Effect of Pyrethroids on the Colony Growth and Metabolic Activity of Entomopathogenic Fungi of the Beauveria Genus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. The Insecticides Used in the Experiment
- A—dose 10 times lower than recommended
- B—recommended field dose
- C—dose 10 times higher than recommended
2.3. Effect on Colony Growth
2.4. Metabolic Activity of Beauveria Strains
2.5. Statistical Analyses
- -
- m is the population average;
- -
- yij is the value of the examined trait (colony growth);
- -
- ai is the effect of the ith level of factor A (fungal species, dose of preparation, observation time); and
- -
- eij is the random error.
3. Results
3.1. Effect on Colony Growth of EPF
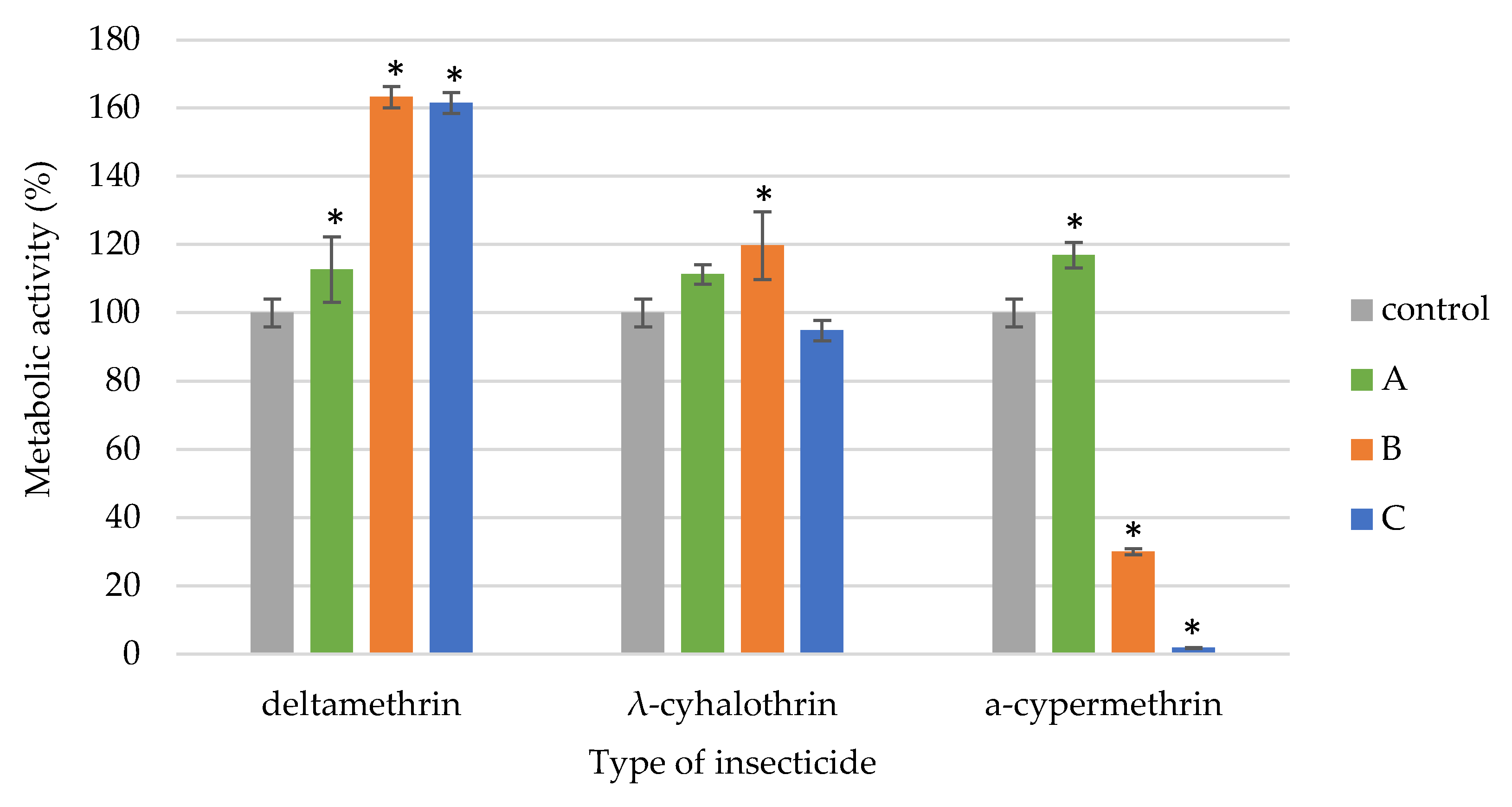
3.2. Insecticides Effects on Metabolic Activity of EPF
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sosnowska, D. The contribution of conservation biological control method to integrated plant protection and organic farming. Prog. Plant Prot. 2018, 58, 288–293. [Google Scholar] [CrossRef]
- Ginter, A. Small Farms Facing the Challenges of Sustainability and the Green Deal; Scientific Dissertation; UPH: Siedlce, Poland, 2021; ISBN 978-83-66541-76-4. [Google Scholar]
- Majchrowska-Safaryan, A.; Tkaczuk, C.; Wrzosek, M. The effect of humic substances on the colony growth and conidial germination of entomopathogenic fungi from the genus Metarhizium. Sustainability 2024, 16, 3616. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Chukwuneme, C.F.; Samuel, O.; Olanrewaju, M.A.; Adegboyega, T.T.; Babalola, O.O. Contribution of microbial inoculants in sustainable maintenance of human health, including test methods and evaluation of safety of microbial pesticide microorganisms. In Botanicals and Microorganisms for Improving Agriculture and Human Health; Logos Verlag Berlin GmbH: Berlin, Germany, 2021; Volume 1, pp. 207–240. [Google Scholar]
- Ayilara, M.S.; Adeleke, B.S.; Babalola, O.O. Bioprospecting and challenges of plant microbiome research for sustainable agriculture, a review on soybean endophytic bacteria. Microb. Ecol. 2022, 85, 1113–1135. [Google Scholar] [CrossRef]
