Ultrastructural Characterization of Developmental Stages and Head Sensilla in Portici okadai, Vector of Thelazia callipaeda
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Scanning Electron Microscopy Observation
2.3. Statistical Analysis
3. Results
3.1. A General Description of the Stages of P. okadai
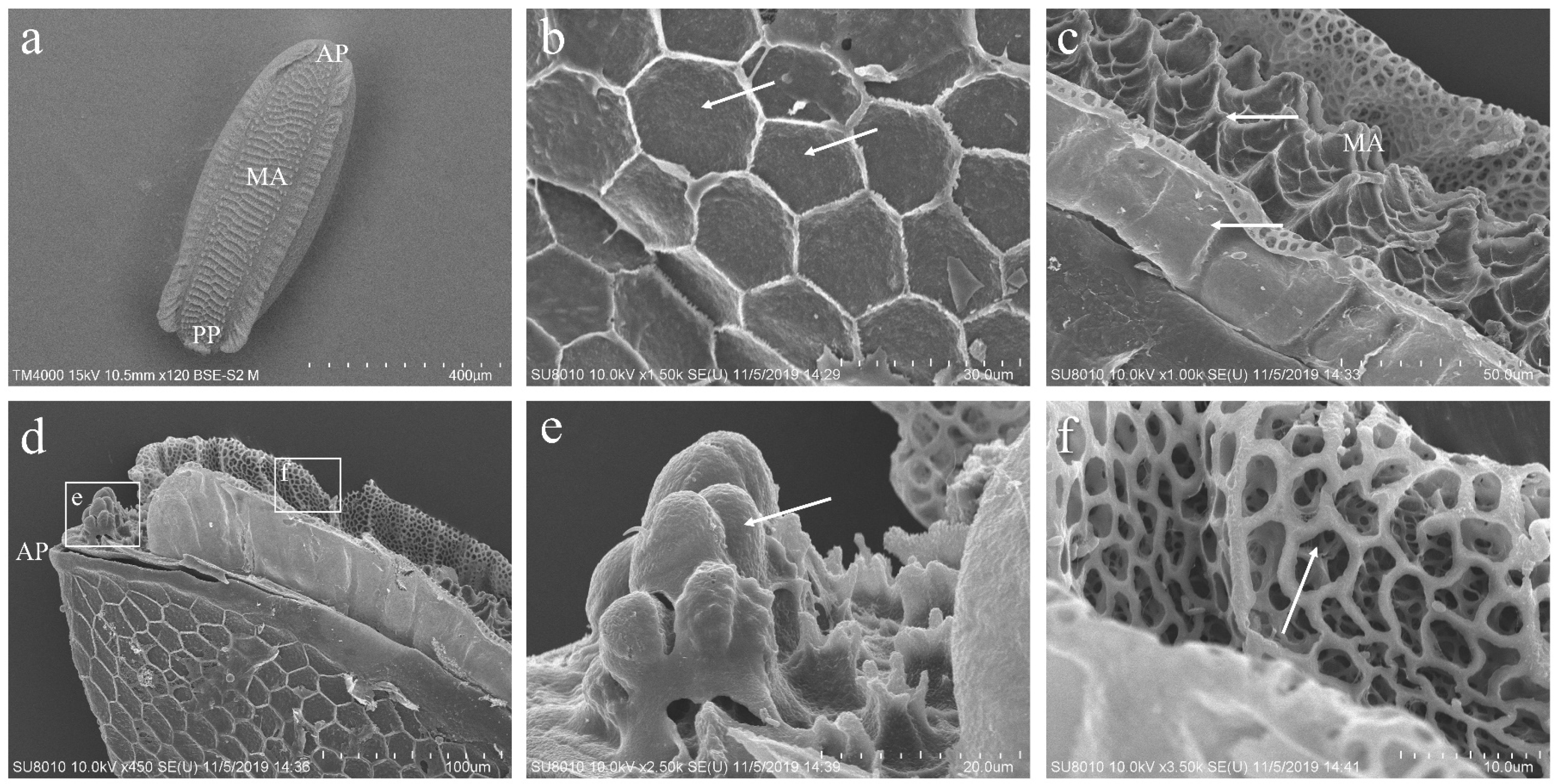
3.2. The Morphology of the Eggs of P. okadai
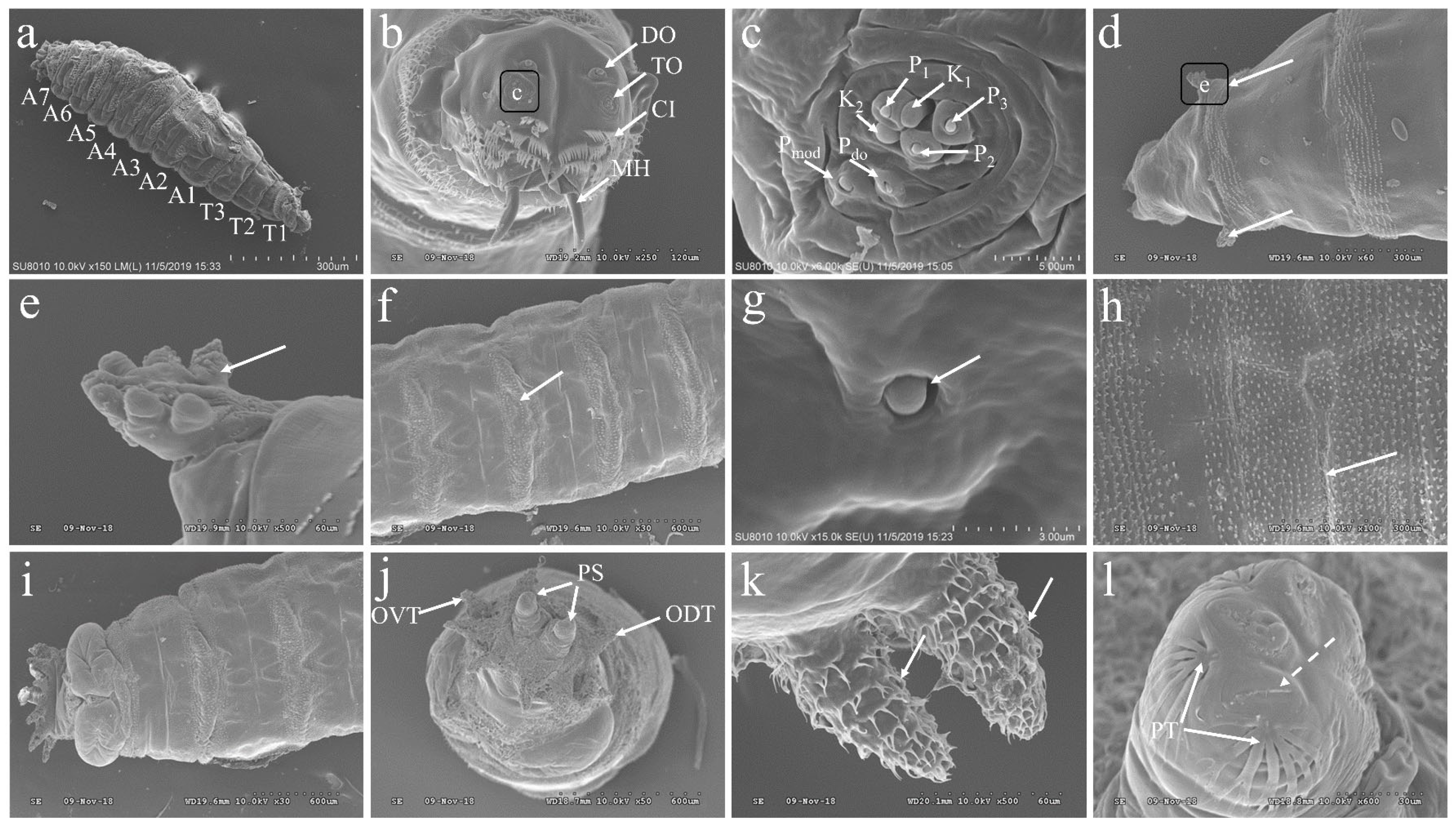
3.3. The Morphology of the Larvae of P. okadai
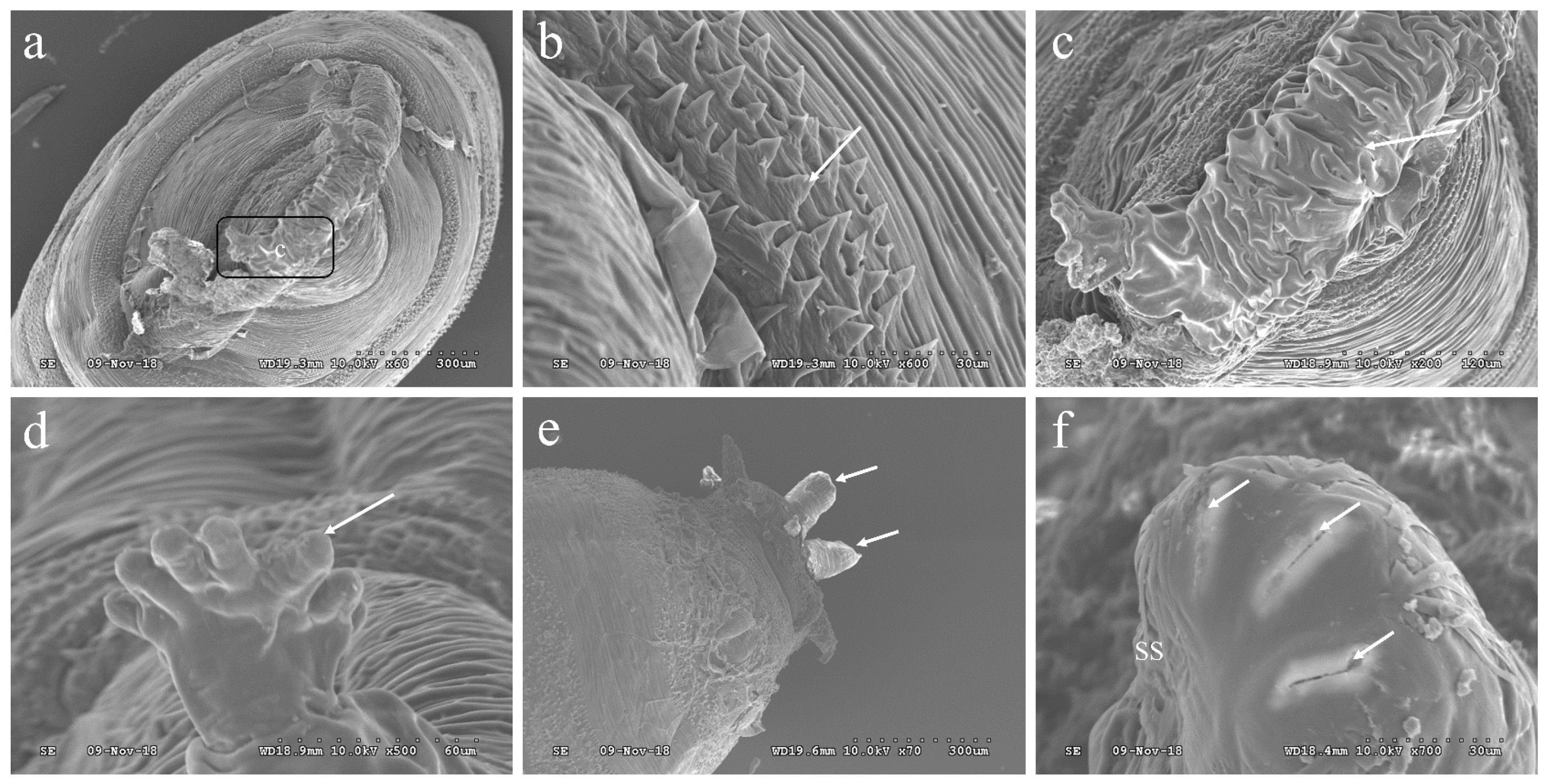
3.4. The Morphology of the Pupae of P. okadai
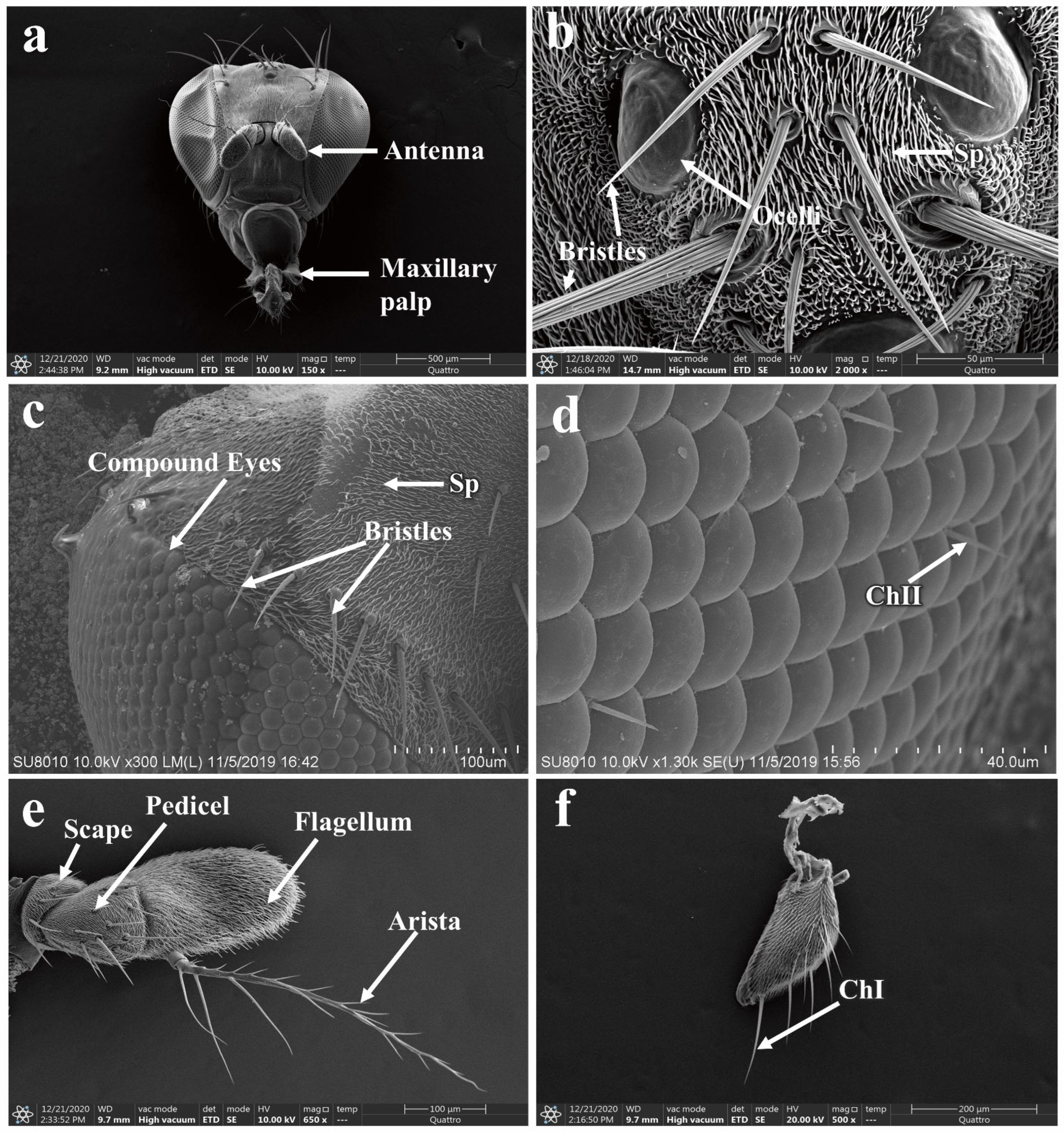
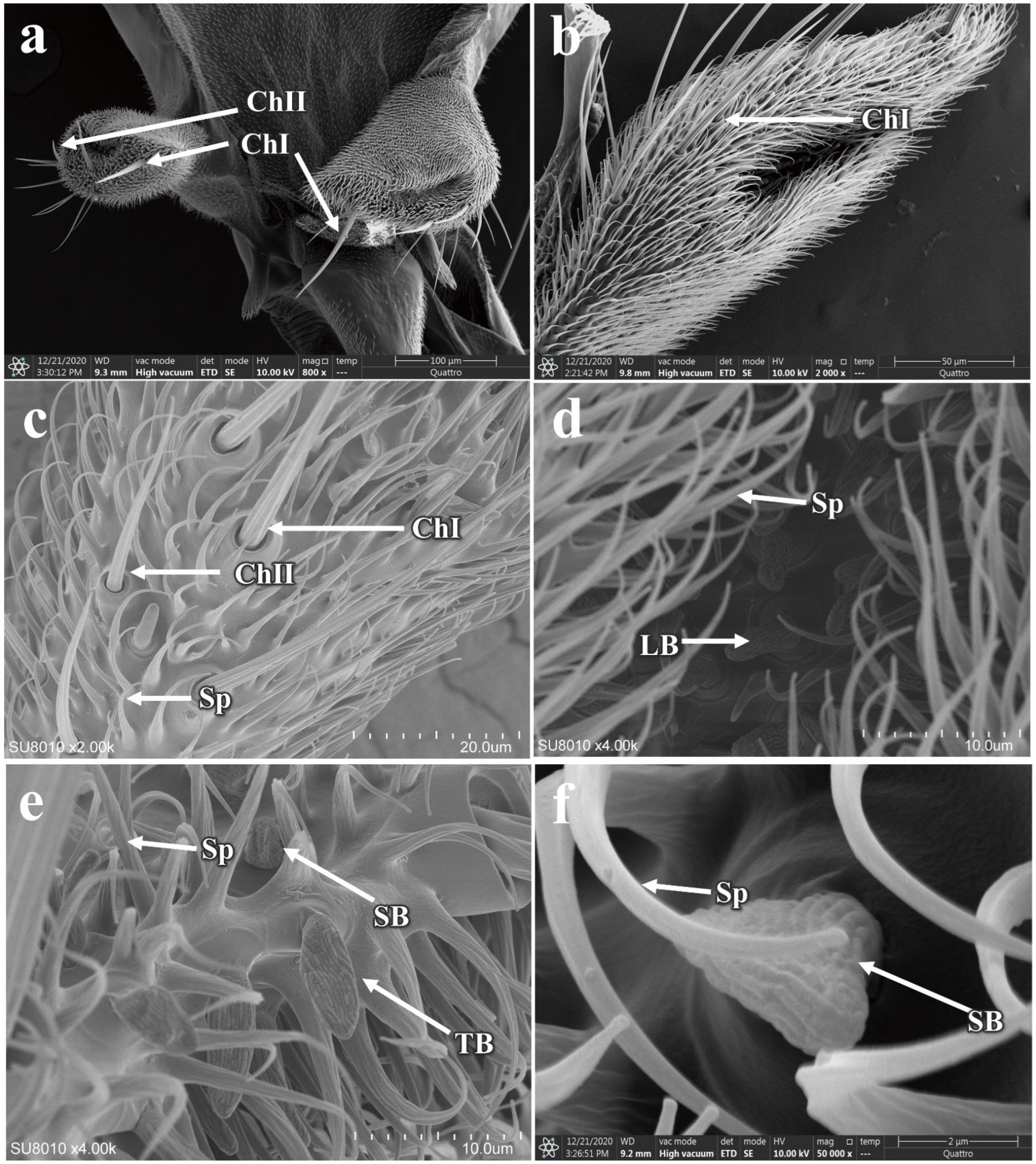
3.5. Morphology of the Adult P. okadai Head
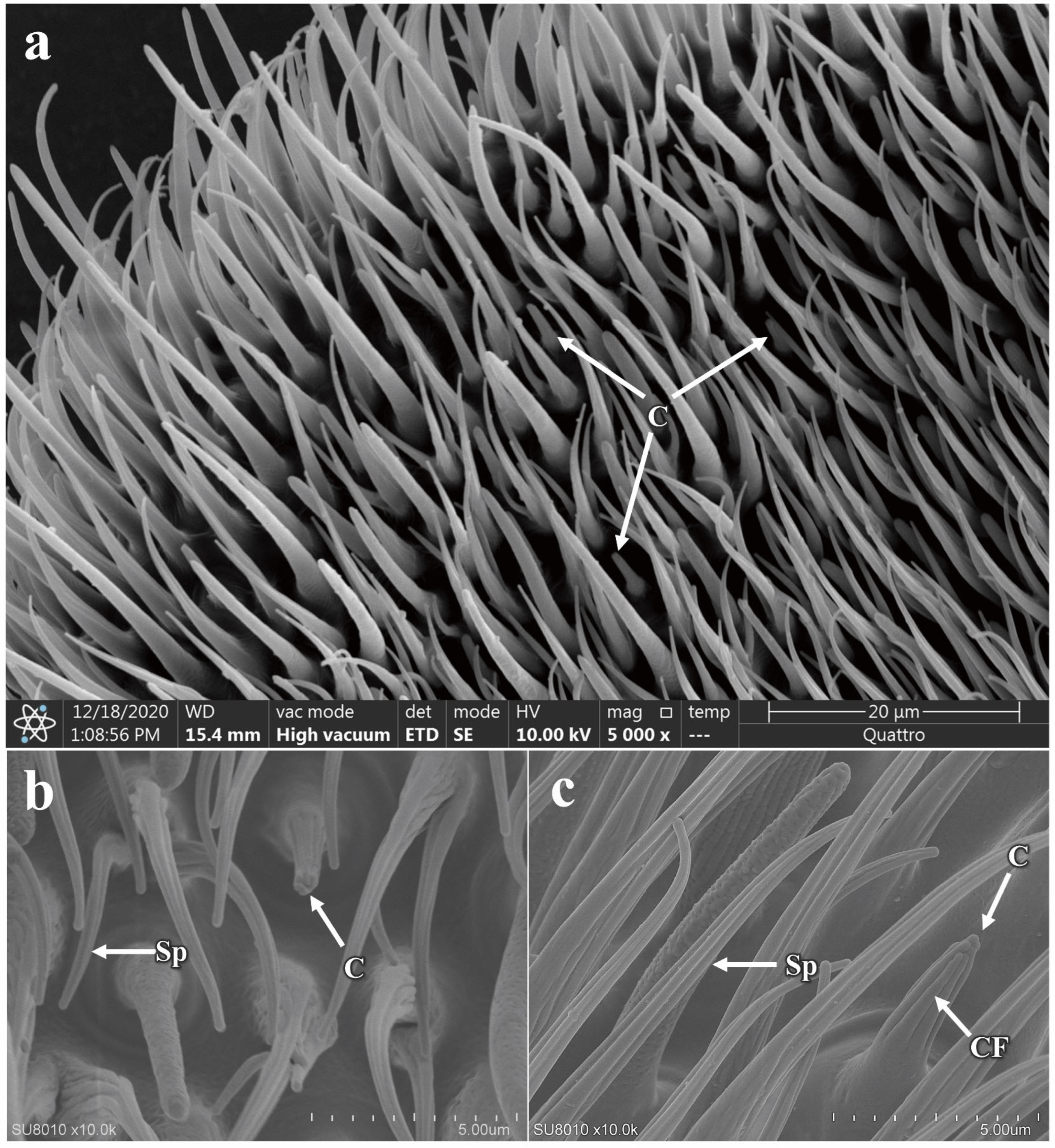
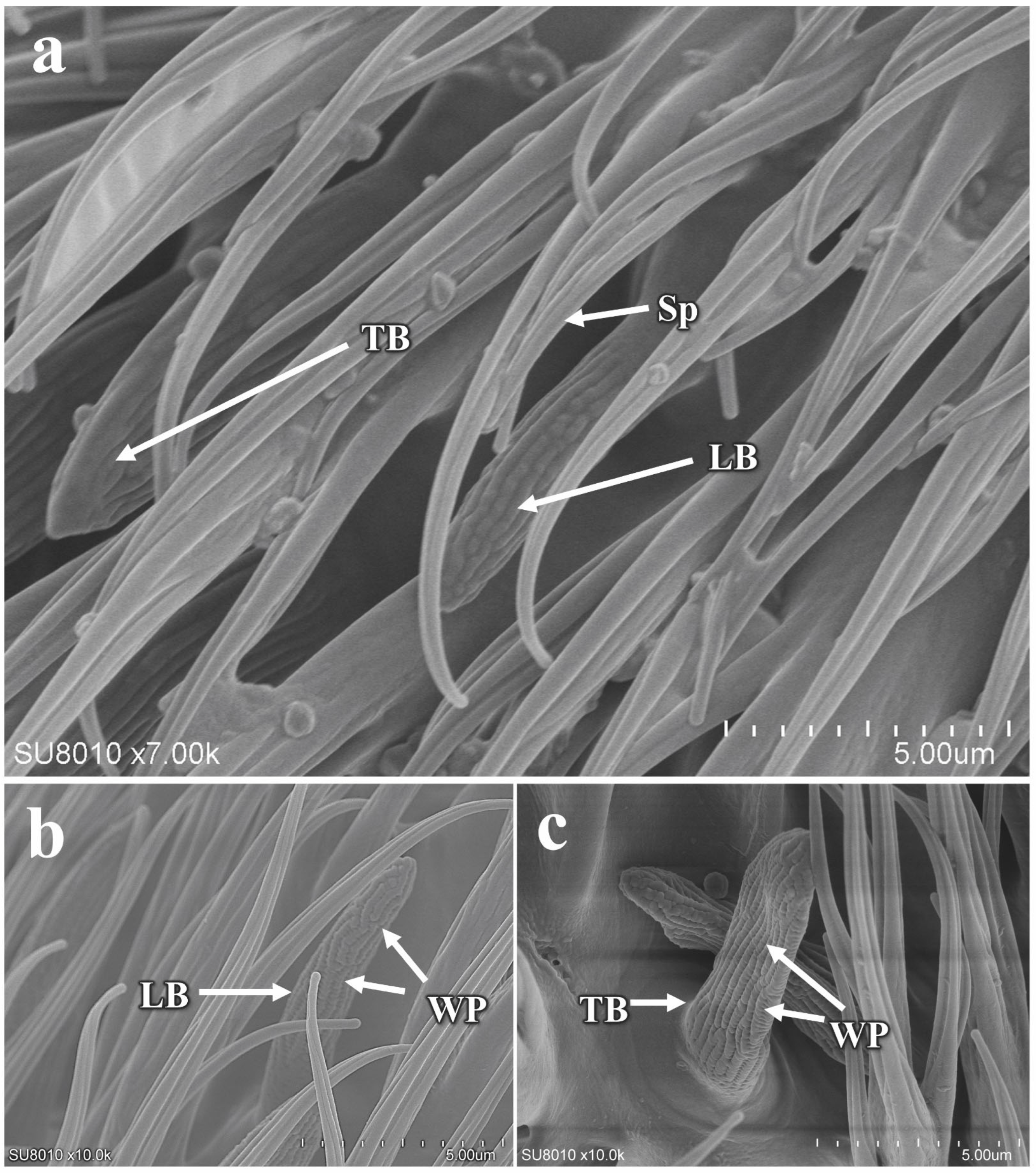
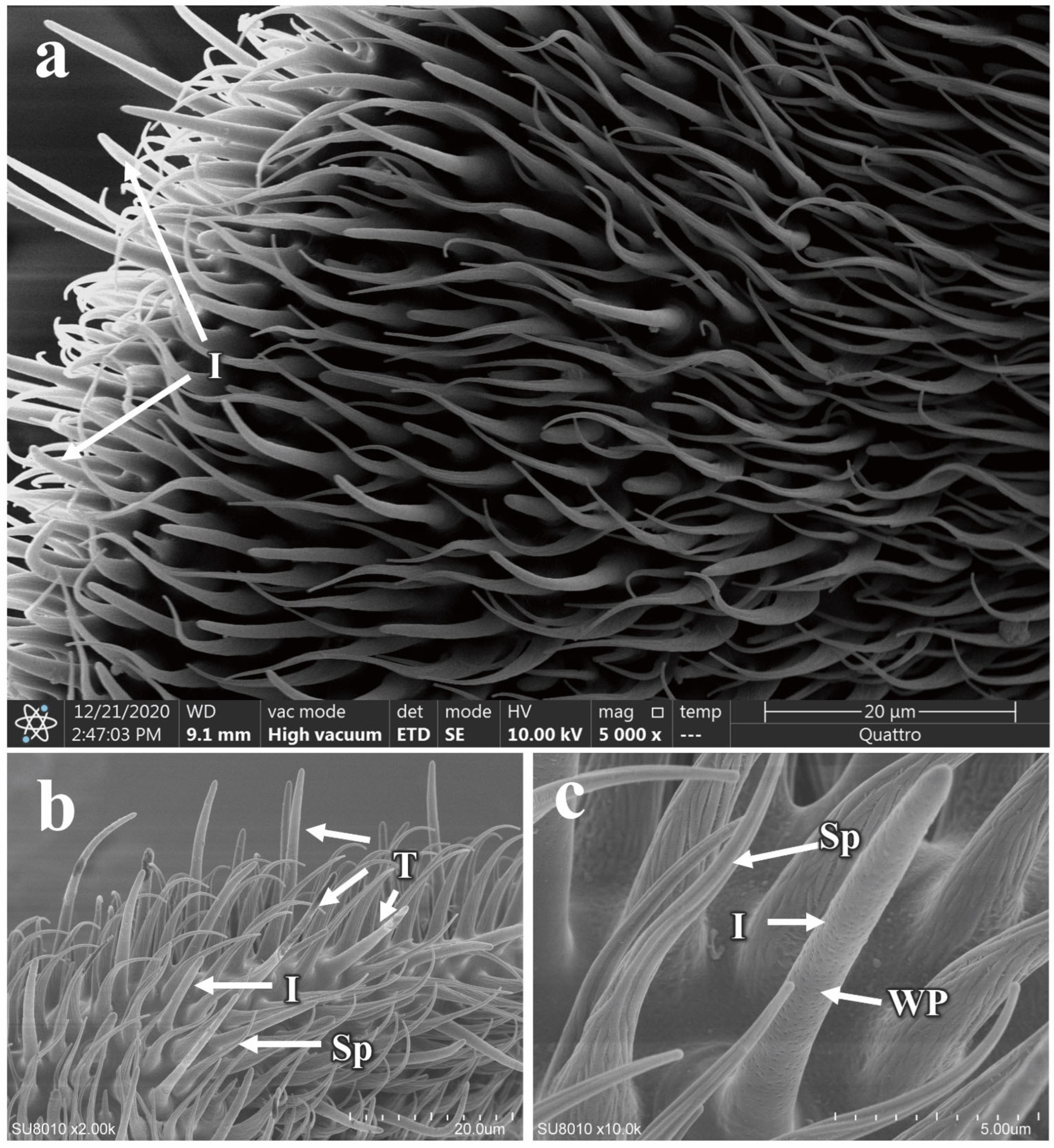
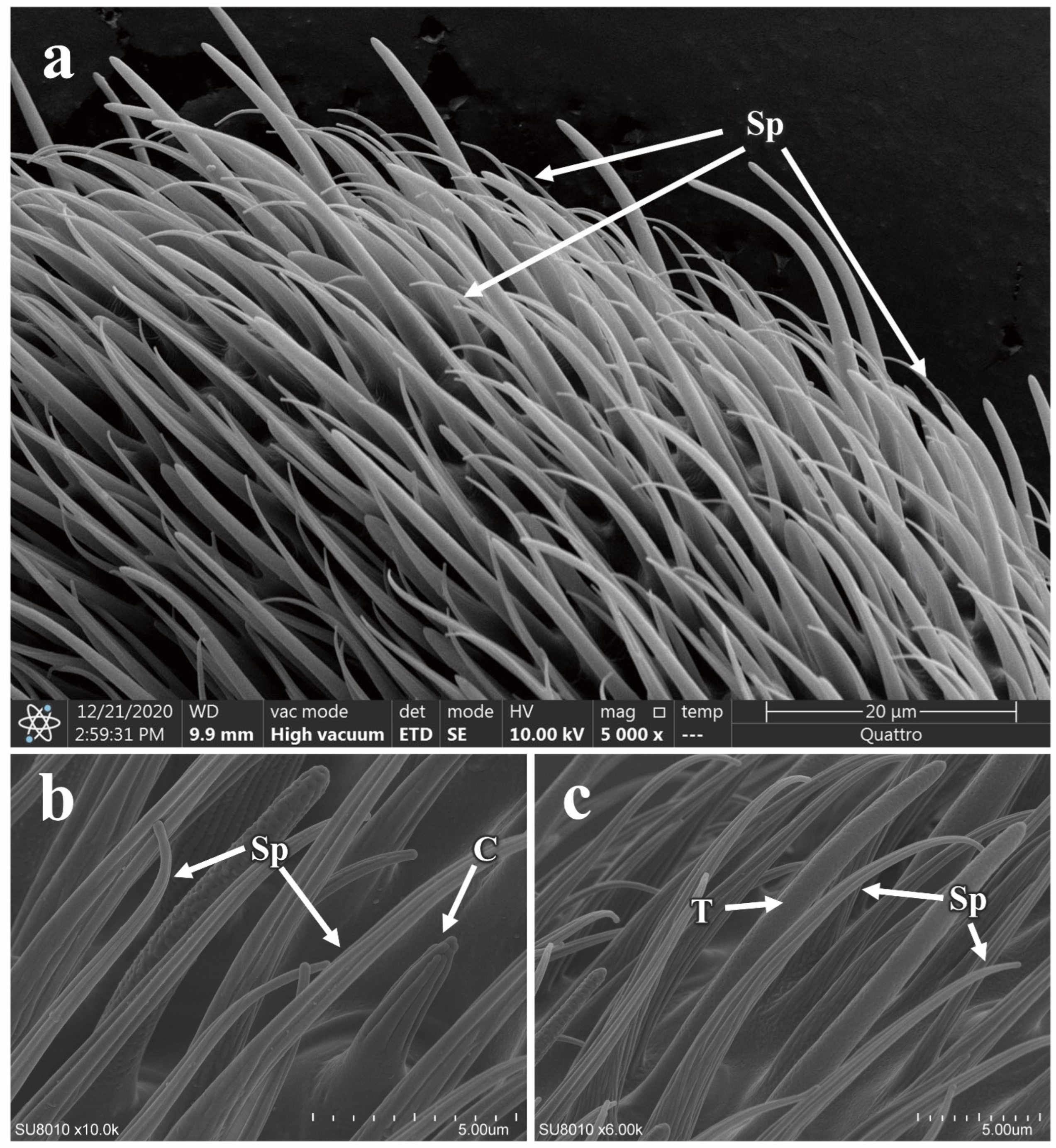
3.5.1. Morphology and Structure of Antennal Sensilla in P. okadai
3.5.2. The Morphology and Structure of the Maxillary Palp Sensilla in P. okadai
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBS | Phosphate-buffered saline |
| MA | Median area |
| Pdo | Papillum sensilla |
| ChI | Type I chaetica sensilla |
| ChII | Type II chaetica sensilla |
| SB | Small basiconic sensilla |
| TB | Thin basiconic sensilla |
| LB | Large basiconic sensilla |
| OBPs | Odorant-binding proteins |
| SEM | Scanning electron microscopy |
References
- Jin, Y.; Liu, Z.; Wei, J.; Wen, Y.; He, N.; Tang, L.; Lin, D.; Lin, J. A first report of Thelazia callipaeda infection in Phortica okadai and wildlife in national nature reserves in China. Parasites Vectors 2021, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Lia, R.P.; Cantacessi, C.; Testini, G.; Troccoli, A.; Shen, J.L.; Wang, Z.X. Nematode biology and larval development of Thelazia callipaeda (Spirurida, Thelaziidae) in the drosophilid intermediate host in Europe and China. Parasitology 2005, 131, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhou, Y.; Wen, H.; Wang, Z.; Shen, J. Ecological habits of Amiota (Phortica) okadai, a vector of Thelazia callipaeda. Chin. J. Zoonoses 2008, 24, 548–550. [Google Scholar]
- Shen, J.; Gasser, R.B.; Chu, D.; Wang, Z.; Yuan, X.; Cantacessi, C.; Otranto, D. Human thelaziosis—A neglected parasitic disease of the eye. J. Parasitol. 2006, 92, 872–875. [Google Scholar] [CrossRef]
