grain Sorghum (Sorghum bicolor). Int. J. Pest. Manag. 2025, 1–10. [Google Scholar] [CrossRef]
- Biru, F.N.; Nayak, J.J.; Waterman, J.M.; Cazzonelli, C.I.; Elbaum, R.; Johnson, S.N. Elevated Atmospheric CO2 and Silicon Antagonistically Regulate Anti-Herbivore Phytohormone and Defence Gene Expression Levels in Wheat. Environ. Exp. Bot. 2024, 227, 105950. [Google Scholar] [CrossRef]
- Qi, X.; Xue, X.; Wang, Z.; Li, S.; Zhang, Z.; Han, Y.; Wang, Y.; Jiang, Y. Silicon Application Enhances Wheat Defence Against Sitobion avenae F. by Regulating Plant Physiological-Biochemical Responses. Basic Appl. Ecol. 2024, 74, 13–23. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Yan, J.; Wang, Y.; Zhang, X.; Tan, X.; Chen, J. Developmental, Reproduction, and Feeding Preferences of the Sitobion avenae Mediated by Soil Silicon Application. Plants 2023, 12, 989. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.S.; Peñaflor, M.F.G.; Gonçalves, F.G.; Sampaio, M.V.; Korndörfer, A.P.; Silva, W.D.; Bento, J.M.S. Silicon-Induced Changes in Plant Volatiles Reduce Attractiveness of Wheat to the Bird Cherry-Oat Aphid Rhopalosiphum padi and Attract the Parasitoid Lysiphlebus testaceipes. PLoS ONE 2020, 15, e0231005. [Google Scholar] [CrossRef] [PubMed]
- Merck KGaA. Specification: Silicic Acid Precipitated Extra Pure Light; MilliporeSigma: Darmstadt, Germany, 2017. [Google Scholar]
- Fernandes, F.O.; Souza, C.D.S.F.; de Avellar, G.S.; Nascimento, P.T.; Damasceno, N.C.R.; dos Santos, N.M.; Lima, P.F.; dos Santos, M.V.C.; Simeone, M.L.F.; da Costa Parrella, R.A.; et al. (Embrapa Milho e Sorgo, Brasília, DF, Brazil) Manejo do Pulgão da Cana-de-Açúcar (Melanaphis sacchari/sorghi) na Cultura do Sorgo. Comun. Técnico 2021, 249, 1–24. [Google Scholar]
- Van Den Berg, J. Status of Resistance of Sorghum Hybrids to the Aphid, Melanaphis sacchari (Zehntner) (Homoptera: Aphididae). S. Afr. J. Plant Soil 2002, 19, 151–155. [Google Scholar] [CrossRef]
- Guimarães, C.C.; Simeone, M.L.F.; Parrella, R.A.; Sena, M.M. Use of NIRS to Predict Composition and Bioethanol Yield from Cell Wall Structural Components of Sweet Sorghum Biomass. Microchem. J. 2014, 117, 194–201. [Google Scholar] [CrossRef]
- Nogueira, A.R.d.A.; Souza, G.B. Manual de Laboratório: Solo, Água, Nutrição Vegetal, Nutrição Animal e Alimentos; Embrapa Pecuária Sudeste: São Carlos, Brazil, 2005; 334p. [Google Scholar]
- Ferreira, D.F. SISVAR: A Computer Analysis System to Fixed Effects Split Plot Type Designs. Braz. J. Biometrics 2019, 37, 529–535. [Google Scholar] [CrossRef]
- Şahin, M.; Aybek, E. Jamovi: An Easy to Use Statistical Software for the Social Scientists. Int. J. Assess. Tools Educ. 2019, 6, 670–692. [Google Scholar] [CrossRef]
- Minitab LLC. Minitab, Version 21; Minitab LLC: State College, PA, USA, 2021.
