Diversity and the Origin of Perlodinella Klapálek 1912 (Plecoptera: Perlodidae) in Qinghai Province, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Filed Observation and Specimen Preparation
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Genetic Analyses
3. Results and Discussion
3.1. Genetic Diversity and Species Identification Based on COI Sequences
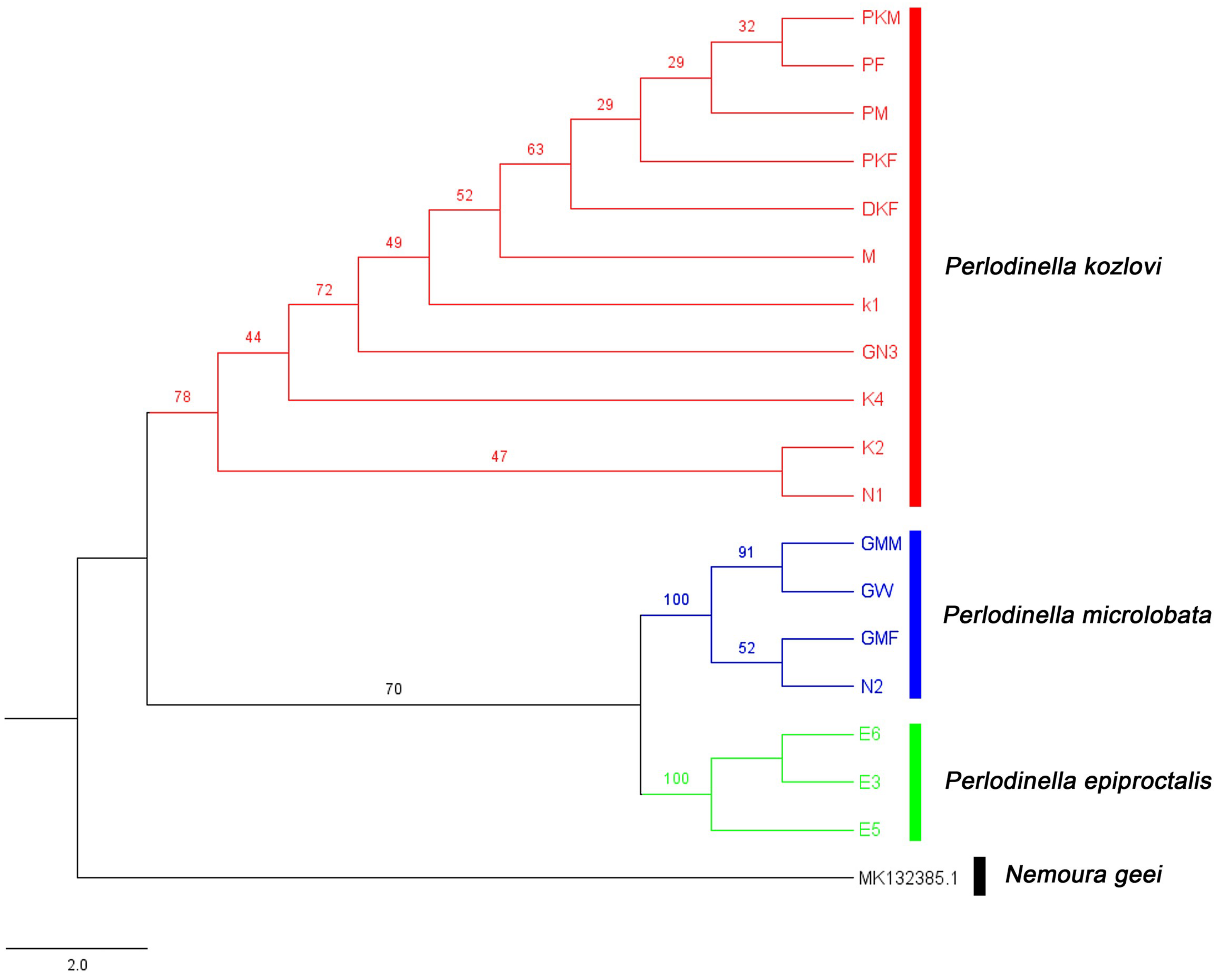
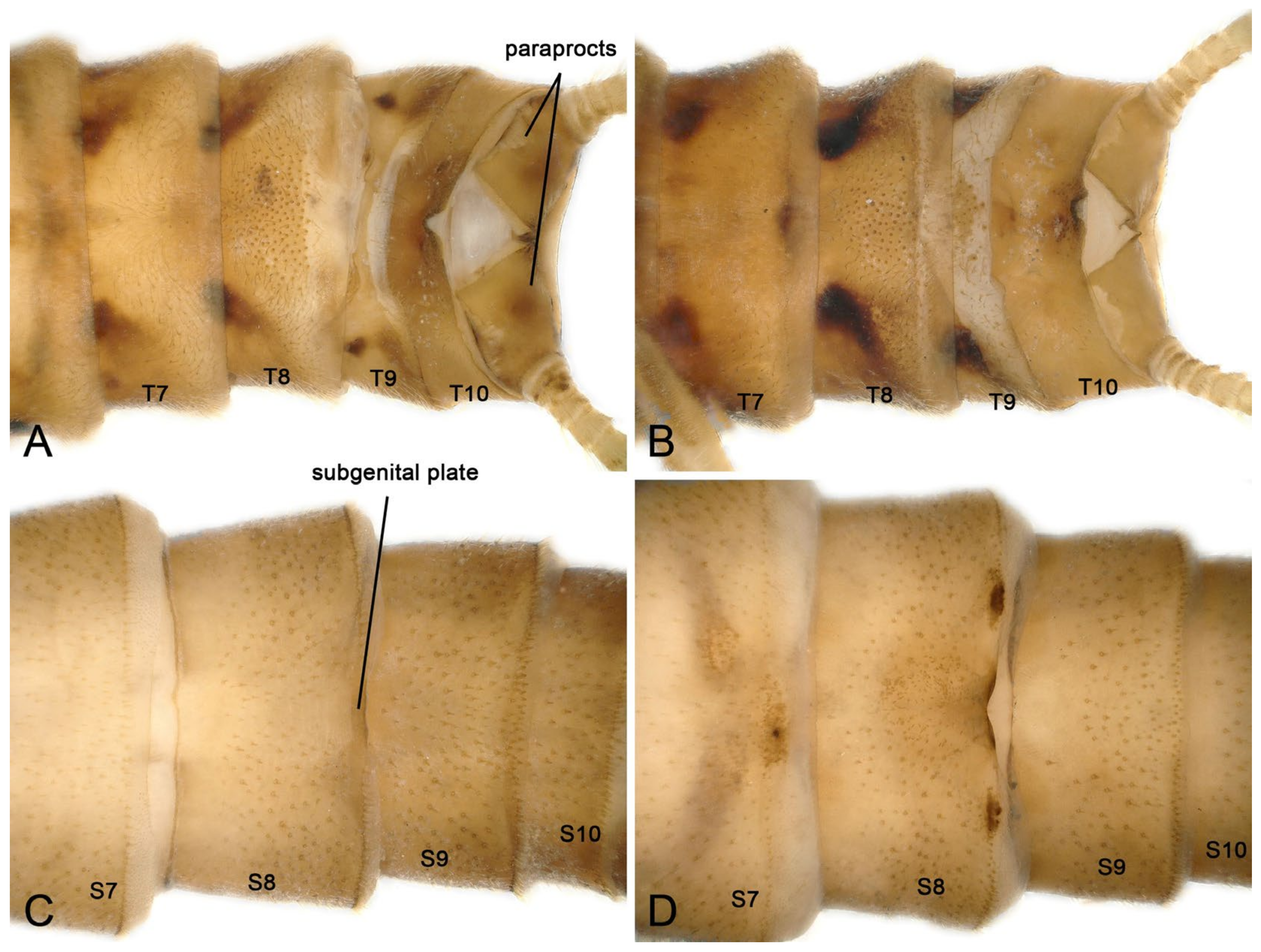
3.2. Species Diversity and the Intraspecific Morphological Variability
- Perlodinella epiproctalis (Zwick, 1997) (Figure 2)
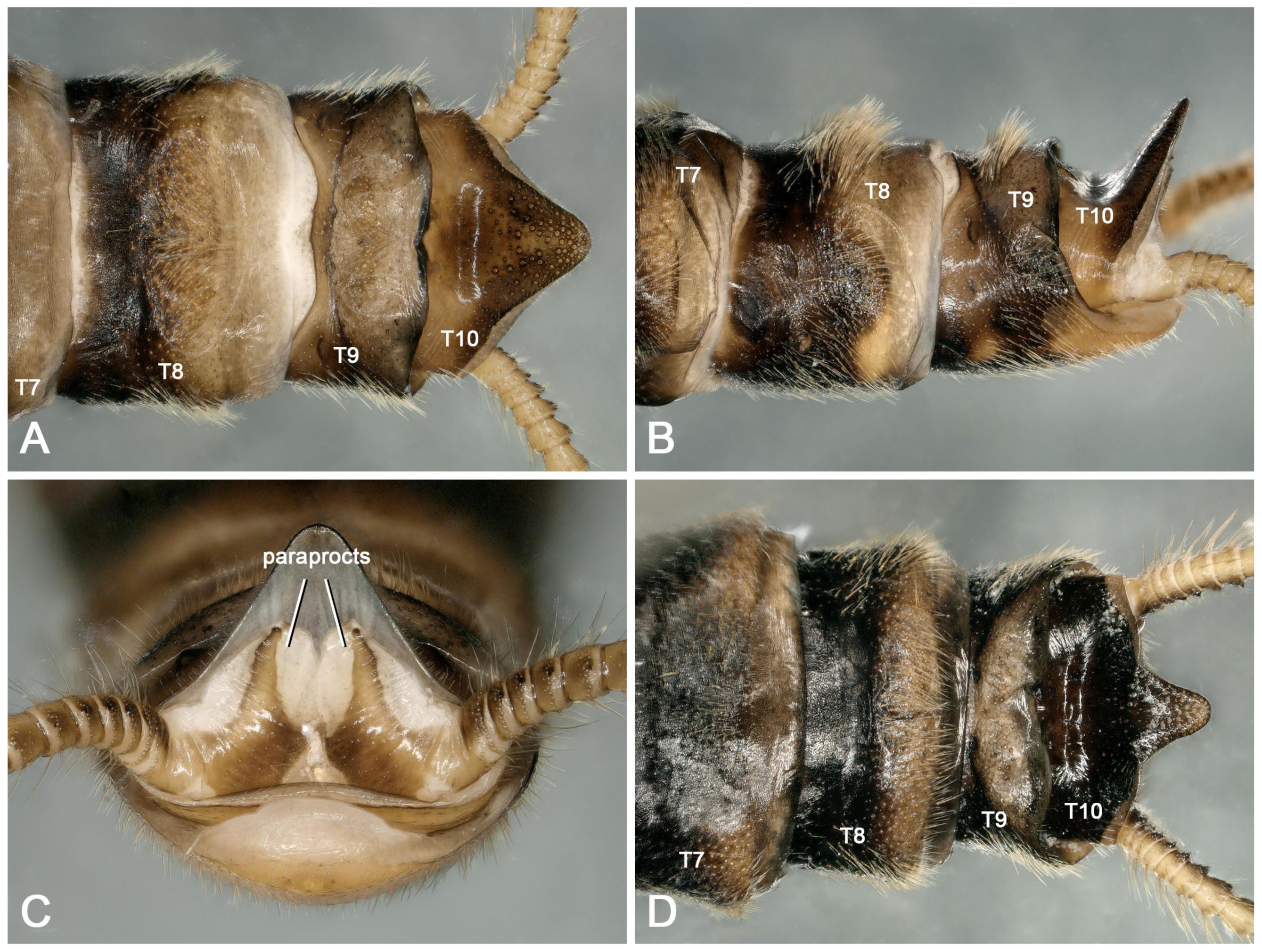
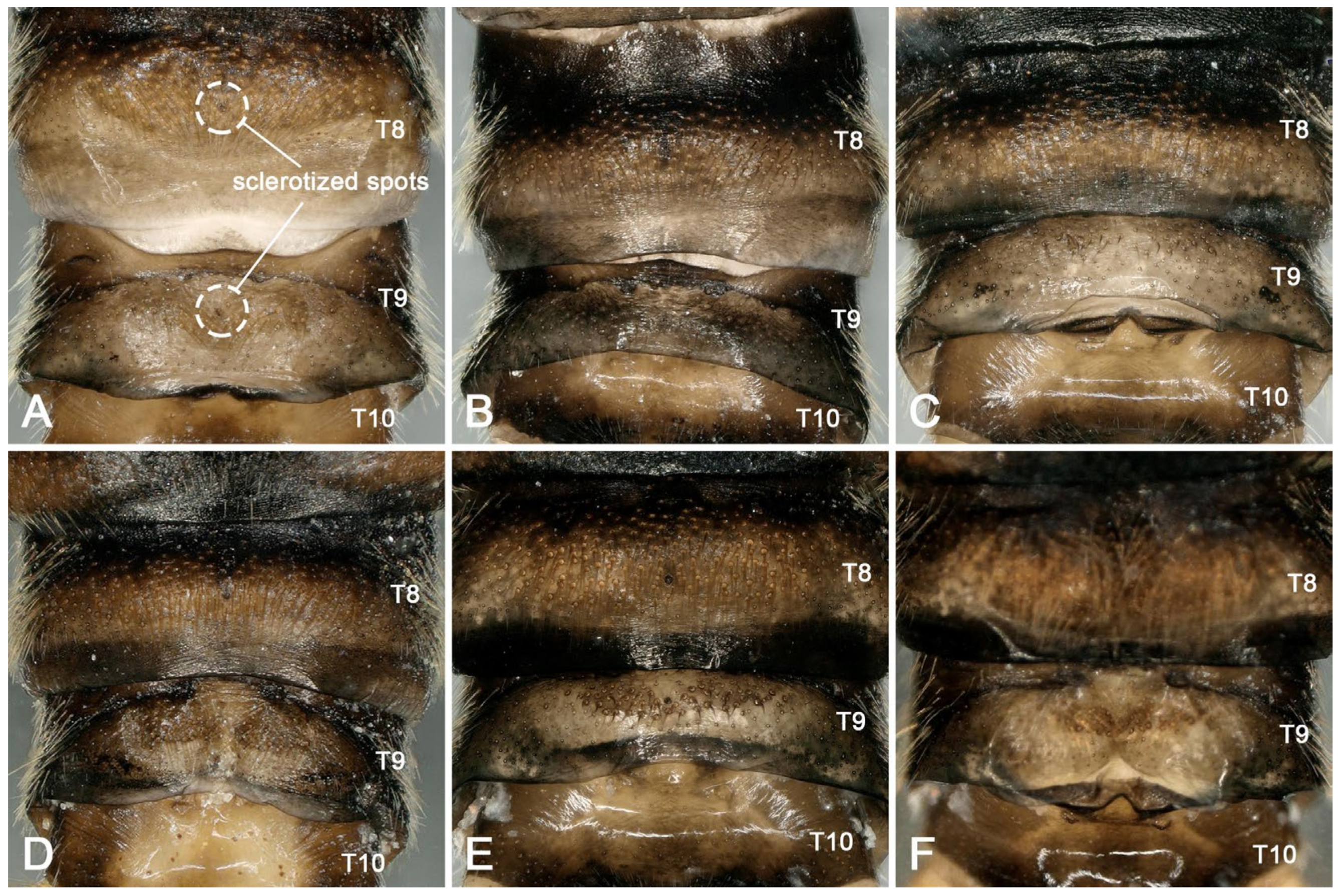


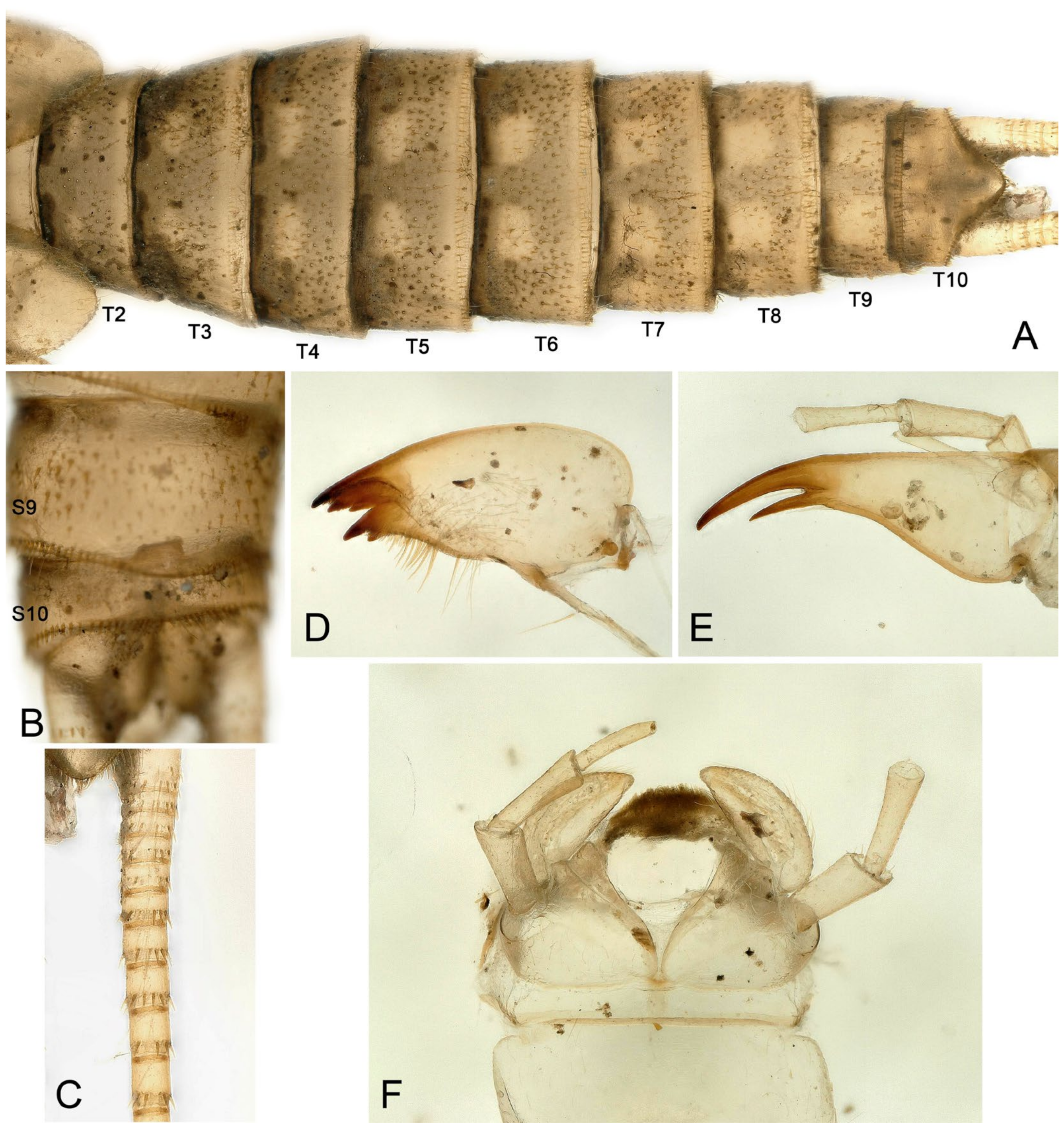
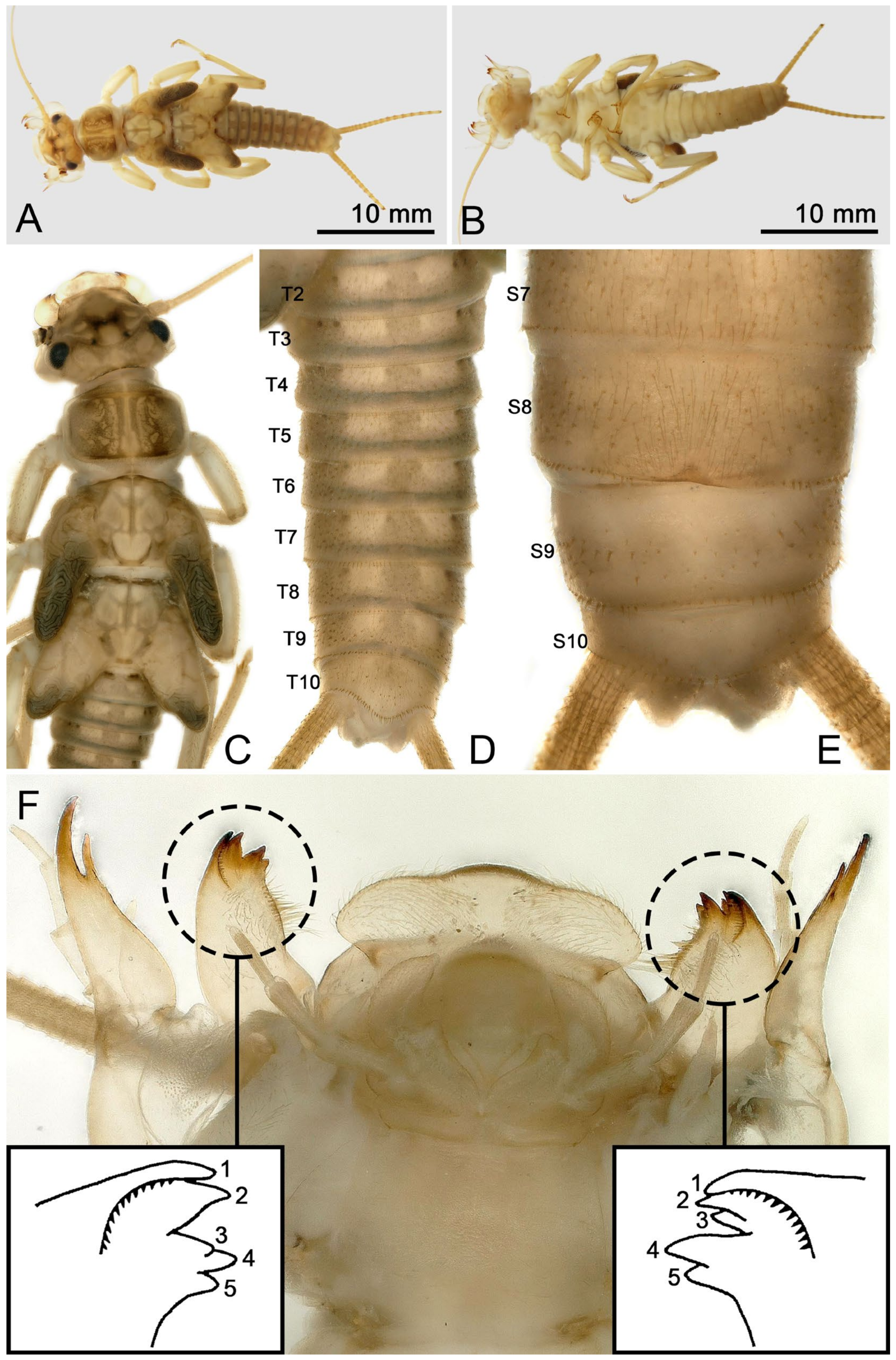
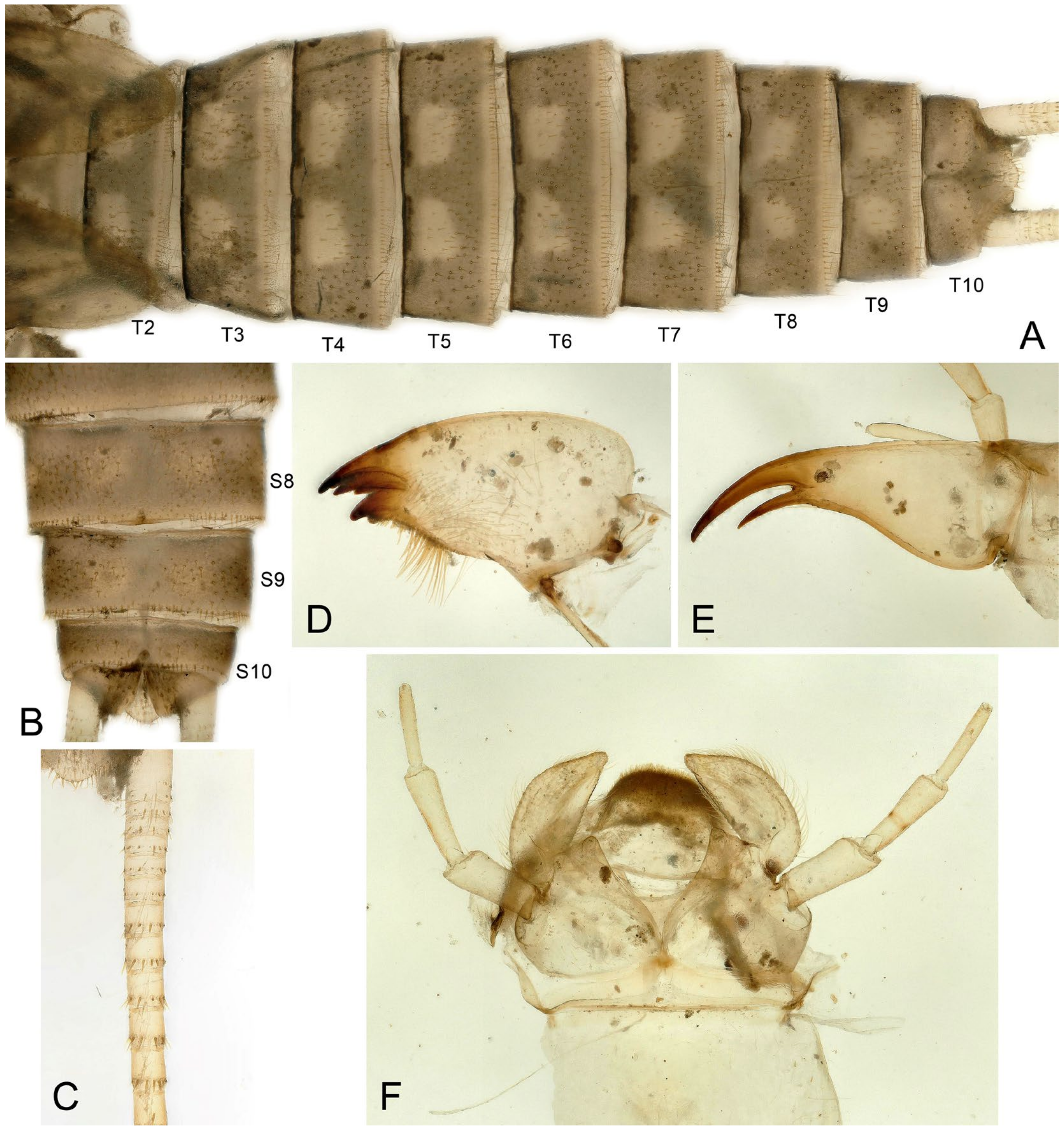