- Tkaczuk, C. Occurrence and Infective Potential of Entomopathigenic Fungi in Soils of Agrocenoses and Seminatural Habitats in the Agricultural Landscape; Scientific Dissertation No. 94; AP: Siedlce, Poland, 2008. [Google Scholar]
- Tomalak, M. Market for biological control agents and their legal regulation. Prog. Plant Prot. 2010, 50, 1053–1063. [Google Scholar]
- Fenibo, E.O.; Grace, N.I.; Weiz, N.; Tonderayi, M. The potential and green chemistry attributes of biopesticides for sustainable agriculture. Sustainability 2022, 14, 14417. [Google Scholar] [CrossRef]
- Šunjka, D.; Špela, M. An Alternative Source of Biopesticides and Improvement in Their Formulation—Recent Advances. Plants 2022, 11, 3172. [Google Scholar] [CrossRef] [PubMed]
- Rıos-Moreno, A.; Garrido-Jurado, I.; Resquın-Romero, G.; Arroyo-Manzanares, N.; Arce, L.; Quesada-Morenga, E. Destruxin A production by Metarhizium brunneum strains during transient endophytic colonisation of Solanum tuberosum. Biocontrol Sci. Technol. 2016, 26, 1574–1585. [Google Scholar] [CrossRef]
- Gao, B.J.; Mou, Y.N.; Tong, S.M. Subtilisin-like Pr1 proteases marking the evolution of pathogenicity in a wide-spectrum insect-pathogenic fungus. Virulence 2020, 11, 365–380. [Google Scholar] [CrossRef]
- Holka, M.; Kowalska, J. The potential of adjuvants used with microbiological control of insect pests with emphasis on organic farming. Agriculture 2023, 13, 1659. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 117. [Google Scholar] [CrossRef]
- Różalska, S.; Soliwoda, K.; Długoński, J. Synthesis of silver nanoparticles from Metarhizium robertsii waste biomass extract after nonylphenol degradation, and their antimicrobial and catalytic potential. RSC Adv. 2016, 6, 21475–21485. [Google Scholar] [CrossRef]
- Litwin, A.; Nowak, M.; Różalska, S. Entomopathogenic fungi: Unconventional applications. Rev. Environ. Sci. Biotechnol. 2020, 19, 23–42. [Google Scholar] [CrossRef]
- Litwin, A.; Bernat, P.; Nowak, M.; Słaba, M.; Różalska, S. Lipidomic response of the entomopathogenic fungus Beauveria bassiana to pyrethroids. Sci. Rep. 2021, 11, 21319. [Google Scholar] [CrossRef]
