Mechanisms of Impact of Alnus ferdinandi-coburgii Odor Substances on Host Location of Tomicus yunnanensis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Tissue Collection
2.2. Identification of Chemosensory-Related Genes of T. yunnanensis
2.3. Sequence Analysis and Phylogenetic Tree Construction
2.4. Influence of Non-Host Odorants on Chemosensory-Related Genes of T. yunnanensis
2.5. Molecular Docking and Molecular Dynamic Simulation
3. Results
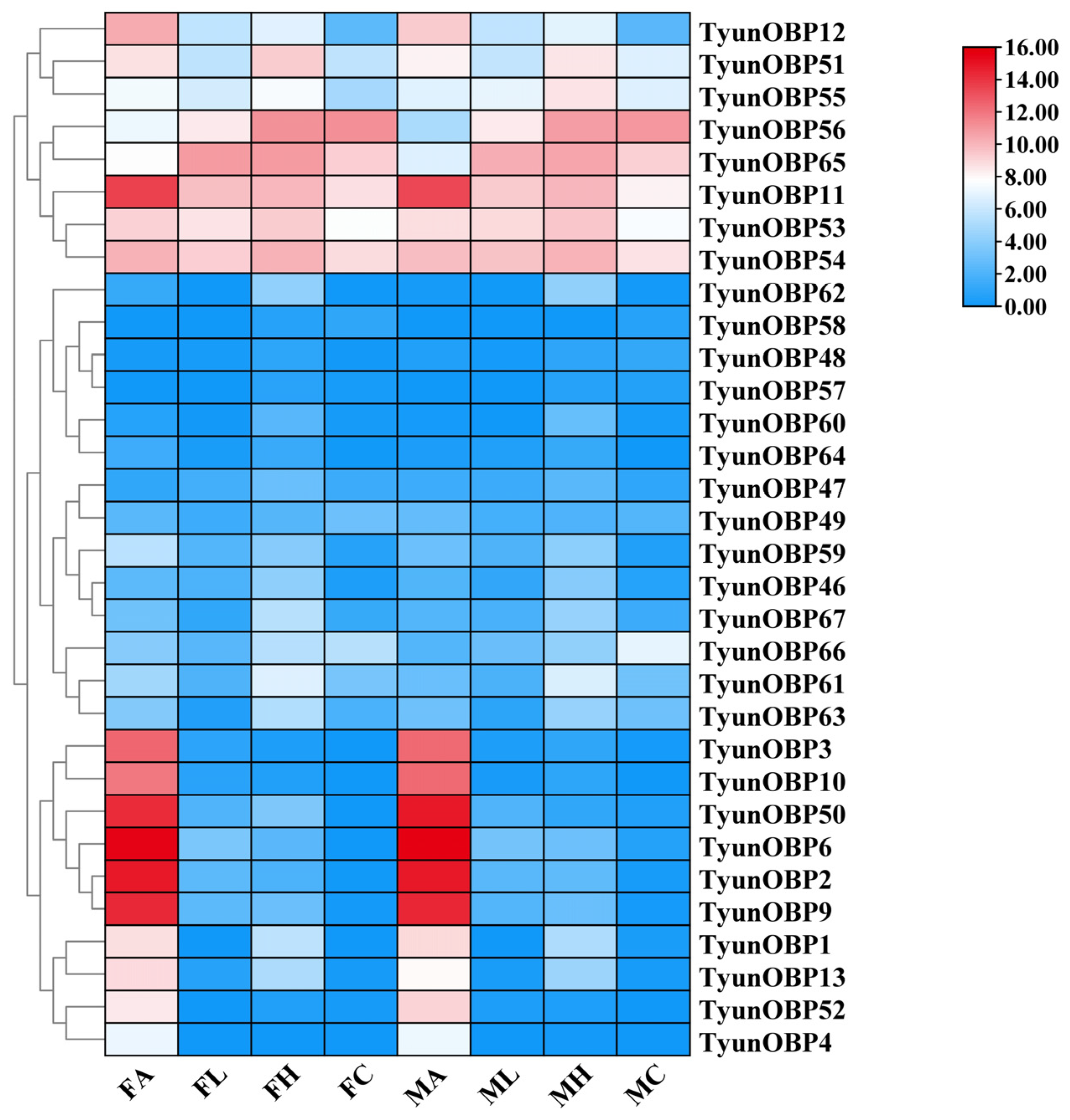
3.1. Basic Information and Expression Patterns of Chemosensory-Related Genes
3.2. Phylogenetic Analysis of Chemosensory-Related Proteins
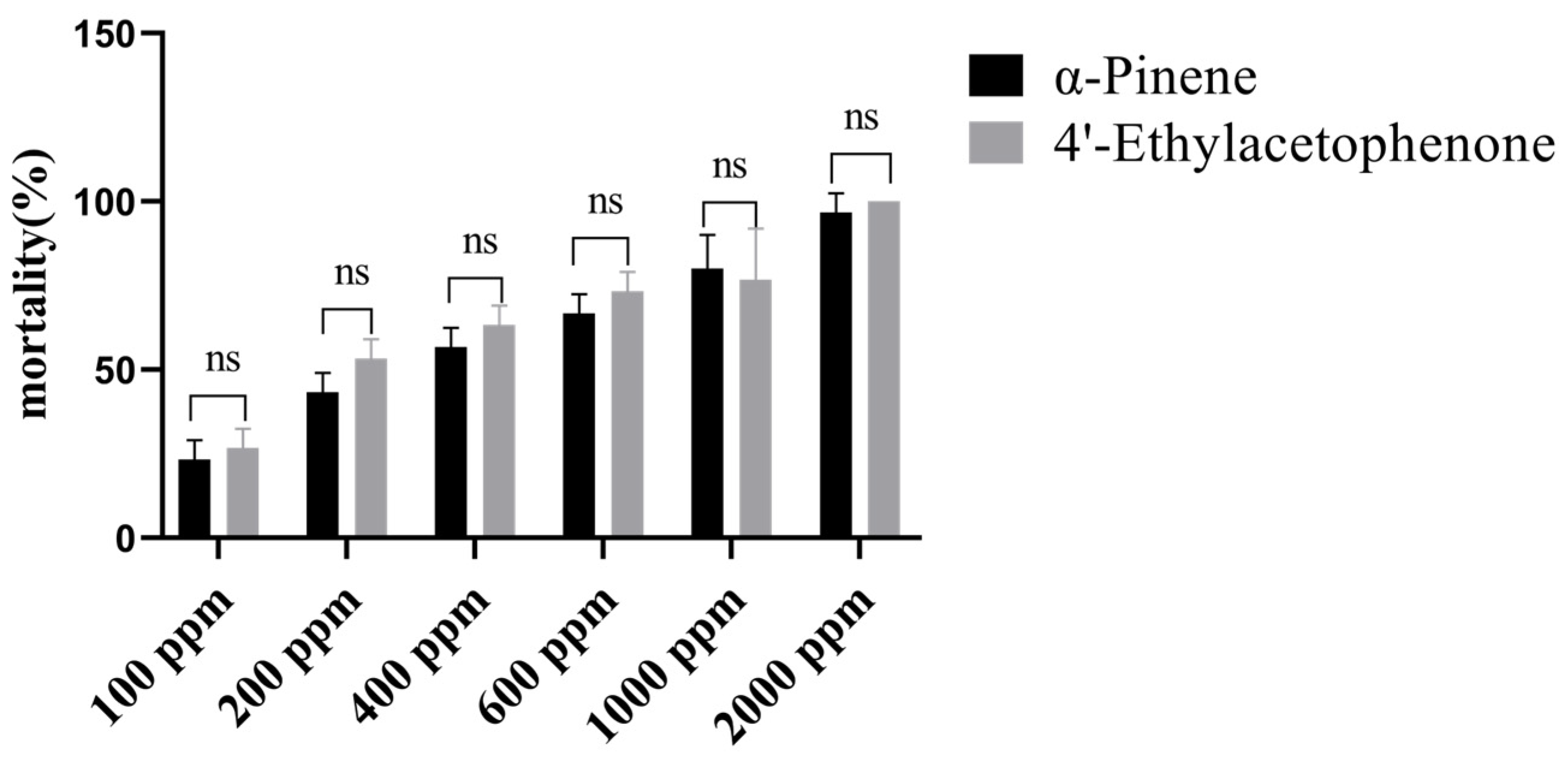
3.3. Compound Concentration Screening
3.4. Expression of Chemosensory-Related Genes Was Affected by Odor Substances
3.5. Analysis of Binding Information Between Compounds and Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OBP | odorant-binding proteins |
| T. yunnanensis | Tomicus yunnanensis |
| CSP | chemosensory protein |
| OR | olfactory receptor |
| GR | gustatory receptor |
| IR | ionotropic receptor |
| SNMP | sensory neuron membrane protein |
| ODEs | odorant-degrading enzymes |
| CYP | cytochrome P450 monooxygenase |
| GST | glutathione S-transferase |
| CCE | carboxylesterase |
| aa | amino acid |
| ORFs | open reading frames |
References
- Qie, X.; Yan, X.; Wang, H.; Li, F.; Hu, L.; Hao, C.; Ma, L. Identification, expression profiles, and binding properties of chemosensory protein 18 in Plutella xylostella (Lepidoptera: Plutellidae). J. Insect Sci. 2024, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Smadja, C.; Butlin, R.K. On the scent of speciation: The chemosensory system and its role in premating isolation. Heredity 2009, 102, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.H.; Hanada, T.; Hayashi, Y.; Shigenobu, S.; Maekawa, K.; Hojo, M.K. Gene expression profiles of chemosensory genes of termite soldier and worker antennae. Insect Mol. Biol. 2023, 32, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, W.; Wang, S.; Miao, W.; Liu, Z.; Wu, F.; Wang, J.; Sheng, S. Binding characteristics and structural dynamics of two general odorant-binding proteins with plant volatiles in the olfactory recognition of Glyphodes pyloalis. Insect Biochem. Mol. Biol. 2024, 173, 104177. [Google Scholar] [CrossRef]
- Hao, E.; Yang, X.; Ma, M.; Lu, P.; Qiao, H. Investigating SnocCSP4 expression and key compound interactions with SnocOBP4 in Sirex noctilio Fabricius (Hymenoptera: Siricidae). Int. J. Biol. Macromol. 2023, 247, 125827. [Google Scholar] [CrossRef]
- Han, X.; Weng, M.; Shi, W.; Wen, Y.; Long, Y.; Hu, X.; Ji, G.; Zhu, Y.; Wen, X.; Zhang, F.; et al. The Neurotranscriptome of Monochamus alternatus. Int. J. Mol. Sci. 2024, 25, 4553. [Google Scholar] [CrossRef]
