Detection of Di- and Tri-Locus kdr Mutations in Aedes aegypti and Aedes albopictus from Texas, USA, and the Implications for Insecticide Resistance
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
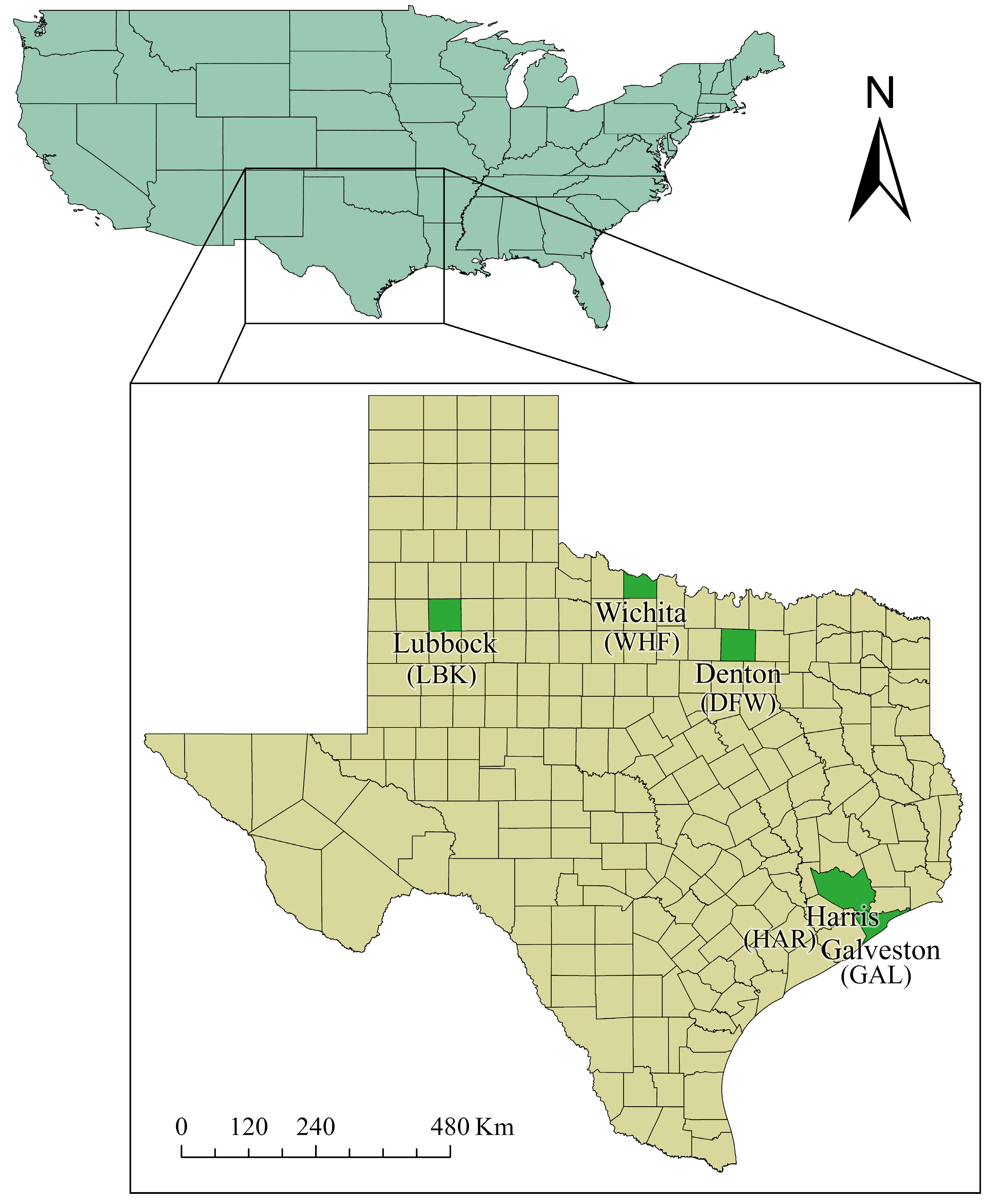
2.1. Mosquito Collection and Rearing
2.2. DNA Extraction
2.3. Knockdown Resistance Genotyping
2.4. Analysis
3. Results
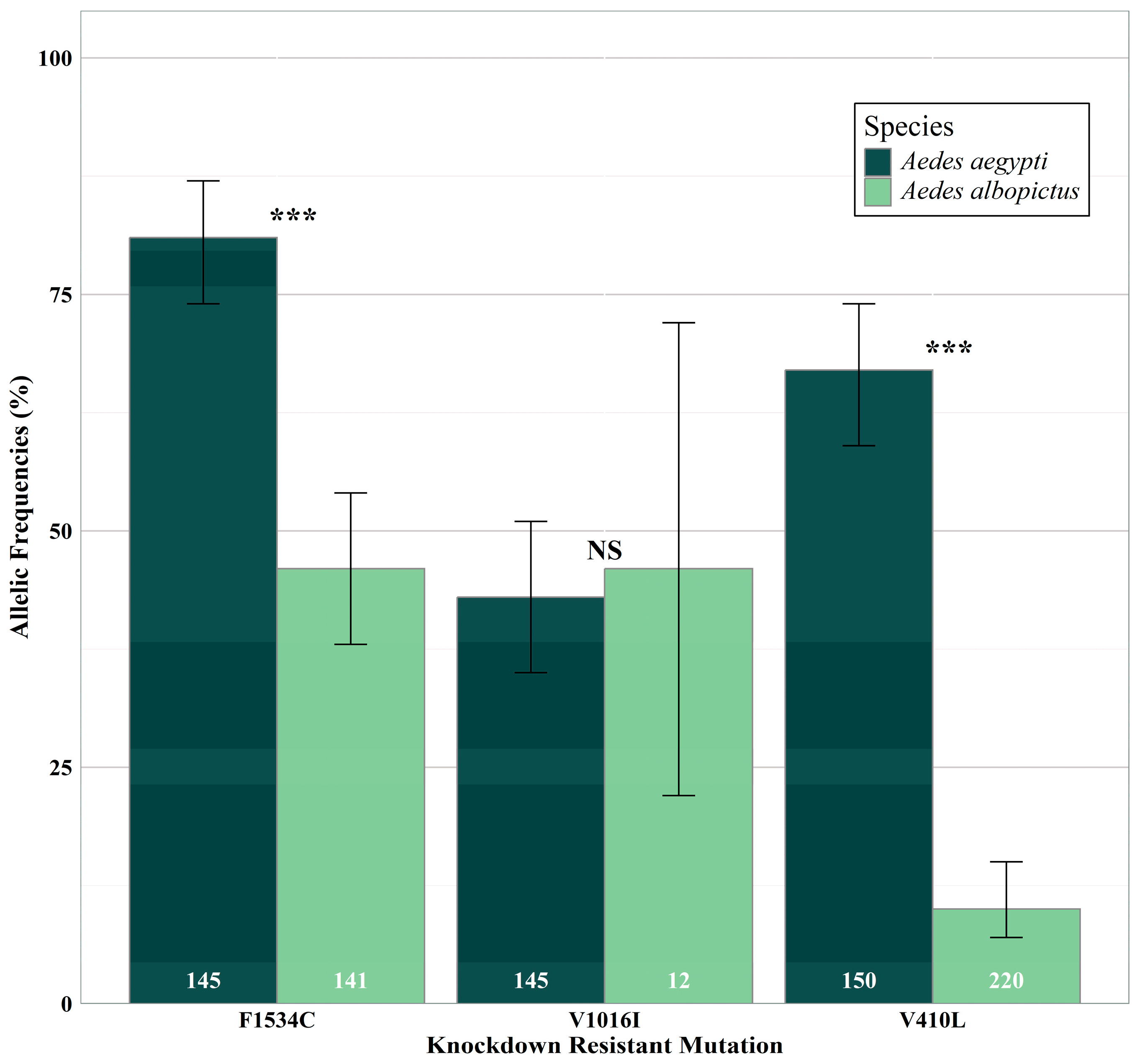
3.1. Species Differences