- Máca, J.; Otranto, D. Drosophilidae feeding on animals and the inherent mystery of their parasitism. Parasites Vectors 2014, 7, 516. [Google Scholar] [CrossRef]
- Juhász, H.; Thury, G.; Szécsényi, M.; Tóth-Molnár, E.; Burián, K.; Deim, Z.; Terhes, G. Human thelaziosis caused by Thelazia callipaeda eyeworm, hungary. Emerg. Infect. Dis. 2022, 28, 2559–2561. [Google Scholar] [CrossRef]
- do Vale, B.; Lopes, A.P.; da Conceição Fontes, M.; Silvestre, M.; Cardoso, L.; Coelho, A.C. Thelaziosis due to Thelazia callipaeda in Europe in the 21st century—A review. Vet. Parasitol. 2019, 275, 108957. [Google Scholar] [CrossRef]
- Otranto, D.; Mendoza-Roldan, J.A.; Dantas-Torres, F. Thelazia callipaeda. Trends Parasitol. 2021, 37, 263–264. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, P.; Liu, R.-D.; Long, S.-R.; Cui, J.; Wang, Z.-Q. Advances in etiology, epidemiology and genetic diversity of Thelazia callipaeda. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2019, 31, 86–93. [Google Scholar]
- Li, D.; Wang, L.; Wang, L.; Gou, Y.; Luo, B.; Yan, R.; Liu, H. The species and abundance of gut bacteria both positively impact Phortica okadai behavior. Parasites Vectors 2024, 17, 217. [Google Scholar] [CrossRef]
- Tyagi, V.; Sharma, A.K.; Dhiman, S.; Srivastava, A.R.; Yadav, R.; Sukumaran, D.; Agrawal, O.P.; Veer, V. Malaria vector Anopheles culicifacies sibling species differentiation using egg morphometry and morphology. Parasites Vectors 2016, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Wipfler, B.; Schneeberg, K.; Löffler, A.; Hünefeld, F.; Meier, R.; Beutel, R.G. The skeletomuscular system of the larva of Drosophila melanogaster (Drosophilidae, Diptera): A contribution to the morphology of a model organism. Arthropod Struct. Dev. 2013, 42, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Cortinhas, L.B.; Martins Mendonça, P.; Braga, M.V.; Queiroz, M.M.C. Ultrastructure of the immature stages of Musca domestica (Diptera: Muscidae: Muscinae). J. Med. Entomol. 2020, 57, 1712–1721. [Google Scholar] [CrossRef]