- Li, J.-B.; Yin, M.-Z.; Yao, W.-C.; Ma, S.; Dewer, Y.; Liu, X.-Z.; Wang, Y.-Y.; Wang, C.-W.; Li, B.-P.; Zhu, X.-Y. Genome-Wide Analysis of Odorant-Binding Proteins and Chemosensory Proteins in the Bean bug Riptortus pedestris. Front. Physiol. 2022, 13, 949607. [Google Scholar] [CrossRef]
- Brown, N.C.; Gordon, B.; McDonough-Goldstein, C.E.; Misra, S.; Findlay, G.D.; Clark, A.G.; Wolfner, M.F. The seminal odorant binding protein Obp56g is required for mating plug formation and male fertility in Drosophila melanogaster. Elife 2023, 12, e86409. [Google Scholar] [CrossRef]
- Vogt, R.G.; Riddiford, L.M. Pheromone binding and inactivation by moth antennae. Nature 1981, 293, 161–163. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Z.; Cui, G.; Du, Z.; Qian, Y.; Yang, S.; Liu, M.; Guo, J. Binding Properties of Odorant-Binding Protein 4 of Tirathaba rufivena to Areca catechu Volatiles. Plants 2022, 11, 167. [Google Scholar] [CrossRef]
- Abendroth, J.A.; Moural, T.W.; Wei, H.; Zhu, F. Roles of insect odorant binding proteins in communication and xenobiotic adaptation. Front. Insect Sci. 2023, 3, 1274197. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, J.A.; Hamiaux, C.; Faraone, N.; Löfstedt, C.; Carraher, C. Structure of an antennally-expressed carboxylesterase suggests lepidopteran odorant degrading enzymes are broadly tuned. Curr. Res. Insect Sci. 2023, 3, 100062. [Google Scholar] [CrossRef] [PubMed]
- Rainio, M.J.; Margus, A.; Tikka, S.; Helander, M.; Lindström, L. The effects of short-term glyphosate-based herbicide exposure on insect gene expression profiles. J. Insect Physiol. 2023, 146, 104503. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Boichot, V.; Fraichard, S.; Muradova, M.; Senet, P.; Nicolai, A.; Lirussi, F.; Bas, M.; Canon, F.; Heydel, J.M.; et al. Role of insect and mammal glutathione transferases in chemoperception. Biomolecules 2023, 13, 322. [Google Scholar] [CrossRef]
- Kuang, Y.; Xiong, Y.; Chen, X.D.; Yu, X. Antennae-abundant expression of candidate cytochrome P450 genes associated with odorant degradation in the asian citrus psyllid, Diaphorina citri. Front. Physiol. 2022, 13, 1004192. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, X.; Gui, L.; Wang, F.; Zhang, G. Molecular and functional characterization of an antenna-enriched glutathione S-transferase BminGSTd3 involved in undecanol degradation in the citrus fruit fly, Bactrocera minax (Enderlein) (Diptera Tephritidae). Int. J. Biol. Macromol. 2024, 256, 128514. [Google Scholar] [CrossRef]
- Cruse, C.; Moural, T.W.; Zhu, F. Dynamic roles of insect carboxyl/cholinesterases in chemical adaptation. Insects 2023, 14, 194. [Google Scholar] [CrossRef]