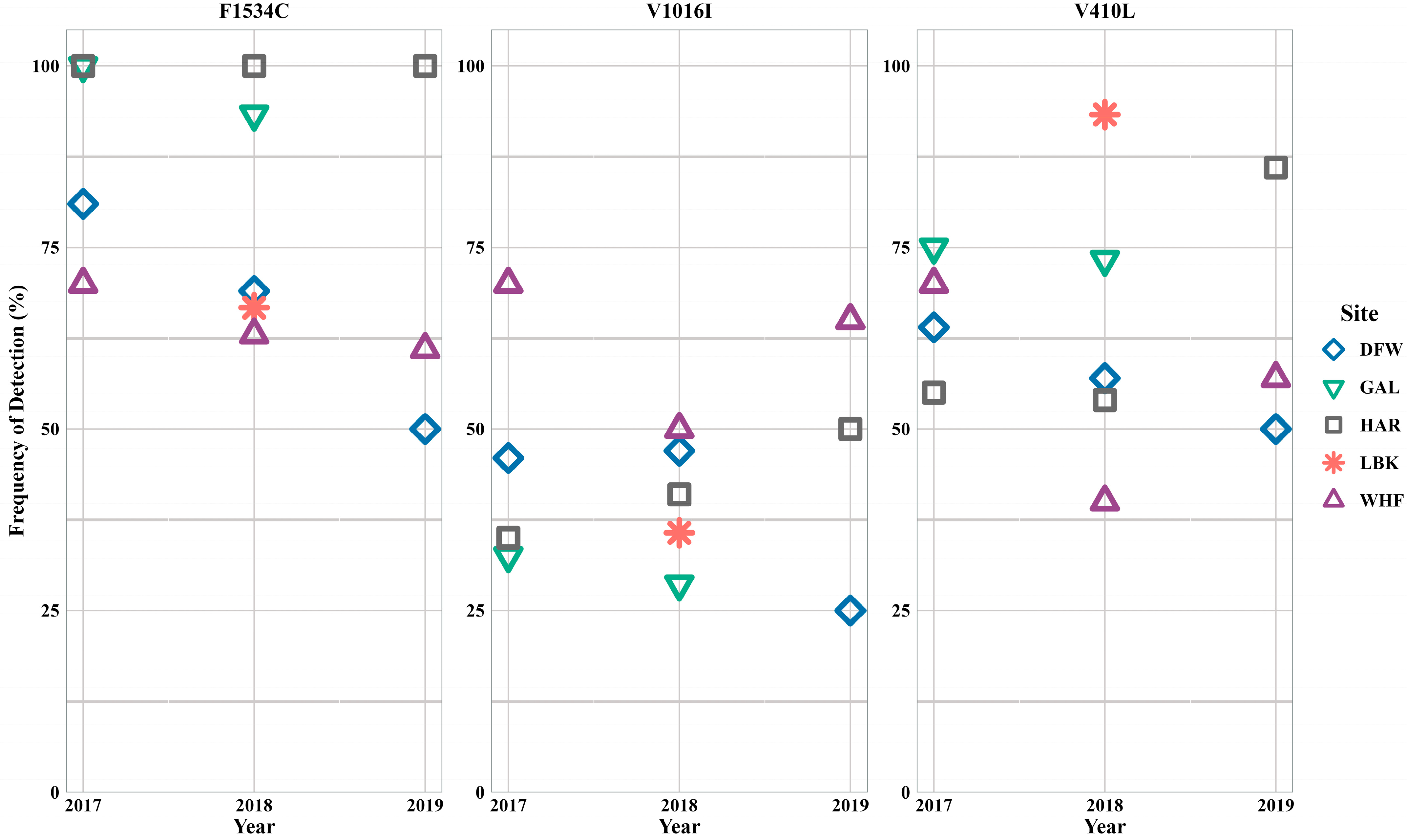
3.2. Aedes aegypti
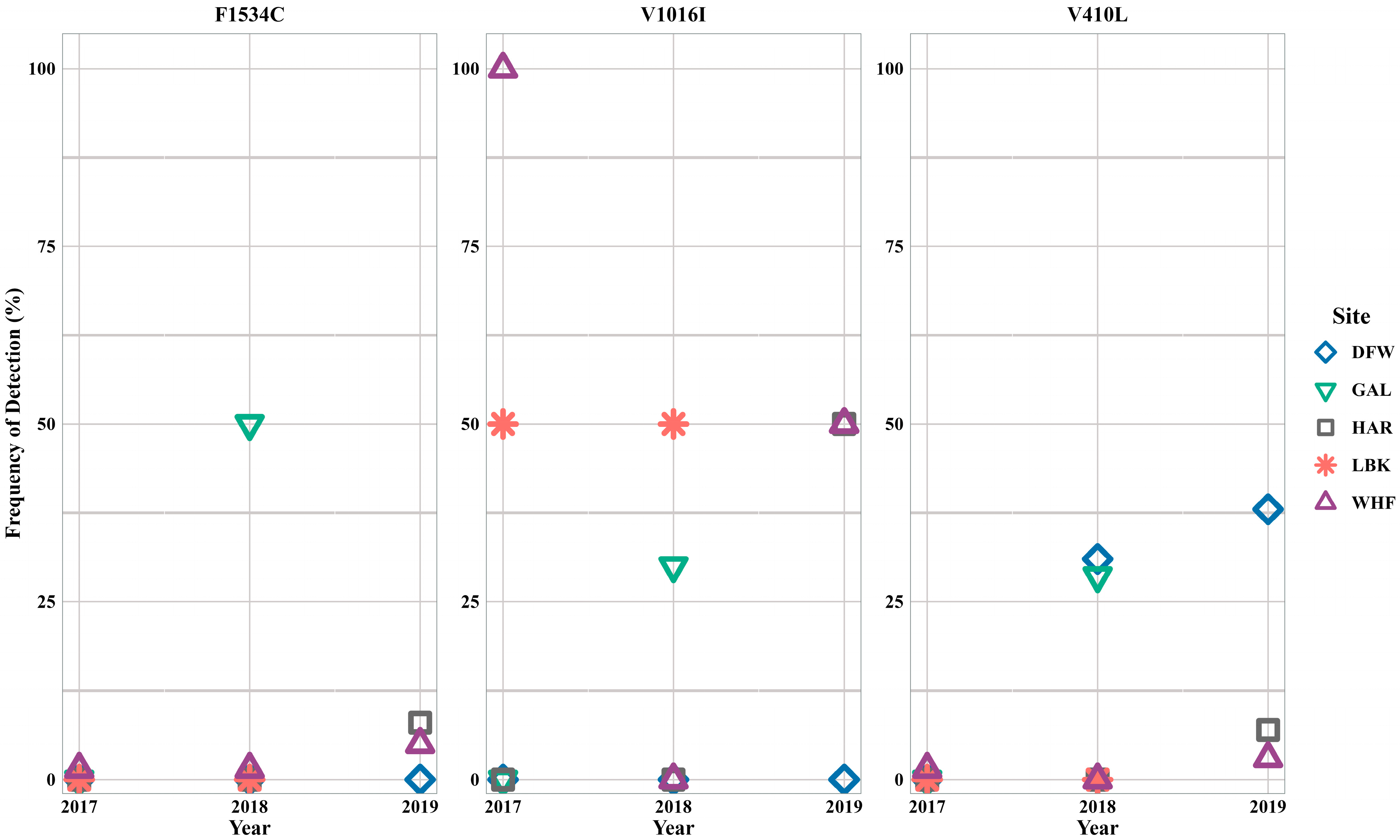
3.3. Aedes albopictus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Chala, B.; Hamde, F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: A review. Front. Public Health 2021, 9, 715759. [Google Scholar] [CrossRef]
- Petersen, L.R.; Beard, C.B.; Visser, S.N. Combatting the increasing threat of vector-borne disease in the United States with a national vector-borne disease prevention and control system. Am. J. Trop. Med. Hyg. 2019, 100, 242–245. [Google Scholar] [CrossRef]
- Texas Department of State Health Services. Arbovirus Activity in Texas, 2019 Surveillance Report. 2022. Available online: https://www.dshs.texas.gov/sites/default/files/IDCU/disease/arboviral/westnile/Reports/2019-DSHS-Arbovirus-Activity-Report-Final.pdf (accessed on 21 June 2023).
- McGregor, B.L.; Connelly, C.R. A review of the control of Aedes aegypti (Diptera: Culicidae) in the continental United States. J. Med. Entomol. 2021, 58, 10–25. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Pesticides Used to Control Adult Mosquitoes. 2024. Available online: https://www.epa.gov/mosquitocontrol/pesticides-used-control-adult-mosquitoes (accessed on 3 December 2024).