- Nowak, M.; Soboń, A.; Litwin, A.; Rożalska, S. 4-nnonylphenol degradation by the genus Metarhizium with cytochrome P450 involvement. Chemosphere 2019, 220, 324–334. [Google Scholar] [CrossRef]
- Dara, S.K. Entomopathogenic Microorganisms: Modes of Action and Role in IPM. Strawberries and Vegetables; ANR Blogs: Davis, CA, USA, 2017; pp. 1–7. [Google Scholar]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Tkaczuk, C.; Krzyczkowski, T.; Głuszczak, B.; Król, A. The influence of selected pesticides on the colony growth and conidial germination of the entomopathogenic fungus Beauveria bassiana (Bals.) Vuill. Prog. Plant Prot. 2012, 52, 969–974. [Google Scholar]
- Cycoń, M.; Piotrowska-Seget, Z. Pyrethroid-degrading microorganisms and their potential for the bioremediation of contaminated soils: A review. Front. Microbiol. 2016, 7, 1463. [Google Scholar] [CrossRef]
- Tkaczuk, C.; Majchrowska-Safaryan, A.; Głuszczak, B. The effect of selected pesticides approved for use in organic farming on the growth of entomopathogenic fungi. Prog. Plant Prot. 2016, 56, 265–271. [Google Scholar]
- Skolarczyk, J.; Pekar, J.; Nieradko-Iwanicka, B. Immune disorders induced by exposure to pyrethroid insecticides. Postep. Hig. Med. Dosw. 2017, 71, 446–453. [Google Scholar] [CrossRef]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef]
- Litwin, A.; Mironenka, J.; Bernat, P.; Soboń, A.; Różalska, S. Accumulation of pyrethroids induces changes in metabolism of the entomopathogenic fungus Beauveria bassiana—Proteomic and lipidomic background. Ecotoxicol. Environ. Saf. 2023, 249, 114418. [Google Scholar] [CrossRef] [PubMed]
- Forlani, L.; Juarez, M.P.; Lavarías, S.; Pedrini, N. Toxicological and biochemical response of the entomopathogenic fungus Beauveria bassiana after exposure to deltamethrin. Pest Manag. Sci. 2014, 70, 751–756. [Google Scholar] [CrossRef]
- Mayerhofer, J.; Enkerli, J.; Zelger, R.; Strasser, H. Biological control of the European cockchafer: Persistence of Beauveria brongniartii after long-term applications in the Euroregion Tyrol. BioControl 2015, 60, 617–629. [Google Scholar] [CrossRef]
- Samson, R.A.; Evans, H.C. Two new Beauveria spp. from South America. J. Invertebr. Pathol. 1982, 39, 93–97. [Google Scholar] [CrossRef]