- Shangguan, C.; Kuang, Y.; Gao, L.; Zhu, B.; Chen, X.D.; Yu, X. Antennae-enriched expression of candidate odorant degrading enzyme genes in the turnip aphid, Lipaphis erysimi. Front. Physiol. 2023, 14, 1228570. [Google Scholar] [CrossRef]
- Fraichard, S.; Legendre, A.; Lucas, P.; Chauvel, I.; Faure, P.; Neiers, F.; Artur, Y.; Briand, L.; Ferveur, J.F.; Heydel, J.M. Modulation of sex pheromone discrimination by a UDP-Glycosyltransferase in Drosophila melanogaster. Genes 2020, 11, 237. [Google Scholar] [CrossRef]
- Xuan, N.; Guo, X.; Xie, H.-Y.; Lou, Q.-N.; Lu, X.-B.; Liu, G.-X.; Picimbon, J.-F. Increased expression of CSP and CYP genes in adult silkworm females exposed to avermectins. Insect Sci. 2015, 22, 203–219. [Google Scholar] [CrossRef]
- Steiner, C.; Chertemps, T.; Maïbèche, M. Diversity of biotransformation enzymes in insect antennae: Possible roles in odorant inactivation and xenobiotic processing. In Olfactory Concepts of Insect Control—Alternative to Insecticides; Picimbon, J.-F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; Volume 2, pp. 115–145. [Google Scholar]
- Guo, X.; Xuan, N.; Liu, G.; Xie, H.; Lou, Q.; Arnaud, P.; Offmann, B.; Picimbon, J.-F. An expanded survey of the moth PBP/GOBP clade in bombyx mori: New insight into expression and functional roles. Front. Physiol. 2021, 12, 712593. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.X.; Liu, F.; Zhang, S.F.; Kong, X.B.; Zhang, Z. Semiochemical regulation of the intraspecific and interspecific behavior of Tomicus yunnanensis and Tomicus minor during the shoot-feeding phase. J. Chem. Ecol. 2019, 45, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Ye, H.; Wang, H.; Clarke, S.R.; Jun, L. Response of Tomicus yunnanensis (Coleoptera: Scolytinae) to Infested and Uninfested Pinus yunnanensis Bolts. J. Econ. Entomol. 2010, 103, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-Y.; Zhao, N.; Yang, B. Global transcriptome profiling of the pine shoot beetle, Tomicus yunnanensis (Coleoptera: Scolytinae). PLoS ONE 2012, 7, e32291. [Google Scholar] [CrossRef]
- Lu, T.-T.; Yin, N.-N.; Yang, A.-J.; Yao, Y.-J.; Li, Z.-Q.; Liu, N.-Y. Comparative transcriptomics reveals the conservation and divergence of reproductive genes across three sympatric Tomicus bark beetles. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 49, 101168. [Google Scholar] [CrossRef]
- Yue, F.; Yang, B.; Feng, D.; Zhou, X. Study on the effect of mixed Pinus yunnanensis forests on the resistance to Tomicus yunnanensis. Jiangsu Agric. Sci. 2011, 39, 3. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, N.; Ze, S.; Yang, B. Interruption of host-location behavior in the Yunnan pine shoot beetle, Tomicus yunnanesis (Coleoptera: Scolytidae), with three green leaf volatiles. Acta Entomol. Sin. 2013, 56, 570–574. [Google Scholar]