- Reedy, M.C.; Beall, C. Ultrastructure of developing flight muscle in Drosophila. Ii. Formation of the myotendon junction. Dev. Biol. 1993, 160, 466–479. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, L.; Long, J.; Chang, Z.; Mu, Y.; Zhou, Z.; Chen, X. Morphology of the antennal sensilla of the nymphal instars and adults in Notobitus meleagris (Hemiptera: Heteroptera: Coreidae). Insects 2023, 14, 351. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, T.; Guo, H.W.; Liang, X.F.; Cheng, Y.Q. Ultrastructure of the olfactory sensilla across the antennae and maxillary palps of Bactrocera dorsalis (Diptera: Tephritidae). Insects 2021, 12, 289. [Google Scholar] [CrossRef]
- Huang, F.; Srisuka, W.; Aupalee, K.; Yasanga, T.; Phuackchantuck, R.; Pitasawat, B.; Junkum, A.; Limsopatham, K.; Sanit, S.; Saingamsook, J.; et al. Ultrastructure of sensilla on the antennae and maxillary palps of the human-biting black flies, Simulium nigrogilvum and Simulium umphangense, (Diptera: Simuliidae) in Thailand. Acta Trop. 2022, 232, 106494. [Google Scholar] [CrossRef]
- Li, S.; Li, B.; Gao, L.; Wang, J.; Yan, Z. Humidity response in drosophila olfactory sensory neurons requires the mechanosensitive channel tmem63. Nat. Commun. 2022, 13, 3814. [Google Scholar] [CrossRef]
- Giglio, A.; Mazzei, A.; Vommaro, M.L.; Brandmayr, P. Antennal sensilla in an anophthalmic wood-dwelling species, Clinidium canaliculatum, costa 1839 (Coleoptera, Rhysodidae). Microsc. Res. Tech. 2022, 85, 1005–1015. [Google Scholar] [CrossRef]
- Souza, A.C.; Catalá, S.; Carbajal de la Fuente, A.L.; Junqueira, A.C.V. Phenotypic variability of the amazonian species Rhodnius brethesi (Hemiptera: Reduviidae). J. Med. Entomol. 2017, 54, 909–916. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Xu, F.; Yang, M.; Wang, X.; Wu, C. Ultrastructure of the sensilla on the antennae and mouthparts of Bean weevils, Megabruchidius dorsalis (Coleoptera: Bruchinae). Insects 2021, 12, 1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Liu, Y.; Li, X.J.; Cheng, W.N.; Zhu-Salzman, K. Morphology, ultrastructure and possible functions of antennal sensilla of Sitodiplosis mosellana géhin (Diptera: Cecidomyiidae). J. Insect Sci. 2016, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.F.; Gendre, N. Peripheral and central nervous effects of lozenge3: A drosophila mutant lacking basiconic antennal sensilla. Dev. Biol. 1988, 127, 12–24. [Google Scholar] [CrossRef]