- Humber, R.A. Manual of Techniques in Invertebrate Pathology. In Identification of Entomopathogenic Fung; Lacey, L.A., Ed.; Academic Press: London, UK, 2012; pp. 151–187. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Kovač, M.; Gorczak, M.; Wrzosek, M.; Tkaczuk, C.; Pernek, M. Identification of entomopathogenic fungi as naturally occurring enemies of the invasive oak lace bug, Corythucha arcuata (Say) (Hemiptera: Tingidae). Insects 2020, 11, 679. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new 432 generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Miętkiewski, R.T.; Pell, J.; Clark, S.J. Influence of pesticides use on the natural occurrence of etomopathogenic fungi in arable soils in the UK: Field and laboratory comparisons. Biocontrol Sci. Technol. 1997, 7, 565–575. [Google Scholar] [CrossRef]
- Rashid, M.; Baghdadi, A.; Sheikhi, A.; Pourian, H.R.; Gacavi, M. Compatability of Metarhizium anisopilae (Ascomycota: Hypocreales) with several insceticides. J. Plant Prot. Res. 2010, 50, 22–27. [Google Scholar] [CrossRef]
- Miętkiewski, R.; Miętkiewska, Z.; Sapieha, A.; Badowska-Czubik, T. Wpływ herbicydów na patogeniczność grzybów entomopatogennych. Zesz. Naukowe. Ser. Rolnictwo. Wyższa Szk. Rol. Pedagog. Siedlcach 1995, 37, 179–184. [Google Scholar]
- Miętkiewski, R.; Ignatowicz, S.; Górski, R. Porównanie wpływu BioNEMTM, insektycydu pochodzenia roślinnego i wybranych insektycydów syntetycznych na wzrost grzybni grzybów owadobójczych. Pestycydy 1996, 1, 15–27. [Google Scholar]
- Tkaczuk, C. Wpływ wybranych pestycydów stosowanych w ochronie sadów na wzrost grzybów owadobójczych. Biul. Nauk. 2001, 12, 375–383. (In Polish) [Google Scholar]
- Amutha, M.; Banu, G.J.; Surulivelu, T.; Gopalakrishnan, N. Effect of commonly used insecticides on the growth of white Muscardine fungus, Beauveria bassiana under laboratory conditions. J. Biopest. 2010, 3, 143–146. [Google Scholar]
- Widyaningsih, S.; Triasih, H.U.; Agustina, D. Effect pesticides to entomopathogen fungi from citrus orchard in vitro. IOP Conf. Ser. Earth Environ. Sci. 2021, 803, 012021. [Google Scholar] [CrossRef]