- Harizanova, A.S. Silicon Application Unveiled: A Review of Insights into Plant Defense Mechanisms Under Biotic Challenges. Agrar. Nauk. 2024, 16, 41. [Google Scholar] [CrossRef]
- Pereira, S.; Monteiro, A.; Moutinho-Pereira, J.; Dinis, L.T. Silicon, an Emergent Strategy to Lighten the Effects of (A)biotic Stresses on Crops: A Review. J. Agron. Crop Sci. 2024, 210, e12762. [Google Scholar] [CrossRef]
- Djikanović, D.; Jovanović, J.; Kalauzi, A.; Maksimović, J.D.; Radotić, K. Effects of Silicon Concentration and Synthesis Duration on Lignin Structure: A Spectroscopic and Microscopic Study. Biopolymers 2025, 116, e23640. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Bahrami-Rad, S.; Poschenrieder, C. Silicon Modifies Both a Local Response and a Systemic Response to Mechanical Stress in Tobacco Leaves. Biol. Plant. 2017, 61, 187–191. [Google Scholar] [CrossRef]
- Vega, I.; Rumpel, C.; Ruíz, A.; Mora, M.D.L.L.; Calderini, D.F.; Cartes, P. Silicon Modulates the Production and Composition of Phenols in Barley Under Aluminum Stress. Agronomy 2020, 10, 1138. [Google Scholar] [CrossRef]
- Zhang, Z.; Rao, Y.; Ye, M.; Zou, D.; Liu, R.; Liu, Y. Reduction of Inhibitory Effect of Lignin via Degradation and Improvement of Anaerobic Digestion Performance of Maize Straw by Oxidative Pretreatment with Fe2+-Activated Persulfate. Renew. Energy 2024, 231, 120795. [Google Scholar] [CrossRef]
- Baez, L.A.; Tichá, T.; Hamann, T. Cell Wall Integrity Regulation Across Plant Species. Plant Mol. Biol. 2022, 109, 483–504. [Google Scholar] [CrossRef]
- Xue, Y.; Li, H.; Kang, X. Molecular Unraveling of Polysaccharide Digestion in Wood-Feeding Termites: A Solid-State NMR Perspective. Carbohydr. Polym. 2024, 331, 121843. [Google Scholar] [CrossRef]
- Jang, S.K.; Jeong, H.; Choi, I.G. The Effect of Cellulose Crystalline Structure Modification on Glucose Production from Chemical-Composition-Controlled Biomass. Sustainability 2023, 15, 5869. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Jincy, E.M.; Femina, K.S. Heteropolymer in Biomass: Hemicellulose Extraction and Modifications. In Handbook of Biomass; Springer: Singapore, 2023; pp. 1–32. [Google Scholar] [CrossRef]
- Rodrigues, P.D.O.; Corrêa, A.G.; Baffi, M.A.; Pasquini, D. Potential Applications of Hemicellulose. In Handbook of Biomass; Springer: Singapore, 2024; pp. 697–727. [Google Scholar] [CrossRef]
- Nechita, P.; Roman, M.; Năstac, S.M. Green Approaches on Modification of Xylan Hemicellulose to Enhance the Functional Properties for Food Packaging Materials—A Review. Polymers 2023, 15, 2088. [Google Scholar] [CrossRef]
- Zexer, N.; Kumar, S.; Elbaum, R. Silica Deposition in Plants: Scaffolding the Mineralization. Ann. Bot. 2023, 131, 897–908. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, V.; Sharma, A. Interaction of Silicon with Cell Wall Components in Plants: A Review. J. Appl. Nat. Sci. 2023, 15, 480–497. [Google Scholar] [CrossRef]
- Coutinho, W.B.G.; da Silva, F.C.; Barrigossi, J.A.F.; de Sousa Almeida, A.C.; Gonçalves de Jesus, F. Silicon Applications in Rice Plants Alter the Stylet Probing Behaviors of Glyphepomis spinosa (Hemiptera: Pentatomidae). J. Insect Sci. 2024, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Tenguri, P.; Chander, S.; Ellur, R.K.; Patil, E.; Sundaran, A.P.; Yele, Y.; Thube, S.H.; Naik, V.C.; Nagaraju, M.T. Effect of Silicon Application on Rice Plant Defence Against Brown Planthopper Feeding Under Climate Change Conditions. Anim. Biol. 2024, 74, 163–179. [Google Scholar] [CrossRef]