- Insecticide Resistance Action Committee. Prevention and Management of Insecticide Resistance in Vectors and Pests of Public Health Importance. 2014. Available online: https://croplife.org/wp-content/uploads/pdf_files/IRAC-Prevention-management-of-insecticide-resistance-in-vectors-pests-of-public-health-importance.pdf (accessed on 6 December 2022).
- Cornel, A.J.; Holeman, J.; Nieman, C.C.; Lee, Y.; Smith, C.; Amorino, M.; Brisco, K.K.; Barrera, R.; Lanzaro, G.C.; Mulligan, F.S. Surveillance, insecticide resistance and control of invasive Aedes aegypti (Diptera: Culicidae) population in California. F1000Research 2016, 5, 194. [Google Scholar] [CrossRef]
- Estep, A.S.; Sanscrainte, N.D.; Waits, C.M.; Bernard, S.J.; Lloyd, A.M.; Lucas, K.J.; Buckner, E.A.; Vaidyanathan, R.; Morreale, R.; Conti, L.A.; et al. Quantification of permethrin resistance and kdr alleles in Florida strains of Aedes aegypti (L.) and Aedes albopictus (Skuse). PLoS Negl. Trop. Dis. 2018, 12, e0006544. [Google Scholar] [CrossRef]
- Kandel, Y.; Vulcan, J.; Rodrigues, S.D.; Moore, E.; Chung, H.-N.; Mitra, S.; Cordova, J.J.; Martinez, K.J.L.; Moon, A.S.; Kulkarni, A.; et al. Widespread insecticide resistance in Aedes aegypti L. from New Mexico, U.S.A. PLoS ONE 2019, 14, e0212693. [Google Scholar] [CrossRef]
- Nauen, R. Insecticide resistance in disease vectors of public health importance. Pest Manag. Sci. 2007, 63, 628–633. [Google Scholar] [CrossRef]
- Dong, K. Insect sodium channels and insecticide resistance. Invert. Neurosci. 2007, 7, 17–30. [Google Scholar] [CrossRef]
- Chen, M.; Du, Y.; Nomura, Y.; Zhorov, B.S.; Dong, K. Chronology of sodium channel mutations associated with pyrethroid resistance in Aedes aegypti. Arch. Insect Biochem. Physiol. 2020, 104, e21686. [Google Scholar] [CrossRef]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef] [PubMed]
- Endersby-Harshman, N.M.; Schmidt, T.L.; Hoffmann, A.A. Diversity and distribution of sodium channel mutations in Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2024, 6, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.R.; Longnecker, M.; Fredregill, C.L.; Debboun, M.; Pietrantonio, P.V. Kdr genotyping (V106I, F1534C) of the Nav channel of Aedes aegypti (L.) mosquito populations in Harris County (Houston), Texas, USA, after Permanone 31-66 field tests and its influence on probability of survival. PLoS Negl. Trop. Dis. 2021, 15, e0009833. [Google Scholar] [CrossRef]
- Mack, L.K.; Kelly, E.T.; Lee, Y.; Brisco, K.K.; Shen, K.V.; Zahid, A.; van Shoor, T.; Cornel, A.J.; Attardo, G.M. Frequency of sodium channel genotypes and association with pyrethrum knockdown time in populations of California Aedes aegypti. Parasites Vectors 2021, 14, 141. [Google Scholar] [CrossRef]
- Haddi, K.; Tomé, H.V.V.; Du, Y.; Valbon, W.R.; Nomura, Y.; Martins, G.F.; Dong, K.; Oliveira, E.E. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: A potential challenge from mosquito control. Sci. Rep. 2017, 7, 46549. [Google Scholar] [CrossRef]
- Fan, Y.; O’Grady, P.; Yoshimizu, M.; Pnlawat, A.; Kaufman, P.E.; Scott, J.G. Evidence of both sequential mutations and recombination in the evolution of kdr alleles in Aedes aegypti. PLoS Negl. Trop. Dis. 2020, 14, e0008154. [Google Scholar] [CrossRef]