- Yue, F.; Yang, B.; Zhou, Q. Effects of 2 kinds of non-hosts essential oil on olfactory behavior of Tomicus yunnanensis. Southwest China J. Agric. Sci. 2013, 26, 4. [Google Scholar] [CrossRef]
- Liu, J. Effects and Evaluation of Plant Volatiles of Alnus Ferdinandi-Coburgii on Ovary and Post-Embrtonic Development of Tomicus Yunnanensis (Coleoptera: Olytidae); Southwest Forest University: Kunming, China, 2019. [Google Scholar]
- Ze, S.; Ji, M.; Yang, B.; Zhao, N.; Zhu, J.; Wang, D.; Hu, L.; Wang, L.; Lin, X.; Huang, R. A method of using Alnus ferdinandi-coburgii to control Tomicus yunnanensis. CN201410434687.X, 10 December 2014. [Google Scholar]
- Li, W.; Yang, B.; Liu, N.; Zhu, J.; Li, Z.; Ze, S.; Yu, J.; Zhao, N. Identification and characterization of the detoxification genes based on the transcriptome of Tomicus yunnanensis. Diversity 2022, 14, 23. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.J.; Chen, W.-H.; Hu, S. Evolview v2: An online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016, 44, W236–W241. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Ma, M.; Wang, C.; Shi, Q.; Zhang, R.; Chen, H. Cytochrome P450s from the Chinese white pine beetle, Dendroctonus armandi (Curculionidae: Scolytinae): Expression profiles of different stages and responses to host allelochemicals. Insect Biochem. Mol. Biol. 2015, 65, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, T.; Khashaveh, A.; Yi, C.; Liu, X.; Zhang, Y. Identification and Evaluation of Suitable Reference Genes for RT-qPCR Analysis in Hippodamia variegata (Coleoptera: Coccinellidae) Under Different Biotic and Abiotic Conditions. Front. Physiol. 2021, 12, 669510. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Mooers, B.H.M.; Brown, M.E. Templates for writing PyMOL scripts. Protein Sci. A Publ. Protein Society 2021, 30, 262–269. [Google Scholar] [CrossRef]
- Kim, H.; Fábián, B.; Hummer, G. Neighbor list artifacts in molecular dynamics simulations. J. Chem. Theory Comput. 2023, 19, 8919–8929. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L., III; Mackerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Simmonett, A.C.; Brooks, B.R. A compression strategy for particle mesh Ewald theory. J. Chem. Phys. 2021, 154, 054112. [Google Scholar] [CrossRef]
- Su, J.; Sun, T.; Wang, Y.; Shen, Y. Conformational dynamics of glucagon-like peptide-2 with different electric field. Polymers 2022, 14, 2722. [Google Scholar] [CrossRef]
- Zhao, N.; Li, K.; Ma, H.; Hu, L.; Yang, Y.; Liu, L. Molecular characterization of odorant-binding protein genes associated with host-seeking behavior in Oides leucomelaena. Int. J. Mol. Sci. 2024, 25, 9436. [Google Scholar] [CrossRef]