- Bhatt, P.; Huang, Y.; Zhan, H.; Chen, S. Insight into microbial applications for the biodegradation of pyrethroid insecticides. Front. Microbiol. 2019, 10, 1778. [Google Scholar] [CrossRef]
- Vänninen, I.; Hokkanen, H. Effect of pesticides on four species of entomopathogenic fungi in vitro. Ann. Agric. Fenn. 1998, 27, 345–353. [Google Scholar]
- Bajan, C.; Kmitowa, K. Thirty year studies on entomopathogenic fungi in the Institute of Ecology, PAS. Pol. Ecol. Stud. 1997, 23, 133–154. [Google Scholar]
- Mayerhofer, J.; Lutz, A.; Franco Widmer, F.; Rehner, S.A.; Leuchtmann, A.; Enkerli, J. Multiplexed microsatellite markers for seven Metarhizium species. J. Invertebr. Pathol. 2015, 132, 132–134. [Google Scholar] [CrossRef]
- Clark, R.A.; Casagrande, R.A.; Wallace, D.B. Influence of pesticides on Beauveria bassiana, a pathogen of the Colorado potato beetle. Environ. Entomol. 1982, 11, 67–70. [Google Scholar] [CrossRef]
- Bednarek, A.; Popowska-Nowak, E.; Pezowicz, E.; Kamionek, M. Integrated methods in pest control: Effect of insecticides on entomopathogenic fungi [Beauveria bassiana [Bals] Vuill., B. brongniartii [Sacc.]] and nematodes [Heterorhabditis megidis Poinar, Jackson, Klein, Steinernema feltiae Filipjev, S.Glaseri Steiner]. Pol. J. Ecol. 2004, 52, 223–228. [Google Scholar]
- Srinivasulu, M.; Ortiz, D.R. Effect of pesticides on bacterial and fungal populations in ecuadorian tomato cultivated soils. Environ. Process. 2017, 4, 93–105. [Google Scholar] [CrossRef]
- James, R.R.; Elzen, G.W. Anatgonism between Beauveria bassiana and imidachloprid when combined for Bemisia argentifolia (Homoptera: Aleuyrodidae) control. J. Econ. Entomol. 2001, 94, 357–361. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Dutta, S.; Dhar, T. In vitro compatibility of different entomopathogens to pesticides, plant growth regulators and micronutrients. Ann. Plant Prot. Sci. 2004, 12, 199–202. [Google Scholar]
- Kumar, S.S.; Monga, D.; Kumar, R.; Nagrale, D.T.; Hiremani, N.S.; Kranthi, S. Compatibility of entomopathogenic fungi with insecticides and their efficacy for IPM of Bemisia tabaci in cotton. J. Pestic. Sci. 2019, 44, 97–105. [Google Scholar] [CrossRef]