- Hernandez, J.R.; Liu, S.; Fredregill, C.L.; Pietrantonio, P.V. Impact of the V410L kdr mutation and co-occurring genotypes at kdr sites 1016 and 1534 in the VGSC on the probability of survival of the mosquito Aedes aegypti (L.) to Permanone in Harris County, TX, USA. PLoS Negl. Trop. Dis. 2023, 17, e0011033. [Google Scholar] [CrossRef]
- Marcombe, S.; Farajollahi, A.; Healy, S.P.; Clark, G.G.; Fonseca, D.M. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS ONE 2014, 9, e101992. [Google Scholar] [CrossRef]
- Xu, J.; Bonizzoni, M.; Zhong, D.; Zhou, G.; Cai, S.; Li, Y.; Wang, X.; Lo, E.; Lee, R.; Sheen, R.; et al. Multi-country survey revealed prevalent and novel F1534S mutation in voltage-gated sodium channel (VGSC) gene in Aedes albopictus. PLoS Negl. Trop. Dis. 2016, 10, e0004696. [Google Scholar] [CrossRef]
- Abernathy, H.A.; Hollingsworth, B.D.; Giandomenico, D.A.; Moser, K.A.; Juliano, J.J.; Bowman, N.M.; George, P.J.; Reiskind, M.H.; Boyce, R.M. Prevalence of knock-down resistance F1534S mutations in Aedes albopictus (Skuse) (Diptera: Culicidae) in North Carolina. J. Med. Entomol. 2022, 59, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Peper, S.T.; Wilson-Fallon, A.; Haydett, K.; Greenberg, H.; Presley, S.M. First record of Aedes aegypti and Aedes albopictus in thirteen Panhandle region counties of Texas, U.S.A. J. Vector Ecol. 2017, 42, 352–354. [Google Scholar] [CrossRef] [PubMed]
- FAO. Guidelines for Routine Colony Maintenance of Aedes Mosquito Species; FAO: Rome, Italy, 2017. [Google Scholar]
- Morlan, H.B.; Hayes, R.O.; Schoof, H.F. Methods of mass rearing of Aedes aegypti (L.). Public Health Rep. 1963, 78, 711–719. [Google Scholar] [CrossRef]
- Qiagen. Purification of Total DNA from Insects Using the DNeasy® Blood & Tissue Kit; Qiagen: Hilden, Germany, 2006; Available online: https://www.qiagen.com/us/resources/resourcedetail?id=cabd47a4-cb5a-4327-b10d-d90b8542421e&lang=en (accessed on 13 April 2023).
- Melo Costa, M.; Campos, K.B.; Brito, L.P.; Roux, E.; Melo Rodovalho, C.; Bellinato, D.F.; Lima, J.B.P.; Martins, A.J. Kdr genotyping in Aedes aegypti from Brazil on a nation-wide scale from 2017 to 2018. Sci. Rep. 2020, 10, 13267. [Google Scholar] [CrossRef]
- Life Technologies. TaqMan® SNP Genotyping Assays TaqMan® Predesigned SNP Genotyping Assays, TaqMan® Custom SNP Genotyping Assays, and TaqMan® Drug Metabolism Enzyme Genotyping Assays. 2014. Available online: https://tools.thermofisher.com/content/sfs/manuals/TaqMan_SNP_Genotyping_Assays_man.pdf (accessed on 12 April 2022).
- Rahman, R.U.; Souza, B.; Uddin, I.; Carrara, L.; Brito, L.P.; Melo Costa, M.; Mahmood, M.A.; Khan, S.; Lima, J.B.P.; Martins, A.J. Insecticide resistance and underlying targets-site and metabolic mechanisms in Aedes aegypti and Aedes albopictus from Lahore, Pakistan. Sci. Rep. 2021, 11, 4555. [Google Scholar] [CrossRef]
- Agresti, A.; Coull, B.A. Approximate is better than “exact” for interval estimation of binomial proportions. Am. Stat. 1998, 52, 119–126. [Google Scholar]