- Yu, H.; Nong, X.; Huang, W.; Bhanumas, C.; Deng, X.; Ding, Y.; Liu, W. Odorant-binding and chemosensory proteins in fig wasps: Evolutionary insights from comparative studies. J. Mol. Evol. 2024, 92, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Dippel, S.; Oberhofer, G.; Kahnt, J.; Gerischer, L.; Opitz, L.; Schachtner, J.; Stanke, M.; Schütz, S.; Wimmer, E.A.; Angeli, S. Tissue-specific transcriptomics, chromosomal localization, and phylogeny of chemosensory and odorant binding proteins from the red flour beetle Tribolium castaneum reveal subgroup specificities for olfaction or more general functions. BMC Genom. 2014, 15, 1141. [Google Scholar] [CrossRef] [PubMed]
- Engsontia, P.; Sanderson, A.P.; Cobb, M.; Walden, K.K.O.; Robertson, H.M.; Brown, S. The red flour beetle’s large nose: An expanded odorant receptor gene family in Tribolium castaneum. Insect Biochem. Mol. Biol. 2008, 38, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Pei, L.; Gu, S.; Zhu, S.; Wang, Y.; Zhang, Y.; Li, B. Glutathione S-transferase (GST) genes in the red flour beetle, Tribolium castaneum, and comparative analysis with five additional insects. Genomics 2012, 100, 327–335. [Google Scholar] [CrossRef]
- Zhu, F.; Moural, T.W.; Shah, K.; Palli, S.R. Integrated analysis of cytochrome P450 gene superfamily in the red flour beetle, Tribolium castaneum. BMC Genom. 2013, 14, 174. [Google Scholar] [CrossRef]
- Keeling, C.I.; Henderson, H.; Li, M.; Yuen, M.; Clark, E.L.; Fraser, J.D.; Huber, D.P.W.; Liao, N.Y.; Roderick Docking, T.; Birol, I.; et al. Transcriptome and full-length cDNA resources for the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major insect pest of pine forests. Insect Biochem. Mol. Biol. 2012, 42, 525–536. [Google Scholar] [CrossRef]
- Liu, N.-Y.; Li, Z.-B.; Zhao, N.; Song, Q.-S.; Zhu, J.-Y.; Yang, B. Identification and characterization of chemosensory gene families in the bark beetle, Tomicus yunnanensis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 25, 73–85. [Google Scholar] [CrossRef]
- Han, W.K.; Tang, F.X.; Yan, Y.Y.; Wang, Y.; Zhang, Y.X.; Yu, N.; Wang, K.; Liu, Z.W. An OBP gene highly expressed in non-chemosensory tissues affects the phototaxis and reproduction of Spodoptera frugiperda. Insect Mol. Biol. 2024, 33, 81–90. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, M.; Eleftherianos, I.; Mohamed, A.; Cao, Y.; Song, B.; Zang, L.S.; Jia, C.; Bian, J.; Keyhani, N.O.; et al. An odorant binding protein is involved in counteracting detection-avoidance and Toll-pathway innate immunity. J. Adv. Res. 2023, 48, 1–16. [Google Scholar] [CrossRef]