- Kumar, P.T. A Comprehensive Review of Nano-Sized Silica’s Effects on Plant Growth, Molecular Responses, and Biochemical Changes. J. Adv. Biol. Biotechnol. 2024, 27, 421–433. [Google Scholar] [CrossRef]
- Johnson, S.N.; Waterman, J.M.; Hartley, S.E.; Cooke, J.; Ryalls, J.M.; Lagisz, M.; Nakagawa, S. Plant Silicon Defences Suppress Herbivore Performance, but Mode of Feeding Is Key. Ecol. Lett. 2024, 27, e14519. [Google Scholar] [CrossRef]
- Putra, R.; Bünker, M.; Müller, C. Role of Silicon in Legume-Insect Interactions: Insights from a Plant Experiencing Different Levels of Herbivory. Funct. Ecol. 2024, 38, 2693–2708. [Google Scholar] [CrossRef]
- Jiang, Y.; Qi, X.X.; Wang, Z.H.; Liu, X.D.; Han, Y.L.; Li, H.; Wang, Y. Effect of Different Forms of Silicon Application on Wheat Aphid Resistance. Agric. Res. 2023, 12, 179–188. [Google Scholar] [CrossRef]
- Rahman, A.; Hussain, F.; Majeed, S.; Nazeer, M.U.; Sharif, R.M.S.; Asif, M.; Sattar, W. Silicon Applications on Yield Attributes of Wheat (Triticum aestivum L.) and Biology of Wheat-Aphid (Schizaphis Graminum). Silicon 2024, 16, 3165–3172. [Google Scholar] [CrossRef]
- Deka, M.K.; Kalita, P. Impact of Foliar Application of Silicic Acid on Aphid Population Growth, Gas Exchange Parameters and Yield of Rapeseed. Phytoparasitica 2024, 52, 65. [Google Scholar] [CrossRef]
- Ullah, M.S.; Mahmood, A.; Alawadi, H.F.N.; Seleiman, M.F.; Khan, B.A.; Javaid, M.M.; Wasonga, D.O. Silicon-Mediated Modulation of Maize Growth, Metabolic Responses, and Antioxidant Mechanisms under Saline Conditions. BMC Plant Biol. 2025, 25, 3. [Google Scholar] [CrossRef]
- Huang, L.; Tan, Z.; Lei, K.; Wang, L.; Shen, W.; Teng, W. Effect of Silicon Addition on the Growth and Photosynthesis of Castanopsis Hystrix in Manganese Stress. Not. Bot. Horti. Agrobot. Cluj Napoca 2024, 52, 13930. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, M.; Li, T.; Yuan, L.; Zhang, A.; Jiang, D.; Yan, S. Silicon Supplementation Improves Biomass and Direct Defense of Ryegrass: A Multi-Omics Study. Ind. Crops Prod. 2023, 204, 117357. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, X.; Ding, C.; Xia, M.; Xue, R.; Sun, Z.; Chen, D.; Zhu-Salzman, K.; Zeng, R.; Song, Y. Priming of Rice Defense Against a Sap-Sucking Insect Pest Brown Planthopper by Silicon. J. Pest Sci. 2022, 95, 1371–1385. [Google Scholar] [CrossRef]
- Rincón-López, B.; Naveda, A.F.; Lara, U.A.; Hernández, M.E.T.; Tafolla-Arellano, J.C. Sorghum and Aphid (Melanaphis sacchari) Interaction: Plant Physiology, Breeding, and Molecular Overview. In Biocontrol Systems and Plant Physiology in Modern Agriculture; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 237–256. [Google Scholar]
- Du, J.L.; Wu, D.G.; Li, J.Q.; Zhan, Q.W.; Huang, S.C.; Huang, B.H.; Wang, X. Effects of Aphid Disoperation on Photosynthetic Performance and Agronomic Traits of Different Sorghum Varieties. Pak. J. Bot. 2021, 53, 2275–2285. [Google Scholar] [CrossRef]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon with Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Costa, M.G.; Prado, R.d.M.; Sarah, M.M.S.; Palaretti, L.F.; Piccolo, M.d.C.; Souza Júnior, J.P. New Approaches to the Effects of Si on Sugarcane Ratoon Under Irrigation in Quartzipsamments, Eutrophic Red Oxisol, and Dystrophic Red Oxisol. BMC Plant Biol. 2023, 23, 51. [Google Scholar] [CrossRef]