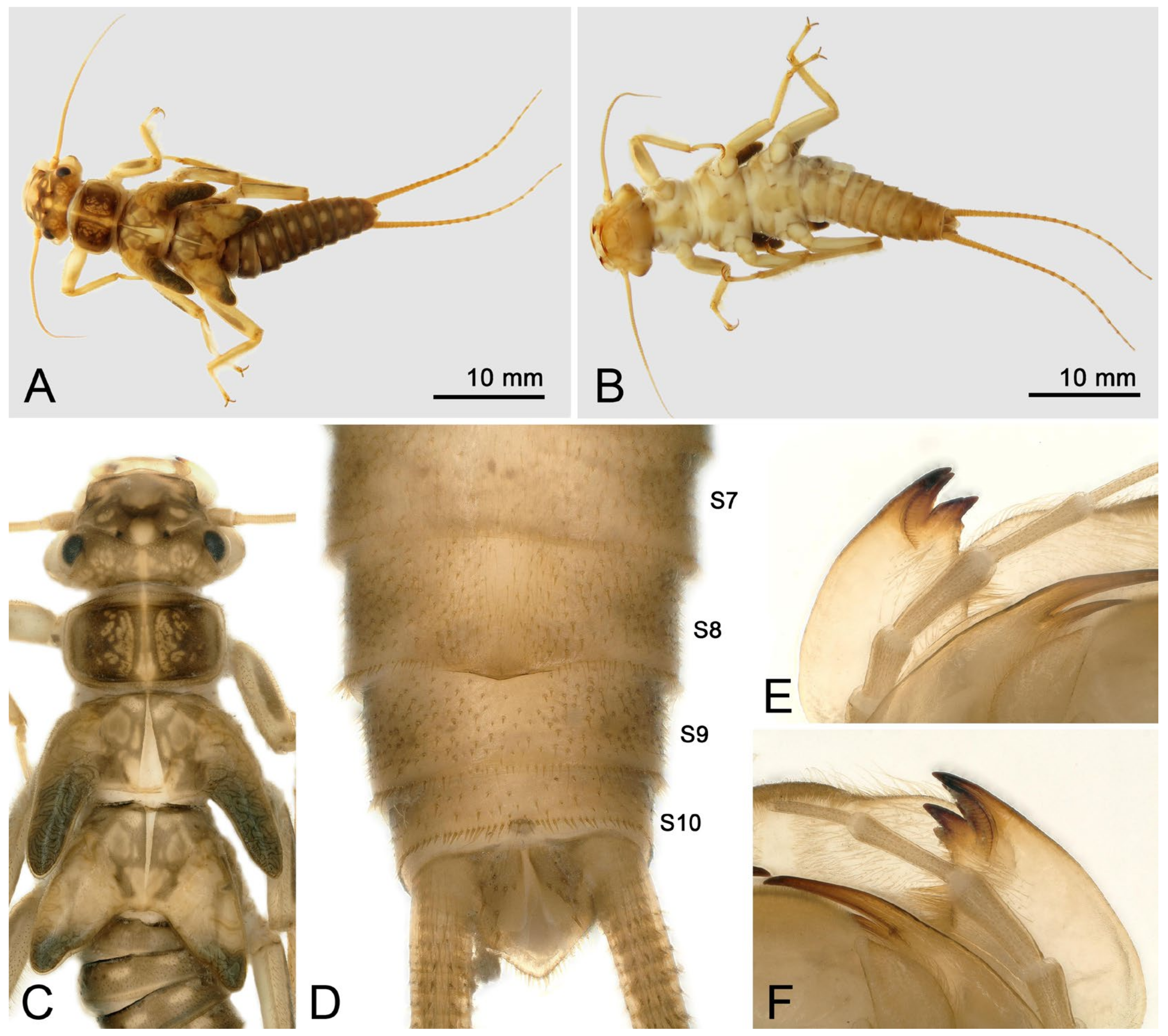
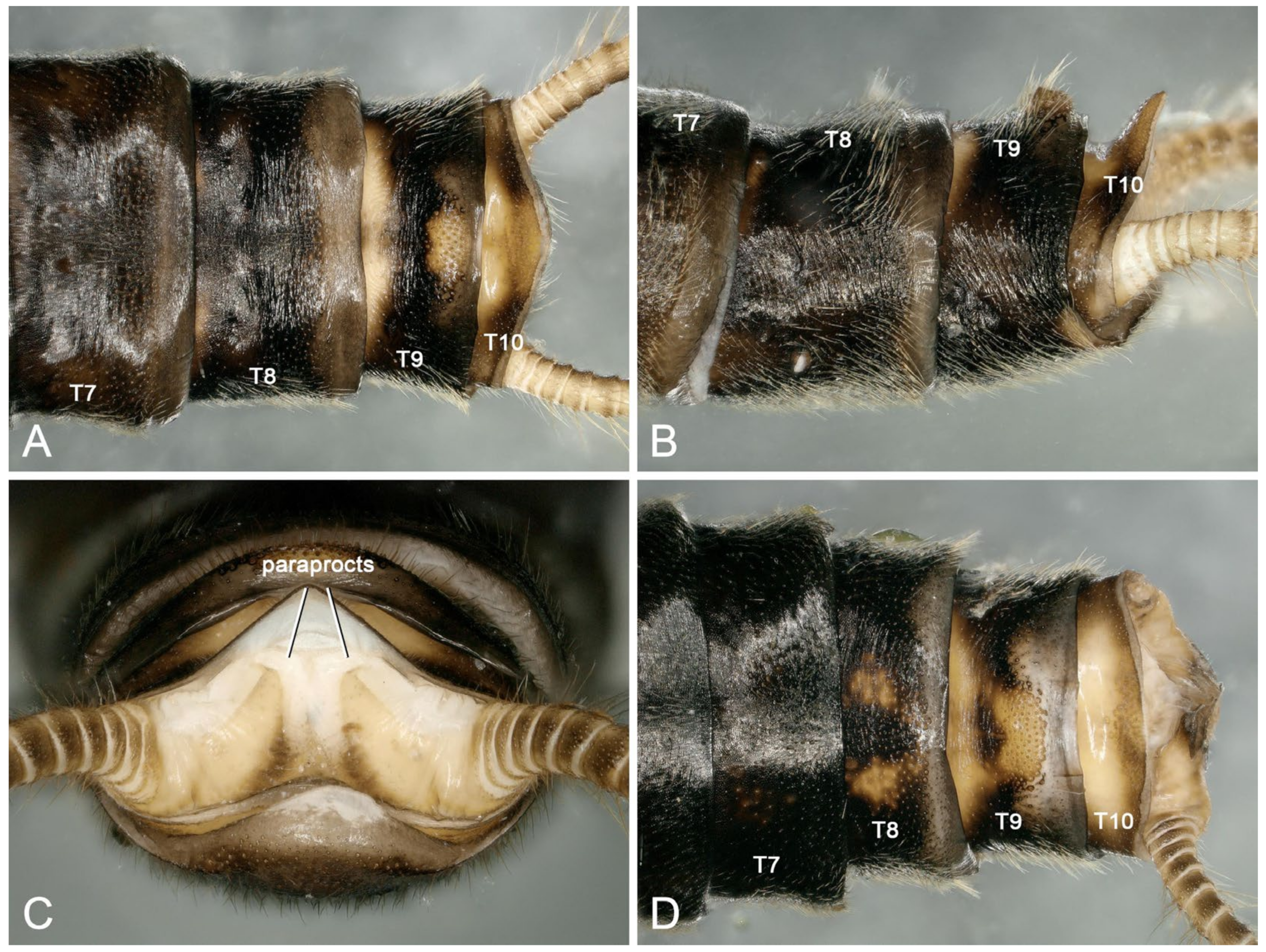


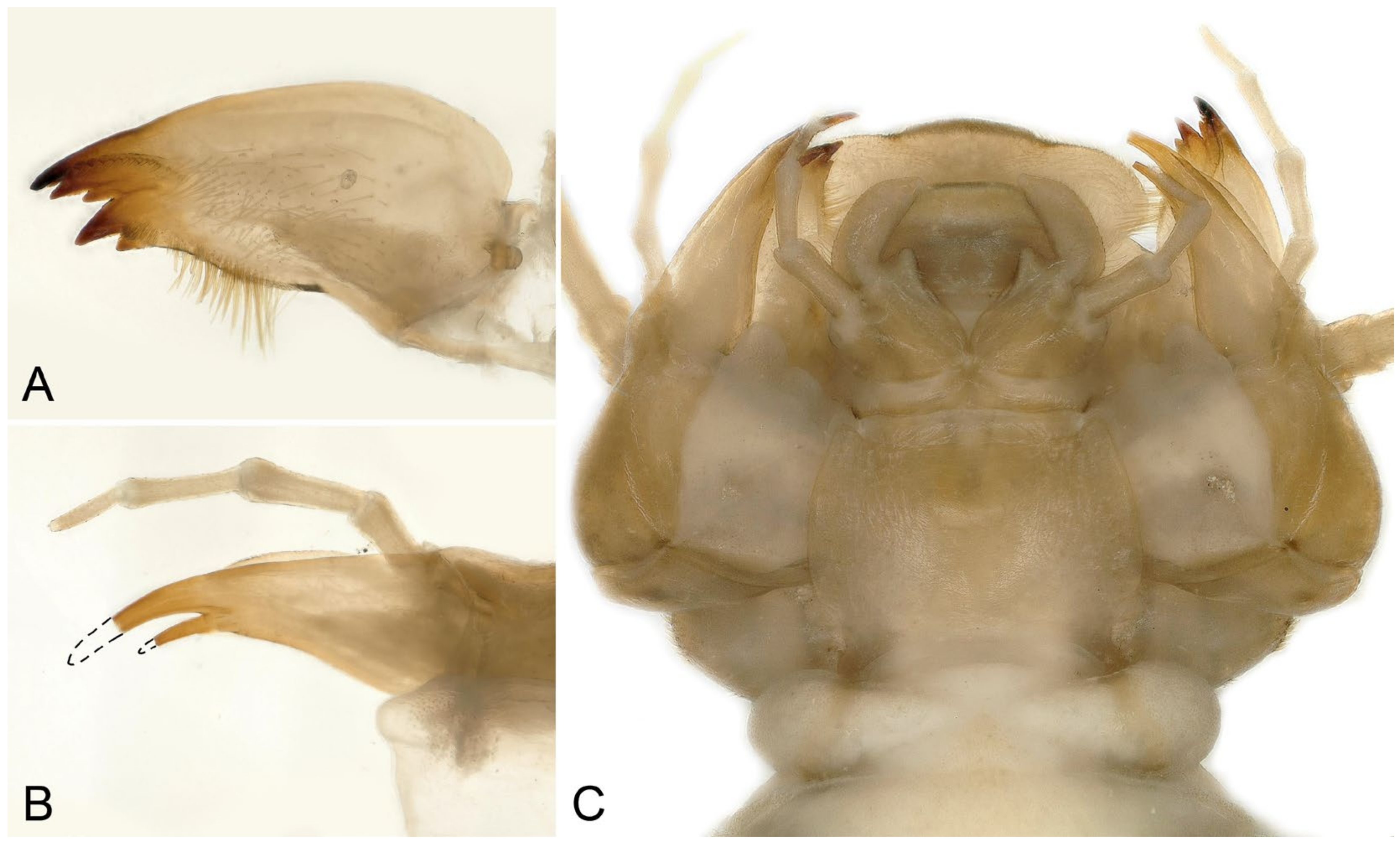
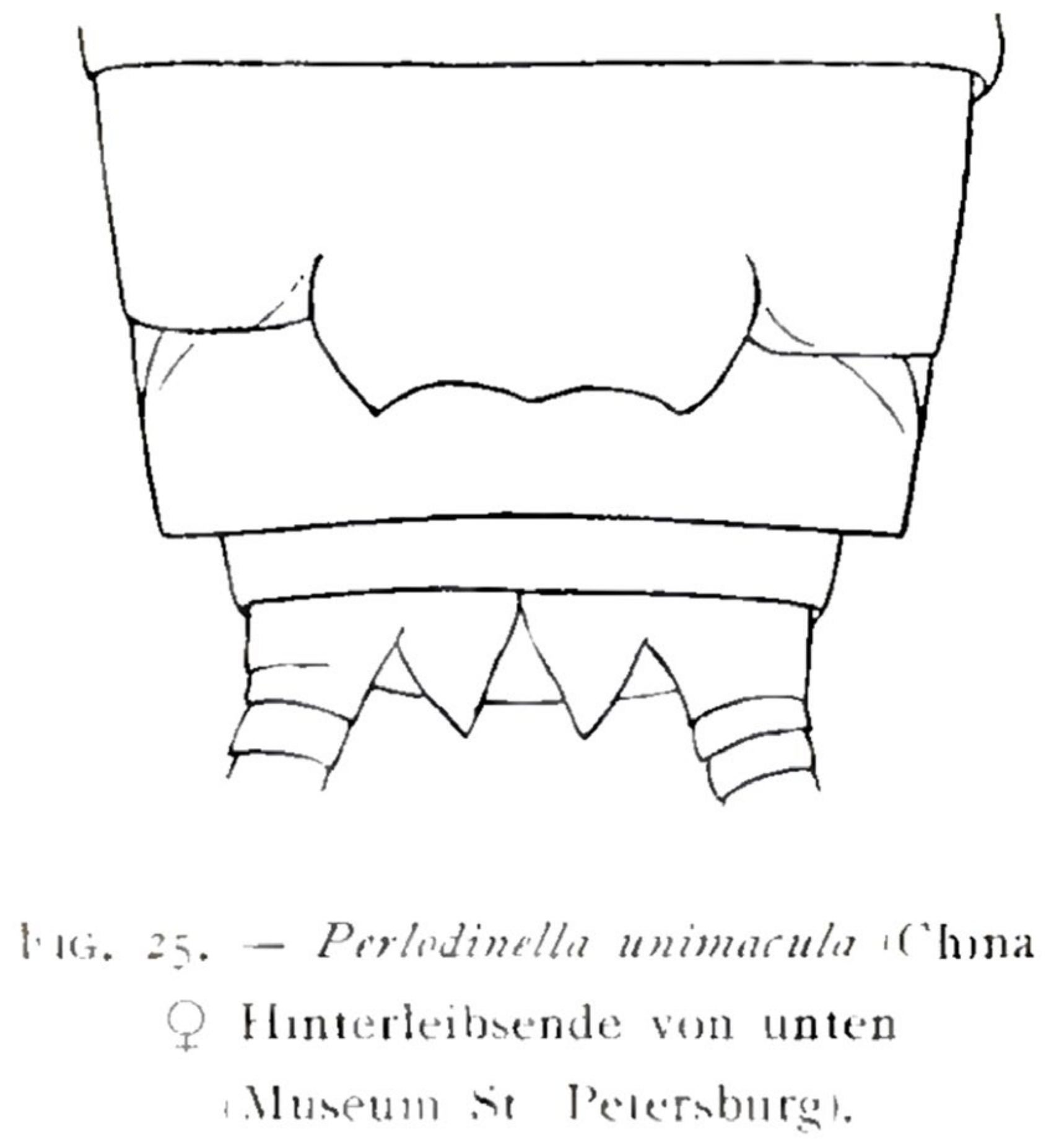
- Perlodinella unimacula Klapálek, 1912 (nomen dubium)
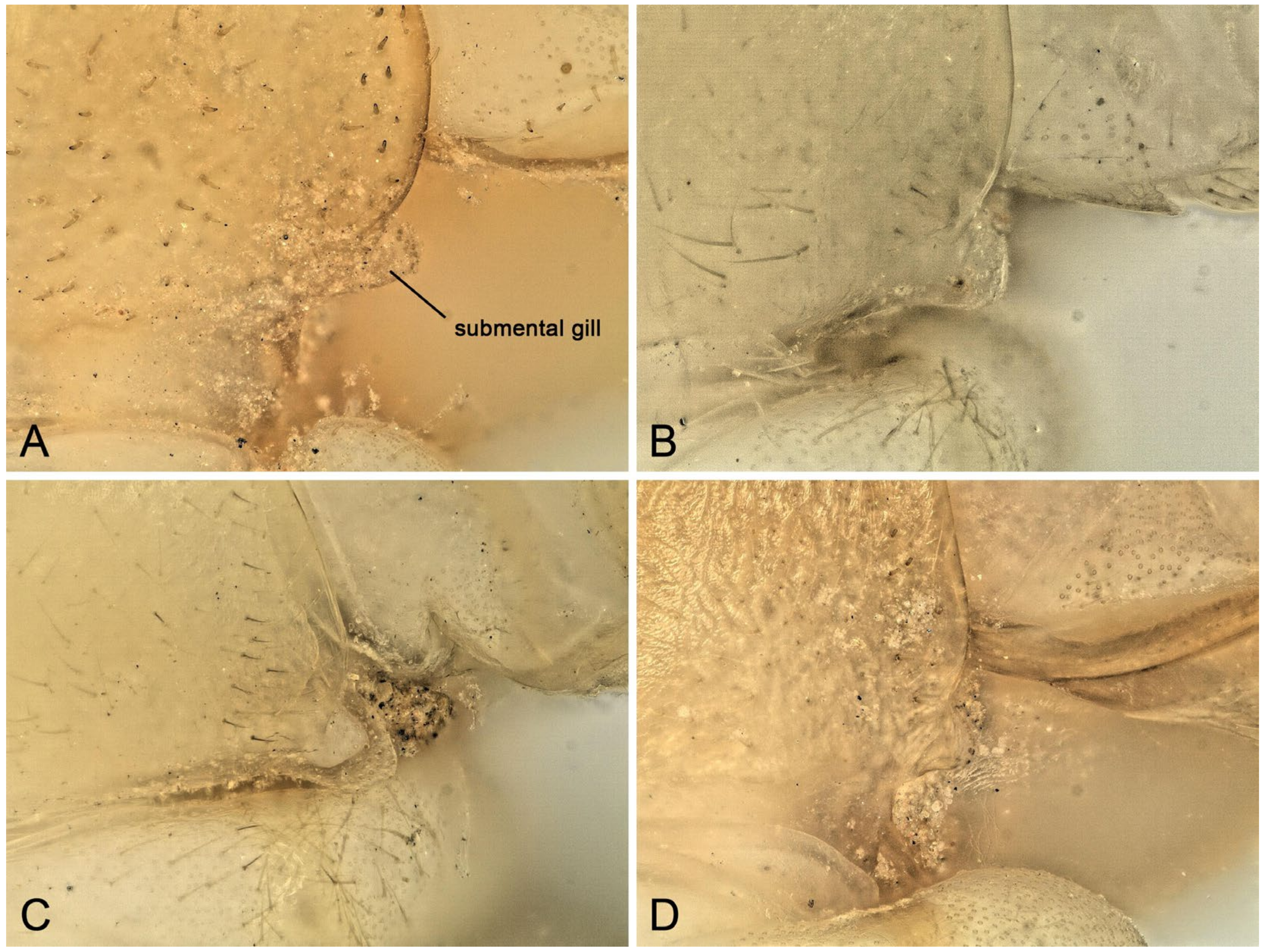
3.3. Biological Adaptations in Natural and Artificial Landscapes

3.4. The Threat from Fungal Infections
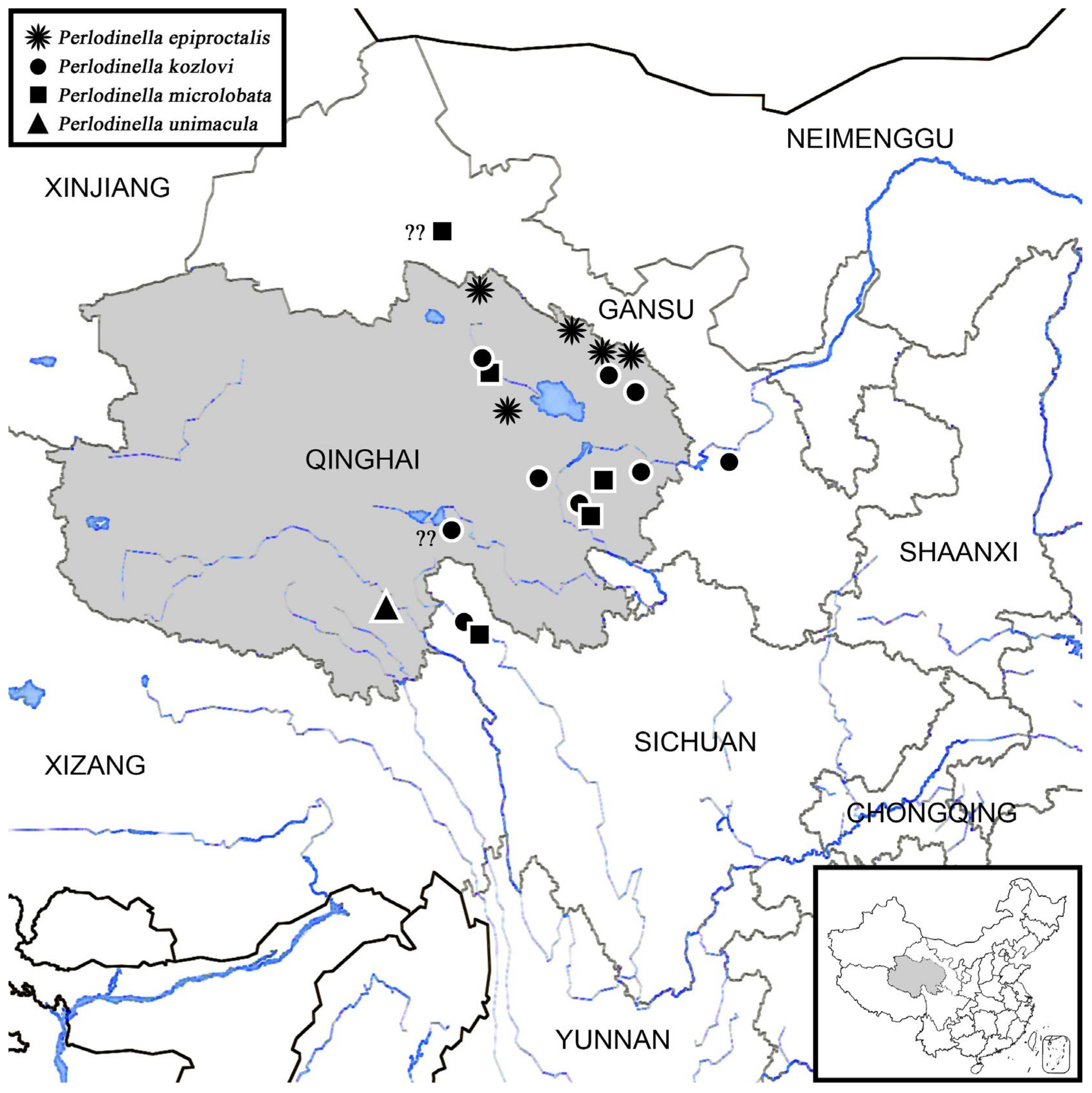
3.5. The Distribution, Origin, and Immigration
- (1)
- The southward expansion of the population is obstructed by mountain ranges, while its northward dispersal is hindered by deserts or arid regions: Perlodinella may not have the ability to cross the higher-altitude Himalayan and Hengduan Mountains to spread southward. Additionally, the regions