- Unterköfler, M.S.; Dengg, P.; Niederbacher, M.; Lindorfer, S.; Eberle, A.; Huck, A.; Staufer, K.; Zittra, C.; Wortha, L.N.; Hodžić, A.; et al. Occurrence of Thelazia callipaeda and its vector Phortica variegata in austria and South tyrol, Italy, and a global comparison by phylogenetic network analysis. Parasites Vectors 2023, 16, 294. [Google Scholar] [CrossRef]
- Cortinhas, L.B.; Mendonça, P.M.; Barbosa, R.R.; Queiroz, M.M.C. Ultrastructure of immature stages of the black dump fly: Ophyra aenescens (Wiedemann, 1830) (Diptera: Muscidae: Azeliinae). Acta Trop. 2016, 158, 125–129. [Google Scholar] [CrossRef]
- Sanit, S.; Sukontason, K.; Kurahashi, H.; Tomberlin, J.K.; Wannasan, A.; Kraisittipanit, R.; Sukontason, K.L. Morphology of immature stages of blow fly, Lucilia sinensis aubertin (Diptera: Calliphoridae), a potential species of forensic importance. Acta Trop. 2017, 176, 395–401. [Google Scholar] [CrossRef]
- Sanit, S.; Limsopatham, K.; Klong-Klaew, T.; Samerjai, C.; Yasanga, T.; Sukontason, K.; Tomberlin, J.K.; Sukontason, K.L. Morphology of immature blow fly Hypopygiopsis infumata (Bigot) (Diptera: Calliphoridae), a potential species of forensic importance. Acta Trop. 2018, 188, 168–179. [Google Scholar] [CrossRef]
- Courtney, G.W.; Sinclair, B.J.; Meier, R. Morphology and terminology of diptera larvae. In Contributions to A Manual of Palaearctic Diptera (with Special Reference to ßies of Economic Importance); Science Herald Press: Budapest, Hungary, 2000; Volume 1, pp. 85–161. [Google Scholar]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Ultrastructure and function of insect chemosensilla. Annu. Rev. Entomol. 1980, 25, 27–47. [Google Scholar] [CrossRef]
- Turner, F.R.; Mahowald, A.P. Scanning electron microscopy of Drosophila melanogaster embryogenesis. Ii. Gastrulation and segmentation. Dev. Biol. 1977, 57, 403–416. [Google Scholar] [CrossRef]
- Landis, G.; Kelley, R.; Spradling, A.C.; Tower, J. The k43 gene, required for chorion gene amplification and diploid cell chromosome replication, encodes the Drosophila homolog of yeast origin recognition complex subunit 2. Proc. Natl. Acad. Sci. USA 1997, 94, 3888–3892. [Google Scholar] [CrossRef] [PubMed]