- Różalska, S.; Glińska, S.; Długoński, J. Metarhizium robertsii morphological fexibility during nonylphenol removal. Int. Biodeterior. Biodegrad. 2014, 95, 285–293. [Google Scholar] [CrossRef]
- Szewczyk, R.; Soboń, A.; Słaba, M.; Długoński, J. Mechanism study of alachlor biodegradation by Paecilomyces marquandii with proteomic and metabolomic methods. J. Hazard. Mater. 2015, 291, 52–64. [Google Scholar] [CrossRef]
- Stolarek, P.; Różalska, S.; Bernat, P. Lipidomic adaptations of the Metarhizium robertsii strain in response to the presence of butyltin compounds. Biochim. Biophys. Acta Biomembr. 2019, 1861, 316–326. [Google Scholar] [CrossRef]
- Subbanna, A.R.N.S.; Chandrashekara, C.; Stanley, J.; Mishra, K.K.; Mishra, P.K.; Pattanayak, A. Bio-efficacy of chitinolytic Bacillus thuringiensis isolates native to northwestern Indian Himalayas and their synergistic toxicity with selected insecticides. Pestic. Biochem. Physiol. 2019, 158, 166–174. [Google Scholar] [CrossRef]
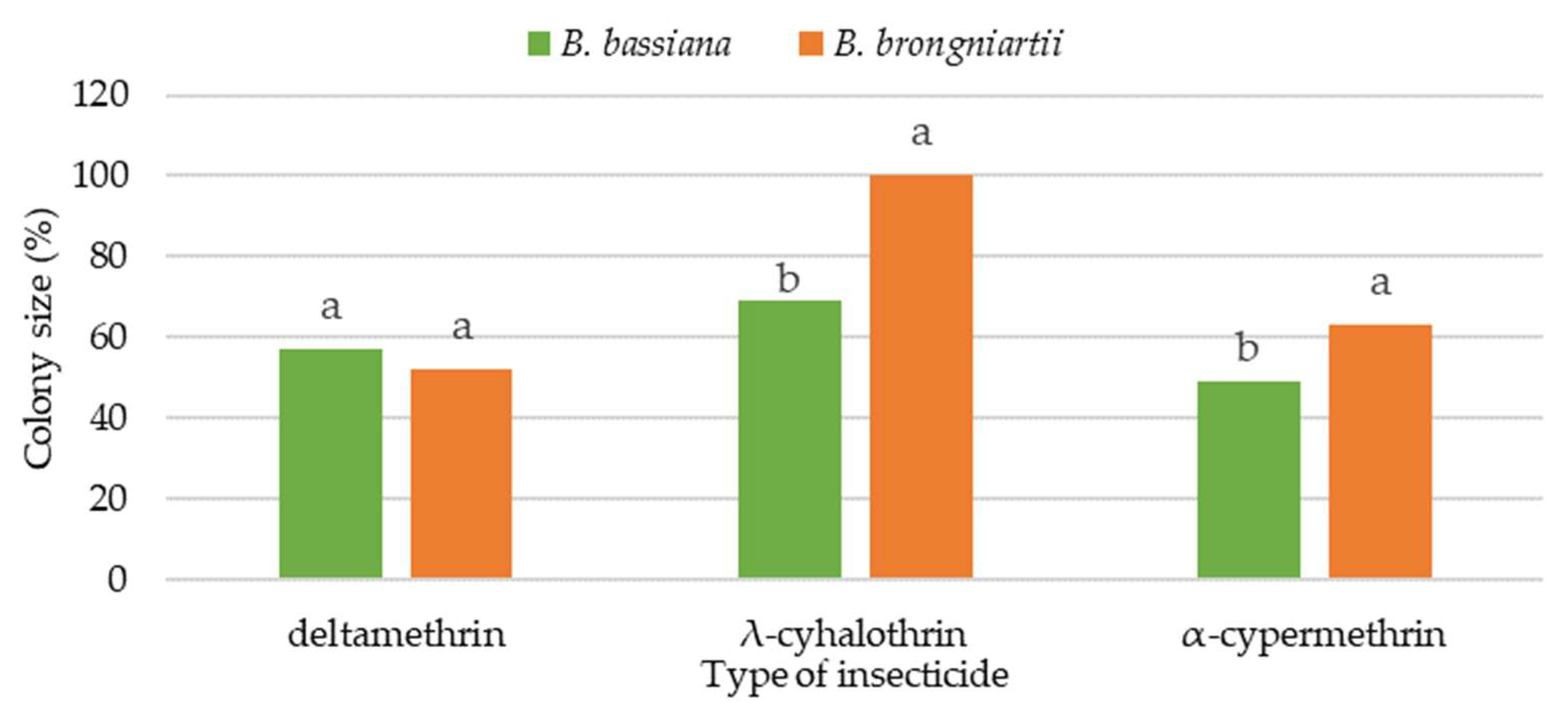

| Insecticide/Active Substance | Dose | Diameter of Fungus Colony (mm) | |||
|---|---|---|---|---|---|
| 5th Day | 10th Day | 15th Day | 20th Day | ||
| DECIS Mega 50 EW (deltamethrin) | A | 7.3 ± 0.47 b | 18.3 ± 1.24 a | 25.6 ± 1.24 a | 33.3 ± 1.84 b |
| B | 3.3 ± 0.47 c | 9.4 ± 1.24 b | 17.0 ± 1.50 b | 25.0 ± 0.81 c | |
| C | n.g. ± 0.0 d | 4.7 ± 1.69 c | 14.6 ± 2.49 b | 19.6 ± 1.24 d | |
| Control | 10.5 ± 0.57 a | 20.3 ± 0.50 a | 28.0 ± 0.81 a | 44.0 ± 1.41 a | |
| KARATE ZEON 050 CS (λ-cyhalothrin) | A | 9.6 ± 0.47 a | 19.3 ± 0.48 a | 24.6 ± 1.25 b | 31.3 ± 1.25 b |
| B | 7.6 ± 0.48 b | 17.7 ± 0.47 b | 23.6 ± 0.49 b | 30.3 ± 0.48 b | |
| C | 3.7 ± 0.94 c | 8.6 ± 0.94 c | 17.3 ± 1.33 c | 23.6 ± 1.42 c | |
| Control | 10.5 ± 0.57 a | 20.3 ± 0.50 a | 28.0 ± 0.81 a | 44.0 ± 1.41 a | |
| CYPERKIL MAX 500 EC (α-cypermethrin) | A | 9.2 ± 0.94 ab | 18.6 ± 0.47 a | 23.3 ± 0.47 b | 32.0 ± 0.81 b |
| B | 7.3 ± 1.70 b | 11.5 ± 1.25 b | 16.4 ± 0.47 c | 21.6 ± 0.94 c | |
| C | n.g. ± 0.0 c | 5.3 ± 1.70 c | 8.6 ± 0.94 d | 12.6 ± 0.94 d | |
| Control | 10.5 ± 0.57 a | 20.3 ± 0.50 a | 28.0 ± 0.81 a | 44.0 ± 1.41 a | |