- Alvarez, L.C.; Ponce, G.; Saavedra-Rodriguez, K.; Lopez, B.; Flores, A.E. Frequency of V1016I and F1534C mutations in the voltage-gated sodium channel gene in Aedes aegypti in Venezuela. Pest. Manag. Sci. 2015, 71, 863–869. [Google Scholar] [CrossRef]
- Vontas, J.; Kioulos, E.; Pavlidi, N.; Morou, E.; della Torre, A.; Ranson, H. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic. Biochem. Physiol. 2012, 104, 126–131. [Google Scholar] [CrossRef]
- Auteri, M.; La Russa, F.; Blanda, V.; Torina, A. Insecticide resistance associated with kdr mutations in Aedes albopictus: An update on worldwide evidences. BioMed. Res. Int. 2018, 2018, 3098575. [Google Scholar] [CrossRef]
- Du, Y.; Nomura, Y.; Zhorov, B.S.; Dong, K. Sodium channel mutations and pyrethroid resistance in Aedes aegypti. Insects 2016, 7, 60. [Google Scholar] [CrossRef]
- Uemura, N.; Itokawa, K.; Komagata, O.; Kasai, S. Recent advance in the study of knockdown resistance mutations in Aedes mosquitoes with a focus on several remarkable mutations. Curr. Opin. Insect. Sci. 2024, 63, 101178. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Du, Y.; Wu, S.; Nomura, Y.; Zhu, G.; Zhorov, B.S.; Dong, K. Molecular evidence of sequential evolution of DDT- and pyrethroid-resistant sodium channel in Aedes aegypti. PLoS Negl. Trop. Dis. 2019, 13, e0007432. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Rodriguez, K.; Vera Maloof, F.; Campbell, C.L.; Garcia-Rejon, J.; Lenhart, A.; Penilla, P.; Rodriguez, A.; Sandoval, A.A.; Flores, A.E.; Ponce, G.; et al. Parallel evolution of vgsc mutations at domains IS6, IIS6 and IIIS6 in pyrethroid resistant Aedes aegypti from Mexico. Sci. Rep. 2018, 8, 6747. [Google Scholar] [CrossRef]
- Kondapaneni, R.; Malcolm, A.N.; Vazquez, B.M.; Zeng, E.; Chen, T.-Y.; Kosinski, K.J.; Romero-Weaver, A.L.; Giordano, B.V.; Allen, B.; Riles, M.T.; et al. Mosquito control priorities in Floridia—Survey results from Florida mosquito control districts. J. Pathog. 2021, 10, 947. [Google Scholar] [CrossRef]
| Location | DFW | GAL | HAR | LBK | WHF | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2017 | 2018 | 2019 | 2017 | 2018 | 2017 | 2018 | 2019 | 2018 | 2017 | 2018 | 2019 | |
| Genotypes * | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| CC+II+LL | 1 (8%) | - | - | - | - | - | - | - | 5 (33%) | 3 (60%) | 1 (7%) | 5 (33%) | 15 (10%) |
| CC+II+vv ** | - | - | - | - | - | - | - | - | - | - | 2 (13%) | - | 2 (1%) |
| CC+VI+LL | 5 (38%) | 2 (13%) | - | 10 (50%) | 8 (53%) | 4 (40%) | 4 (33%) | 10 (71%) | - | - | - | - | 43 (28%) |
| CC+VI | 2 (15%) | - | - | - | - | - | - | - | - | - | - | - | 2 (1%) |
| CC+VI+vL | 1 (8%) | 3 (20%) | - | 3 (15%) | - | 3 (30%) | 5 (42%) | 4 (29%) | - | - | 3 (20%) | - | 22 (15%) |
| CC+VI+vv | - | - | - | - | - | - | - | - | - | - | 1 (7%) | - | 1 (<1%) |
| CC+VV+vL | - | - | - | 7 (35%) | 6 (40%) | - | - | - | 1 (7%) | - | - | - | 14 (9%) |
| CC+VV+vv | - | - | - | - | - | 1 (10%) | - | - | - | - | - | - | 1 (<1%) |
| FC+VI+vL | - | 9 (60%) | 1 (50%) | - | - | - | - | - | - | 1 (20%) | 5 (33%) | 7 (47%) | 23 (15%) |
| FC+VI+vv | 1 (8%) | - | - | - | - | - | - | - | - | - | - | - | 1 (<1%) |
| FC+VV+LL | - | - | - | - | - | - | - | - | 7 (47%) | - | - | - | 7 (5%) |