- Turner, F.R.; Mahowald, A.P. Scanning electron microscopy of Drosophila melanogaster embryogenesis. Iii. Formation of the head and caudal segments. Dev. Biol. 1979, 68, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Cinege, G.; Magyar, L.B.; Kovács, H.; Varga, V.; Bodai, L.; Zsindely, N.; Nagy, G.; Hegedűs, Z.; Hultmark, D.; Andó, I. Distinctive features of Zaprionus indianus hemocyte differentiation and function revealed by transcriptomic analysis. Front. Immunol. 2023, 14, 1322381. [Google Scholar] [CrossRef]
- Vommaro, M.L.; Donato, S.; Caputo, S.; Agostino, R.G.; Montali, A.; Tettamanti, G.; Giglio, A. Anatomical changes of tenebrio molitor and tribolium castaneum during complete metamorphosis. Cell Tissue Res. 2024, 396, 19–40. [Google Scholar] [CrossRef]
- Shanbhag, S.R.; Müller, B.; Steinbrecht, R.A. Atlas of olfactory organs of Drosophila melanogaster 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 1999, 28, 377–397. [Google Scholar] [CrossRef]
- Guillén, L.; López-Sánchez, L.; Velázquez, O.; Rosas-Saito, G.; Altúzar-Molina, A.; Stoffolano, J.G., Jr.; Ramírez-Vázquez, M.; Aluja, M. New insights on antennal sensilla of Anastrepha ludens (Diptera: Tephritidae) using advanced microscopy techniques. Insects 2023, 14, 652. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Zhang, R.; Chen, L. Sensilla on antenna and maxillary palp of Neoceratitis asiatica (Diptera: Tephritidae). Micron 2020, 138, 102921. [Google Scholar] [CrossRef]
- Rani, A.T.; Shashank, P.R.; Meshram, N.M.; Sagar, D.; Srivastava, C.; Pandey, K.K.; Singh, J. Morphological characterization of antennal sensilla of Earias vittella (Fabricius) (Lepidoptera: Nolidae). Micron 2021, 140, 102957. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. The variability of antennal sensilla in Naucoridae (Heteroptera: Nepomorpha). Sci. Rep. 2021, 11, 19651. [Google Scholar] [CrossRef]
- Liu, F.; Li, F.; Zhang, S.; Kong, X.; Zhang, Z. Ultrastructure of antennal sensilla of Erannis ankeraria staudinger (Lepidoptera: Geometridae). Microsc. Res. Tech. 2019, 82, 1903–1910. [Google Scholar] [CrossRef]
- Agrawal, S.; Grimaldi, D.; Fox, J.L. Haltere morphology and campaniform sensilla arrangement across Diptera. Arthropod Struct. Dev. 2017, 46, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Yan, S.C.; Yang, B.; Wang, G.R. Ultrastructural and descriptive study on the adult body surface of Heortia vitessoides (Lepidoptera: Crambidae). Insects 2023, 14, 687. [Google Scholar] [CrossRef]