| Insecticide/Active Substance | Dose | Diameter of Fungus Colony (mm) | |||
|---|---|---|---|---|---|
| 5th Day | 10th Day | 15th Day | 20th Day | ||
| DECIS Mega 50 EW (deltamethrin) | A | 5.0 ± 0.0 b | 17.6 ± 1.52 b | 25.6 ± 0.45 b | 31.6 ± 2.88 b |
| B | 2.3 ± 0.47 c | 7.6 ± 0.47 c | 15.0 ± 1.73 c | 23.3 ± 2.88 c | |
| C | n.g. ± 0.0 d | 4.3 ± 0.52 d | 9.6 ± 0.23 d | 15.3 ± 1.50 d | |
| Control | 13.0 ± 0.81 a | 28.3 ± 0.95 a | 36.7 ± 0.95 a | 45.0 ± 3.74 a | |
| KARATE ZEON 050 CS (λ-cyhalothrin) | A | 12.6 ± 0.57 a | 26.2 ± 1.52 ab | 31.7 ± 2.88 b | 46.0 ± 4.35 a |
| B | 11.6 ± 0.57 a | 24.6 ± 1.52 b | 33.3 ± 2.88 ab | 45.0 ± 3.00 a | |
| C | 2.6 ± 1.15 b | 8.6 ± 1.25 c | 13.5 ± 1.15 c | 19.7 ± 1.52 b | |
| Control | 13.0 ± 0.81 a | 28.3 ± 0.95 a | 36.7 ± 0.95 a | 45.0 ± 3.74 a | |
| CYPERKIL MAX 500 EC (α-cypermethrin) | A | 11.0 ± 1.00 a | 20.6 ± 2.08 b | 28.5 ± 2.08 b | 37.0 ± 1.73 b |
| B | 6.0 ± 0.57 b | 15.5 ± 1.15 c | 21.3 ± 1.52 c | 28.3 ± 2.88 c | |
| C | 2.0 ± 0.0 c | 4.0 ± 0.0 d | 6.6 ± 0.57 d | 10.2 ± 0.52 d | |
| Control | 13.0 ± 0.81 a | 28.3 ± 0.95 a | 36.7 ± 0.95 a | 45.0 ± 3.74 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majchrowska-Safaryan, A.; Różalska, S.; Tkaczuk, C.; Nowak, M. Effect of Pyrethroids on the Colony Growth and Metabolic Activity of Entomopathogenic Fungi of the Beauveria Genus. Insects 2025, 16, 533. https://doi.org/10.3390/insects16050533
Majchrowska-Safaryan A, Różalska S, Tkaczuk C, Nowak M. Effect of Pyrethroids on the Colony Growth and Metabolic Activity of Entomopathogenic Fungi of the Beauveria Genus. Insects. 2025; 16(5):533. https://doi.org/10.3390/insects16050533
Chicago/Turabian StyleMajchrowska-Safaryan, Anna, Sylwia Różalska, Cezary Tkaczuk, and Monika Nowak. 2025. "Effect of Pyrethroids on the Colony Growth and Metabolic Activity of Entomopathogenic Fungi of the Beauveria Genus" Insects 16, no. 5: 533. https://doi.org/10.3390/insects16050533
APA StyleMajchrowska-Safaryan, A., Różalska, S., Tkaczuk, C., & Nowak, M. (2025). Effect of Pyrethroids on the Colony Growth and Metabolic Activity of Entomopathogenic Fungi of the Beauveria Genus. Insects, 16(5), 533. https://doi.org/10.3390/insects16050533