- Costa, M.G.; Prado, R.D.M.; Santos Sarah, M.M.; Souza Júnior, J.P.; de Souza, A.E.S. Silicon, by Promoting a Homeostatic Balance of C:N:P and Nutrient Use Efficiency, Attenuates K Deficiency, Favoring Sustainable Bean Cultivation. BMC Plant Biol. 2023, 23, 213. [Google Scholar] [CrossRef]
- Costa, M.G.; Mello Prado, R.; Palaretti, L.F.; de Souza Júnior, J.P. The Effect of Abiotic Stresses on Plant C:N:P Homeostasis and Their Mitigation by Silicon. Crop J. 2024, 12, 340–353. [Google Scholar] [CrossRef]
- Barão, L.; Teixeira, R.F. Interaction of Silicon-Phosphorus: The Unexplored Connection in the Soil Ecosystem. Preprints 2024, 2024100945. [Google Scholar] [CrossRef]
- Kostic, I.; Nikolic, N.; Milanovic, S.; Milenkovic, I.; Pavlovic, J.; Paravinja, A.; Nikolic, M. Silicon Modifies Leaf Nutriome and Improves Growth of Oak Seedlings Exposed to Phosphorus Deficiency and Phytophthora plurivora Infection. Front. Plant Sci. 2023, 14, 1265782. [Google Scholar] [CrossRef]
- Coquerel, R.; Arkoun, M.; Trouverie, J.; Bernay, B.; Laîné, P.; Etienne, P. Ionomic and Proteomic Changes Highlight the Effect of Silicon Supply on the Nodules Functioning of Trifolium incarnatum L. Front. Plant Sci. 2024, 15, 1462149. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Zehra, A.; Wani, K.I.; Naeem, M.; Hakeem, K.R.; Aftab, T. The Role of Micronutrients in Growth and Development: Transport and Signalling Pathways from Crosstalk Perspective. In Plant Micronutrients: Deficiency and Toxicity Management; Springer: Cham, Switzerland, 2020; pp. 73–81. [Google Scholar] [CrossRef]
- Nietupski, M.; Ludwiczak, E.; Olszewski, J.; Gabryś, B.; Kordan, B. Effect of Aphid Foraging on the Intensity of Photosynthesis and Transpiration of Selected Crop Plants in Its Early Stages of Growing. Agronomy 2022, 12, 2370. [Google Scholar] [CrossRef]
- Ramovha, D. Increased Cell Wall Activity on Tugela Dn1 Wheat Cultivar Infested with Russian Wheat Aphid Biotype 2; University of the Free State: Bloemfontein, South Africa, 2021. [Google Scholar] [CrossRef]
- Wang, Y.; Di, B.; Sun, Z.; Sonali; Donovan-Mak, M.; Chen, Z.H.; Wang, M.Q. Multi-Omics and Physiological Analysis Reveal Crosstalk Between Aphid Resistance and Nitrogen Fertilization in Wheat. Plant Cell Environ. 2024, 47, 2024–2039. [Google Scholar] [CrossRef]
- Srihari, P.; Mehera, B.; Swaroop, B.T.; Kumar, P. Effect of Iron and Silicon on Growth and Yield of Sorghum (Sorghum bicolor L.). Int. J. Environ. Clim. Change 2023, 13, 630–636. [Google Scholar] [CrossRef]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J. Is Silicon a Panacea for Alleviating Drought and Salt Stress in Crops? Front. Plant Sci. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Thorne, S.J.; Stirnberg, P.M.; Hartley, S.E.; Maathuis, F.J. The Ability of Silicon Fertilisation to Alleviate Salinity Stress in Rice Is Critically Dependent on Cultivar. Rice 2022, 15, 8. [Google Scholar] [CrossRef]
- Toledo-Hernández, E.; Peña-Chora, G.; Mancilla-Dorantes, I.; Torres-Rojas, F.I.; Romero-Ramírez, Y.; Palemón-Alberto, F.; Ortega-Acosta, S.Á.; Delgado-Núñez, E.J.; Salinas-Sánchez, D.O.; Tagle-Emigdio, L.J.; et al. A Review of Biological Control One Decade After the Sorghum Aphid (Melanaphis sorghi) Outbreak. Plants 2024, 13, 2873. [Google Scholar] [CrossRef]
- Domingues, R.F.; Barbosa, M.S.; Sampaio, M.V. Silicon amendment to the crop increases the potential of Aphidius platensis to control the invasive pest aphid Melanaphis sorghi. Pest Manag Sci. 2025; ahead of print. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).