- Ren, C.S.; Chang, Z.M.; Zu, Z.Y.; Han, L.; Chen, X.S.; Long, J.K. Comparison of morphological characteristics of antennae and antennal sensilla among four species of Bumblebees (Hymenoptera: Apidae). Insects 2023, 14, 232. [Google Scholar] [CrossRef]
- Pezzi, M.; Munari, C.; Mistri, M.; Scapoli, C.; Chicca, M.; Leis, M.; Scieuzo, C.; Franco, A.; Salvia, R.; Ferracini, C.; et al. Morphological characterization of the antenna of Torymus sinensis (Hymenoptera: Torymidae) and a comparison within the superfamily chalcidoidea. Arthropod Struct. Dev. 2024, 78, 101325. [Google Scholar] [CrossRef]
- Limberger, G.M.; Brugnera, R.; Fonseca, D.B.D. Antennal morphology and sensilla ultrastructure of Ascia monuste (Linnaeus) (Lepidoptera: Pieridae). Micron 2021, 142, 103000. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, J.; Ma, M.; Lu, P.; Liu, S.; Guo, K.; Xu, R.; Qiao, H.; Xu, C.Q. Morphology and distribution of antennal sensilla in the gall midge Gephyraulus lycantha (Diptera: Cecidomyiidae). Micron 2021, 145, 103061. [Google Scholar] [CrossRef]
- Awad, A.A.; Mohamed, H.O.; Ali, N.A. Differences in antennal sensillae of male and female peach fruit flies in relation to hosts. J. Insect Sci. 2015, 15, 178. [Google Scholar] [CrossRef]
- Otranto, D.; Cantacessi, C.; Testini, G.; Lia, R.P. Phortica variegata as an intermediate host of Thelazia callipaeda under natural conditions: Evidence for pathogen transmission by a male arthropod vector. Int. J. Parasitol. 2006, 36, 1167–1173. [Google Scholar] [CrossRef]
- Pusawang, K.; Sriwichai, P.; Aupalee, K.; Yasanga, T.; Phuackchantuck, R.; Zhong, D.; Yan, G.; Somboon, P.; Junkum, A.; Wongpalee, S.P.; et al. Antennal morphology and sensilla ultrastructure of the malaria vectors, Anopheles maculatus and An. Sawadwongporni (Diptera: Culicidae). Arthropod Struct. Dev. 2023, 76, 101296. [Google Scholar] [CrossRef]
- Clyne, P.; Grant, A.; O’Connell, R.; Carlson, J.R. Odorant response of individual sensilla on the Drosophila antenna. Invert. Neurosci. 1997, 3, 127–135. [Google Scholar] [CrossRef]
- Zhang, N.; Simpson, J.H. A pair of commissural command neurons induces Drosophila wing grooming. iScience 2022, 25, 103792. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.F. The organization of the chemosensory system in Drosophila melanogaster: A review. Cell Tissue Res. 1994, 275, 3–26. [Google Scholar] [CrossRef]