| FC+VV | - | 1 (7%) | - | - | - | - | - | - | - | - | - | - | 1 (<1%) |
| FC+VV+vL | 1 (8%) | - | 1 (50%) | - | - | - | - | - | 1 (7%) | - | - | - | 3 (2%) |
| FC+VV+vv | 1 (8%) | - | - | - | - | - | - | - | - | - | - | - | 1 (<1%) |
| FF+LL | - | - | - | - | - | - | - | - | 1 (7%) | - | - | - | 1 (<1%) |
| FF+VV+LL | - | - | - | - | - | - | - | - | - | - | 1 (7%) | - | 1 (<1%) |
| FF+VV+vv | - | - | - | - | - | - | - | - | - | 1 (20%) | 2 (13%) | 1 (7%) | 4 (3%) |
| FF+vv | 1 (8%) | - | - | - | 1 (7%) | - | - | - | - | - | - | 1 (7%) | 3 (2%) |
| VV+vv | - | - | - | - | - | 2 (20%) | 2 (17%) | - | - | - | - | - | 4 (3%) |
| vv | - | - | - | - | - | - | 1 (8%) | - | - | - | - | 1 (7%) | 2 (1%) |
| Total | 13 | 15 | 2 | 20 | 15 | 10 | 12 | 14 | 15 | 5 | 15 | 15 | 151 |
| Location | DFW | GAL | HAR | LBK | WHF | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2017 | 2018 | 2019 | 2017 | 2018 | 2017 | 2018 | 2019 | 2017 | 2018 | 2017 | 2018 | 2019 | |
| Genotypes * | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| CC+VI+LL | - | - | - | - | 3 (21%) | - | - | 1 (7%) | - | - | - | - | - | 4 (2%) |
| CC+VV+vL | - | - | - | - | 2 (14%) | - | - | - | - | - | - | - | - | 2 (1%) |
| FC+VI+vL | - | - | - | - | - | - | - | - | - | - | - | - | 1 (7%) | 1 (<1%) |
| FF+VI+vv ** | - | - | - | - | - | - | - | - | 2 (7%) | 2 (13%) | - | - | - | 4 (2%) |
| FF+vv | 12 (75%) | 1 (8%) | 6 (22%) | 5 (56%) | 5 (36%) | 13 (65%) | 9 (50%) | 11 (73%) | 18 (60%) | 13 (87%) | 18 (75%) | 9 (64%) | 9 (60%) | 129 (56%) |
| FF+vL | - | 1 (8%) | 10 (37%) | - | - | - | - | - | - | - | - | - | - | 11 (5%) |
| II+vv | - | - | - | - | - | - | - | - | - | - | 1 (4%) | - | - | 1 (<1%) |
| FF | - | - | 1 (4%) | - | - | - | - | - | - | - | - | 1 (7%) | - | 2 (1%) |
| vv | 4 (25%) | 4 (31%) | - | 4 (44%) | 4 (29%) | 7 (35%) | 9 (50%) | 3 (20%) | 10 (33%) | - | 5 (21%) | 4 (29%) | 5 (33%) | 59 (26%) |
| vL | - | 7 (54%) | 10 (37%) | - | - | - | - | - | - | - | - | - | - | 17 (7%) |
| Total | 16 | 13 | 27 | 9 | 14 | 20 | 18 | 15 | 30 | 15 | 24 | 14 | 15 | 230 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wimmer, B.M.; Reinoso Webb, C.; Presley, S.M. Detection of Di- and Tri-Locus kdr Mutations in Aedes aegypti and Aedes albopictus from Texas, USA, and the Implications for Insecticide Resistance. Insects 2025, 16, 551. https://doi.org/10.3390/insects16060551
Wimmer BM, Reinoso Webb C, Presley SM. Detection of Di- and Tri-Locus kdr Mutations in Aedes aegypti and Aedes albopictus from Texas, USA, and the Implications for Insecticide Resistance. Insects. 2025; 16(6):551. https://doi.org/10.3390/insects16060551
Chicago/Turabian StyleWimmer, Bianca M., Cynthia Reinoso Webb, and Steven M. Presley. 2025. "Detection of Di- and Tri-Locus kdr Mutations in Aedes aegypti and Aedes albopictus from Texas, USA, and the Implications for Insecticide Resistance" Insects 16, no. 6: 551. https://doi.org/10.3390/insects16060551
APA StyleWimmer, B. M., Reinoso Webb, C., & Presley, S. M. (2025). Detection of Di- and Tri-Locus kdr Mutations in Aedes aegypti and Aedes albopictus from Texas, USA, and the Implications for Insecticide Resistance. Insects, 16(6), 551. https://doi.org/10.3390/insects16060551