- Andersson, M.N.; Keeling, C.I.; Mitchell, R.F. Genomic content of chemosensory genes correlates with host range in wood-boring beetles (Dendroctonus ponderosae, Agrilus planipennis, and Anoplophora glabripennis). BMC Genom. 2019, 20, 690. [Google Scholar] [CrossRef]
- Li, Z.; Dai, L.; Chu, H.; Fu, D.; Sun, Y.; Chen, H. Identification, expression patterns, and functional characterization of chemosensory proteins in Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae). Front. Physiol. 2018, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.N.; Grosse-Wilde, E.; Keeling, C.I.; Bengtsson, J.M.; Yuen, M.M.S.; Li, M.; Hillbur, Y.; Bohlmann, J.; Hansson, B.S.; Schlyter, F. Antennal transcriptome analysis of the chemosensory gene families in the tree killing bark beetles, Ips typographus and Dendroctonus ponderosae(Coleoptera: Curculionidae: Scolytinae). BMC Genom. 2013, 14, 198. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Jiang, H.-B.; Fan, J.-Y.; Liu, T.-Y.; Meng, L.-W.; Liu, Y.; Yu, H.-Z.; Dou, W.; Wang, J.-J. An odorant-binding protein of Asian citrus psyllid, Diaphorina citri, participates in the response of host plant volatiles. Pest Manag. Sci. 2021, 77, 3068–3079. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Mang, D.; Purba, E.R.; Ye, J.; Qian, J.; Rao, F.; Wang, H.; Wu, Z.; Zhang, W.; Zheng, Y.; et al. Identification and Functional Analysis of Odorant Binding Proteins in Apriona germari (Hope). J. Agric. Food Chem. 2024, 72, 17248–17259. [Google Scholar] [CrossRef]
- Li, L.; Wu, L.; Xu, Y.; Liu, F.; Zhao, H. Three odorant-binding proteins of small hive beetles, Aethina tumida, participate in the response of bee colony volatiles. Int. J. Biol. Macromol. 2024, 278, 134905. [Google Scholar] [CrossRef]
- Reid, M.L.; Purcell, J.R.C. Condition-dependent tolerance of monoterpenes in an insect herbivore. Arthropod-Plant Interact. 2011, 5, 331–337. [Google Scholar] [CrossRef]
- Adams, B.; Yusuf, A.A.; Torto, B.; Khamis, F.M. Non-host plant odors influence the tritrophic interaction between tomato, its foliar herbivore Tuta absoluta and mirid predator Nesidiocoris tenuis. Front. Plant Sci. 2023, 14, 1014865. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Liu, H.; Guo, M.; Deng, J. Inhibition Effect of non-host plant volatile extracts on reproductive behaviors in the Diamondback Moth Plutella xylostella (Linnaeus). Insects 2024, 15, 227. [Google Scholar] [CrossRef]
- López, M.F.; Cano-Ramírez, C.; Cesar-Ayala, A.K.; Ruiz, E.A.; Zúñiga, G. Diversity and expression of P450 genes from Dendroctonus valens LeConte (Curculionidae: Scolytinae) in response to different kairomones. Insect Biochem. Mol. Biol. 2013, 43, 417–432. [Google Scholar] [CrossRef]