- Winding, M.; Pedigo, B.D.; Barnes, C.L.; Patsolic, H.G.; Park, Y.; Kazimiers, T.; Fushiki, A.; Andrade, I.V.; Khandelwal, A.; Valdes-Aleman, J.; et al. The connectome of an insect brain. Science 2023, 379, eadd9330. [Google Scholar] [CrossRef]
- Park, S.K.; Shanbhag, S.R.; Wang, Q.; Hasan, G.; Steinbrecht, R.A.; Pikielny, C.W. Expression patterns of two putative odorant-binding proteins in the olfactory organs of Drosophila melanogaster have different implications for their functions. Cell Tissue Res. 2000, 300, 181–192. [Google Scholar] [CrossRef]
- Couto, A.; Alenius, M.; Dickson, B.J. Molecular, anatomical, and functional organization of the drosophila olfactory system. Curr. Biol. 2005, 15, 1535–1547. [Google Scholar] [CrossRef]
- Stocker, R.F. Drosophila as a focus in olfactory research: Mapping of olfactory sensilla by fine structure, odor specificity, odorant receptor expression, and central connectivity. Microsc. Res. Tech. 2001, 55, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Boto, T.; Gomez-Diaz, C.; Alcorta, E. Elements of olfactory reception in adult Drosophila melanogaster. Anat. Rec. 2013, 296, 1477–1488. [Google Scholar] [CrossRef]


| Type | Sample Size/N | Length (μm) | ||||
|---|---|---|---|---|---|---|
| Scape | Pedicel | Flagellum | Total | Arista | ||
| Male | 6 | 40.88 ± 2.00 | 116.30 ± 4.86 *a | 180.1 ± 9.51 *c | 337.2 ± 12.57 | 466.9 ± 33.97 |
| Female | 6 | 37.63 ± 6.53 | 94.28 ± 7.60 *a | 204.7 ± 4.83 *c | 336.6 ± 13.36 | 323.1 ± 57.47 |
| Type of Sensilla | Subtype | Sample Size/N | Sex | Length/μm | Basal Diameter/μm | Tip Diameter/μm |
|---|---|---|---|---|---|---|
| Antennae | ||||||
| Chaetica sensilla | ChI | 10 | Male | 56.53 ± 1.62 *b | 4.71 ± 0.31 *a | 1.53 ± 1.87 |
| Female | 77.34 ± 7.08 *b | 5.79 ± 0.24 *a | 1.74 ± 0.25 | |||
| ChII | 10 | Male | 31.19 ± 2.34 *b | 3.72 ± 0.31 | 0.68 ± 0.22 *c | |
| Female | 24.63 ± 0.95 *b | 3.69 ± 0.27 | 1.00 ± 0.10 *c | |||
| Trichoid sensilla Intermediate sensilla | T | 10 | Male | 23.10 ± 0.82 | 2.40 ± 0.14 *a | 0.53 ± 0.03 |
| Female | 24.63 ± 0.95 | 2.79 ± 0.12 *a | 0.54 ± 0.03 | |||
| I | 10 | Male | 9.94 ± 0.90 *a | 2.06 ± 0.06 *a | 0.45 ± 0.02 *a | |
| Female | 13.65 ± 0.90 *a | 2.55 ± 0.05 *a | 0.52 ± 0.03 *a | |||