north and west of the Altyn Tagh-Qilian Mountains, being close to deserts and lacking streams, have prevented Perlodinella from establishing populations further north (though they may have once inhabited these areas). This suggests that the genus likely originated after the formation of these mountains.
- (2)
- Environmental-change-induced niche mismatch: In Qinghai, Perlodinella inhabits flat, slow-moving rivers that lack predators and riparian vegetation. However, as the latitude and altitude decrease, accompanied by rising temperatures and increasing biodiversity, such habitats and ecosystems become rare in provinces west of Qinghai. Based on our sampling experience, from southeastern Xizang to central/south China, the typical mountainous landscapes predominantly consist of dense forests, heavily shaded river valleys, cascading waterfalls, and more diverse aquatic predators (including natural enemies and aquatic competitors occupying similar ecological niches, at least multiple genera of Perlidae and other Perlodidae).
- (3)
- The lineage within Perlodinella may have dispersed through the Qinling Mountains and finally settled in the northeastern China: Interestingly, in Perlodinella, there is still one species recorded from central China (P. shennongjia Chen, Xu & Shen, 2022, from Hubei Province [31]), and the other two species were recorded in northeastern China (P. mazehaoi Chen, 2019 from eastern Inner Mongolia [7]; P. fuliginosa Wu, 1973 from western Heilongjiang Province).

Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klapálek, F.; Plécoptères, I.; Perlodidae, F. Collections Zoologiques du Baron Edm. de Sélys-Longchamps; Bruxelles, Belgium, 1912; Volume 4, pp. 1–66. [Google Scholar]
- Wu, C.F. The stoneflies of China (Order Plecoptera). Bull. Peking Soc. Nat. Hist. 1938, 13, 53–87. [Google Scholar]
- Wu, C.F. New species of Chinese stoneflies (order Plecoptera). Acta Entomol. Sin. 1973, 16, 97–118. [Google Scholar]
- Kimmins, D.E. New species of Himalayan Plecoptera. Ann. Mag. Nat. Hist. 1946, 13, 721–740. [Google Scholar] [CrossRef]
- Ricker, W.E. Systematic Studies on Plecoptera; Indiana University: Bloomington, IN, USA, 1952; 200p. [Google Scholar]
- Illies, J. Katalog der Rezenten Plecoptera; Das Tierreich: Berlin, Germany, 1966; Volume 82, pp. 1–632. [Google Scholar]
- Chen, Z.T. Perlodinella mazehaoi sp. nov., a new species of Perlodidae (Plecoptera) from Inner Mongolia of China. Zootaxa 2019, 4651, 297–304. [Google Scholar] [CrossRef]
- Huo, Q.B.; Chen, Z.N.; Kong, X.B.; Du, Y.Z. New records and a confirmation of three perlodid species in China, with additional notes and images of Rauserodes epiproctalis (Zwick, 1997) (Plecoptera: Perlodidae). Zootaxa 2020, 4808, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.B.; Du, Y.Z.; Zwick, P.; Murányi, D. Notes on Perlodinella Klapálek, 1912 (Plecoptera: Perlodidae) with a new species and a new synonym. Zootaxa 2022, 5162, 378–396. [Google Scholar] [CrossRef]
- Huo, Q.B.; Rehman, A.; Du, Y.Z.; Chen, Z.N. Rediscovery of Perlodinella microlobata Wu, 1938, with notes on Tibetisoperla sclerotica Yan, Chen, Bozdogan & Li, 2022 (Plecoptera: Perlodidae). Zootaxa 2022, 5205, 26–34. [Google Scholar] [CrossRef]
- Huo, Q.B. Taxonomy and phylogeny of Systellognatha (Plecoptera: Arctoperlaria) of China. Ph.D. Dissertation, Yangzhou University, Yangzhou, China, 2023; 277p. [Google Scholar]
- DeWalt, R.E.; Hopkins, H.; Neu-Becker, U.; Stueber, G. Plecoptera Species File Online. 2024. Available online: https://plecoptera.speciesfile.org/ (accessed on 30 December 2024).
- Zwick, P. Rauserella, a new genus of Plecoptera (Perlodidae), with notes on related genera. In Ephemeroptera & Plecoptera, Biology–Ecology-Systematics; Landolt, P., Sartori, M., Eds.; MTL-Mauron + Tinguel Lachat SA: Fribourg, Switzerland; pp. 489–496.