- Cano-Ramírez, C.; López, M.F.; Cesar-Ayala, A.K.; Pineda-Martínez, V.; Sullivan, B.T.; Zúñiga, G. Isolation and expression of cytochrome P450 genes in the antennae and gut of pine beetle Dendroctonus rhizophagus (Curculionidae: Scolytinae) following exposure to host monoterpenes. Gene 2013, 520, 47–63. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, G.Y.; Li, L.; Song, Q.S.; Stanley, D.; Wei, S.J.; Zhu, J.Y. Genome-wide and expression-profiling analyses of the cytochrome P450 genes in Tenebrionidea. Arch. Insect Biochem. Physiol. 2022, 111, e21954. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wang, C.; Zhang, X.; Yu, J.; Zhang, R.; Chen, H. Two CYP4 genes of the Chinese white pine beetle, Dendroctonus armandi (Curculionidae: Scolytinae), and their transcript levels under different development stages and treatments. Insect Mol. Biol. 2014, 23, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, H. Disruption of CYP6DF1 and CYP6DJ2 increases the susceptibility of Dendroctonus armandi to (+)-α-pinene. Pestic. Biochem. Physiol. 2022, 188, 105270. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, D.; Ning, H.; Tang, M.; Chen, H. Knockdown of CYP6CR2 and CYP6DE5 reduces tolerance to host plant allelochemicals in the Chinese white pine beetle Dendroctonus armandi. Pestic. Biochem. Physiol. 2022, 187, 105180. [Google Scholar] [CrossRef]
- Feyereisen, R. 8—Insect CYP Genes and P450 Enzymes. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 236–316. [Google Scholar]
- Zhang, Y.; Chen, D.; Xu, Y.; Ma, L.; Du, M.; Li, P.; Yin, Z.; Xu, H.; Wu, X. Stereoselective toxicity mechanism of neonicotinoid dinotefuran in honeybees: New Perspective from a Spatial Metabolomics Study. Sci. Total Environ. 2022, 809, 151116. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, J.; Li, W.; Li, X.; Li, Z.; Mao, X.; Yang, B.; Zhao, N. Mechanisms of Impact of Alnus ferdinandi-coburgii Odor Substances on Host Location of Tomicus yunnanensis. Insects 2025, 16, 553. https://doi.org/10.3390/insects16060553
Bo J, Li W, Li X, Li Z, Mao X, Yang B, Zhao N. Mechanisms of Impact of Alnus ferdinandi-coburgii Odor Substances on Host Location of Tomicus yunnanensis. Insects. 2025; 16(6):553. https://doi.org/10.3390/insects16060553
Chicago/Turabian StyleBo, Jingyi, Wen Li, Xiangyi Li, Zongbo Li, Xiangzhong Mao, Bin Yang, and Ning Zhao. 2025. "Mechanisms of Impact of Alnus ferdinandi-coburgii Odor Substances on Host Location of Tomicus yunnanensis" Insects 16, no. 6: 553. https://doi.org/10.3390/insects16060553
APA StyleBo, J., Li, W., Li, X., Li, Z., Mao, X., Yang, B., & Zhao, N. (2025). Mechanisms of Impact of Alnus ferdinandi-coburgii Odor Substances on Host Location of Tomicus yunnanensis. Insects, 16(6), 553. https://doi.org/10.3390/insects16060553