| Coeloconic sensilla | C | 10 | Male | 5.56 ± 0.12 *b | 2.00 ± 0.06 *a | 0.48 ± 0.04 |
| Female | 4.77 ± 0.22 *b | 1.80 ± 0.07 *a | 0.51 ± 0.03 | |||
| Basiconic sensilla | TB | 10 | Male | 9.66 ± 0.34 *a | 2.37 ± 0.08 *c | 0.56 ± 0.03 |
| Female | 6.72 ± 0.32 *a | 2.79 ± 0.17 *c | 0.60 ± 0.02 | |||
| LB | 10 | Male | 8.40 ± 0.27 *b | 2.15 ± 0.07 | 0.61 ± 0.04 | |
| Female | 6.60 ± 0.49 *b | 2.08 ± 0.11 | 0.54 ± 0.03 | |||
| Maxillary palp | ||||||
| Chaetica sensilla | ChI | 10 | Male | 71.93 ± 3.51 *a | 4.18 ± 0.30 | 1.66 ± 0.20 |
| Female | 94.18 ± 3.17 *a | 4.79 ± 0.44 | 1.47 ± 0.07 | |||
| ChII | 10 | Male | 40.83 ± 1.83 | 4.30 ± 0.37 *c | 1.85 ± 0.23 | |
| Female | 41.63 ± 2.32 | 3.10 ± 0.25 *c | 1.38 ± 0.10 | |||
| Basiconic sensilla | SB | 6 | Male | 2.19 ± 0.63 | 1.46 ± 0.37 | 0.64 ± 0.05 *a |
| Female | 1.22 ± 0.05 | 0.80 ± 0.06 | 0.26 ± 0.03 *a | |||
| TB | 10 | Male | 7.25 ± 0.40 *b | 2.57 ± 0.10 *a | 0.79 ± 0.04 | |
| Female | 11.09 ± 0.15 *b | 3.13 ± 0.12 *a | 0.93 ± 0.06 | |||
| LB | 10 | Male | 8.25 ± 1.11 | 2.48 ± 0.09 *a | 0.71 ± 0.03 *b | |
| Female | 10.45 ± 0.57 | 2.85 ± 0.14 *a | 0.91 ± 0.07 *b | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Luo, Y.; Wang, Y.; Cui, H.; Gou, Y.; Zhou, J.; Luo, B.; Liu, H.; Yan, R.; Wang, L. Ultrastructural Characterization of Developmental Stages and Head Sensilla in Portici okadai, Vector of Thelazia callipaeda. Insects 2025, 16, 539. https://doi.org/10.3390/insects16050539
Sun D, Luo Y, Wang Y, Cui H, Gou Y, Zhou J, Luo B, Liu H, Yan R, Wang L. Ultrastructural Characterization of Developmental Stages and Head Sensilla in Portici okadai, Vector of Thelazia callipaeda. Insects. 2025; 16(5):539. https://doi.org/10.3390/insects16050539
Chicago/Turabian StyleSun, Da, Yang Luo, Yikang Wang, Hongle Cui, Yanting Gou, Juan Zhou, Bo Luo, Hui Liu, Rong Yan, and Lingjun Wang. 2025. "Ultrastructural Characterization of Developmental Stages and Head Sensilla in Portici okadai, Vector of Thelazia callipaeda" Insects 16, no. 5: 539. https://doi.org/10.3390/insects16050539
APA StyleSun, D., Luo, Y., Wang, Y., Cui, H., Gou, Y., Zhou, J., Luo, B., Liu, H., Yan, R., & Wang, L. (2025). Ultrastructural Characterization of Developmental Stages and Head Sensilla in Portici okadai, Vector of Thelazia callipaeda. Insects, 16(5), 539. https://doi.org/10.3390/insects16050539