- Zwick, P. Notes on Plecoptera (22) Rauserodes nom. n. replacement name for Rauserella Zwick (Plecoptera: Perlodidae). Aquat. Insects 1999, 21, 168. [Google Scholar] [CrossRef]
- Chen, Z.T.; Jiang, C.; Li, Y.S. DNA barcodes of Plecoptera from China: The preliminary dataset and its performance in species delimitation. Zootaxa 2020, 4751, 345–356. [Google Scholar] [CrossRef]
- Chen, Z.T. DNA barcodes of Plecoptera from China. J. Jiangsu Univ. Sci. Technol. (Nat. Sci. Ed.) 2021, 35, 99–104. [Google Scholar]
- Yang, Y.-B.; Du, Y.-Z. A new synonym species with description of a new species of Rhopalopsole from China (Plecoptera: Leuctridae). Eur. Zool. J. 2022, 89, 190–196. [Google Scholar]
- Huo, Q.-B.; Yang, Y.-B.; Eichert, A.; Du, Y.-Z. Gone with water or mountain: The population genetic diversity of Rhopalopsole sinensis Yang and Yang, 1993 in China. Insects 2025, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Zwick, P. Phylogenetic System and Zoogeography of the Plecoptera. Annu. Rev. Entomol. 2000, 45, 709–746. [Google Scholar] [CrossRef]
- Fochetti, R.; Tierno de Figueroa, J.M. Global diversity of stoneflies (Plecoptera: Insecta) in freshwate. Hydrobiologia 2008, 595, 365–377. [Google Scholar] [CrossRef]
- Park, D.S.; Suh, S.J.; Oh, H.W.; Hebert, P.D.N. Recovery of the mitochondrial COI barcode region in diverse Hexapoda through tRNA-based primers. BMC Genom. 2010, 11, 423. [Google Scholar] [CrossRef]
- Lai, Y.; Li, K.Y.; Liu, X.Y. Comprehensive DNA barcode reference library and optimization of genetic divergence threshold facilitate the exploration of species diversity of green lacewings (Neuroptera: Chrysopidae). Insect Sci. 2023, 31, 613–632. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organiza tion and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021. [Google Scholar] [CrossRef]
- Du, Y.Z. A Taxonomic Study on Plecoptera from China; Postdoctoral Research Report; Zhejiang University: Hangzhou, China, 1999; 324p. [Google Scholar]
- Vega, F.E.; Meyling, N.V.; Luangsa-Ard, J.J.; Blackwell, M. Fungal entomopathogens. In Insect Pathology, 2nd ed.; Vega, F., Kaya, H.K., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 171–220. [Google Scholar]
- Shang, Y.F.; Feng, P.; Wang, C.S. Fungi That Infect Insects: Altering Host Behavior and Beyond. PLoS Pathog. 2015, 11, e1005037. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Huo, Q.B.; Wen, T.; Wang, X.Y.; Zhao, M.Y.; Du, Y.Z. Mechanisms of fungal community assembly in wild stoneflies moderated by host characteristics and local environment. npj Biofilms Microbiomes 2022, 8, 31. [Google Scholar] [CrossRef]
- DeWalt, R.E.; Ower, G.D. Ecosystem services, global diversity, and rate of stonefly species descriptions (Insecta: Plecoptera). Insects 2019, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Fochetti, R. Diversity, threats, decline and conservation of European stoneflies (Plecoptera, Insecta). In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Chen, Z.-T.; Xu, Y.-Y.; Shen, Z.-H. Perlodinella shennongjia sp. nov., a new species of Perlodinella Klapálek (Plecoptera, Perlodidae) from the central area of China. Biodivers. Data J. 2022, 10, e87247. [Google Scholar] [CrossRef]
- Huo, Q.B. Faunal investigation of Plecoptera in Jiangsu, China, with the taxonomy of Chinese roachlike stonefly (Plecoptera: Peltoperlidae). Master’s Dissertation, Yangzhou University, Yangzhou, China, 2019; 77p. [Google Scholar]
- Huo, Q.-B.; Du, Y.-Z. The second species of Neowuia Li & Murányi, 2017 (Plecoptera: Perlodidae) from Fujian, China. Zootaxa 2023, 5339, 594–600. [Google Scholar] [CrossRef] [PubMed]




| No. | Species Name | Voucher | Accession Number | Locality | Collecting Date | Coord | Sex | Stage |
|---|---|---|---|---|---|---|---|---|
| 1 | P. epiproctalis | E3 | PV535523 | Menyuan County | 4 July 2021 | 101.582778° E, 37.585556° N | Male | Adult |
| 2 | E5 | PV535522 | Menyuan County | 4 July 2021 | 101.582778° E, 37.585556° N | Female | Adult | |
| 3 | E6 | PV535521 | Menyuan County | 4 July 2021 | 101.582778° E, 37.585556° N | Female | Adult | |
| 4 | P. kozlovi | k1 | PV535532 | Tianjun County | 13 July 2021 | 37.56869° N, 98.65726° E | Male | Adult |
| 5 | K2 | PV535529 | Tianjun County | 13 July 2021 | 37.56869° N, 98.65726° E | Female | Adult | |
| 6 | K4 | PV535528 | Tianjun County | 13 July 2021 | 37.56869° N, 98.65726° E | Female | Adult | |
| 8 | DKF | PV535530 | Datong County | 27 May 2024 | 37.023000° N, 101.783832° E | Female | Adult | |
| 9 | M | PV535527 | Datong County | 27 May 2024 | 37.023000° N, 101.783832° E | Male | Adult | |
| 10 | PKM | PV535525 | Datong County | 27 May 2024 | 37.023000° N, 101.783832° E | Male | Adult | |
| 11 | PKF | PV535534 | Datong County | 27 May 2024 | 37.023000° N, 101.783832° E | Female | Adult | |
| 12 | GN3 | PV535535 | Tongde County | 4 June 2024 | 35.123291° N, 100.622657° E | Female | Larva | |
| 13 | PM | PV535524 | Tongde County | 4 June 2024 | 35.123291° N, 100.622657° E | Male | Adult | |
| 14 | PF | PV535526 | Tongde County | 4 June 2024 | 35.123291° N, 100.622657° E | Female | Adult | |
| 15 | N1 | PV535531 | Xinghai County | 30 May 2024 | 35.581647° N, 99.863318° E | Female | Larva | |
| 16 | P. microlobata | GMM | PV535536 | Tongde County | 4 June 2024 | 35.123291° N, 100.622657° E | Male | Adult |
| 17 | GMF | PV535537 | Tongde County | 4 June 2024 | 35.123291° N, 100.622657° E | Female | Adult | |
| 18 | GW | PV535539 | Tongde County | 4 June 2024 | 35.123291° N, 100.622657° E | Male | Adult | |
| 19 | N2 | PV535538 | Zeku County | 5 June 2024 | 35.256546° N, 101.050529° E | Female | Larva |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Q.-B.; Fan, S.-X.; Zhu, Y.-F.; Du, Y.-Z. Diversity and the Origin of Perlodinella Klapálek 1912 (Plecoptera: Perlodidae) in Qinghai Province, China. Insects 2025, 16, 520. https://doi.org/10.3390/insects16050520
Huo Q-B, Fan S-X, Zhu Y-F, Du Y-Z. Diversity and the Origin of Perlodinella Klapálek 1912 (Plecoptera: Perlodidae) in Qinghai Province, China. Insects. 2025; 16(5):520. https://doi.org/10.3390/insects16050520
Chicago/Turabian StyleHuo, Qing-Bo, Shi-Xiong Fan, Ya-Fei Zhu, and Yu-Zhou Du. 2025. "Diversity and the Origin of Perlodinella Klapálek 1912 (Plecoptera: Perlodidae) in Qinghai Province, China" Insects 16, no. 5: 520. https://doi.org/10.3390/insects16050520
APA StyleHuo, Q.-B., Fan, S.-X., Zhu, Y.-F., & Du, Y.-Z. (2025). Diversity and the Origin of Perlodinella Klapálek 1912 (Plecoptera: Perlodidae) in Qinghai Province, China. Insects, 16(5), 520. https://doi.org/10.3390/insects16050520







