The Role of Insect-Based Feed in Mitigating Climate Change: Sustainable Solutions for Ruminant Farming
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Effect of Conventional Protein-Based Livestock Feed on the Environment

4. Insects as an Alternative Sustainable Feed Resource

| Feed Type | kg CO2-Equivalent | References |
|---|---|---|
| General insect | −6.42 to 5.3 (food waste) 0.77–12 (manure) | [32] |
| Mealworms | 1.47 | [33] |
| House crickets | 110 | [33] |
| Fishmeal | 2–4 | [24,33,34] |
| Soymeal | 0.65–1 | [33,34,35] |
| Fish meal | 2–4 | [32] |
| Black soldier fly | 0.91 | [34] |
| Cricket | 1.3–2.9 | [36] |
5. Common Types of Insects Used as Livestock Proteins
| Common and Scientific Names | GHG Emissions | Land Use | Water Use | Energy Use | Waste Conversion Efficiency | References |
|---|---|---|---|---|---|---|
| Black soldier fly (Hermetia illucens) | Low | Low | Very low | Moderate to high | Excellent | [56,57] |
| Mealworms (Tenebrio molitor) | Low | Low | Low | Moderate | Moderate | [33] |
| Crickets (Acheta domesticus) | Low | Low | Moderate | Moderate | Good | [14,58] |
| Grasshoppers (Schistocerca gregaria) | Moderate | Very low | Moderate | Low | Good | [59] |
| Housefly (Musca domestica) | Low | Very low | Very low | Moderate | Excellent | [60] |
| Desert locust (Schistocerca gregaria) | Moderate | Very low | Moderate | low | Good | [54] |
6. Legislation and Regulation of Insect-Based Feed in Some Countries
7. Climate Change Mitigation Mechanisms of Insect-Based Feed
8. Insect-Based Feed in Ruminant for Methane Emission Reduction
8.1. Cattle
8.2. Sheep
8.3. Goats
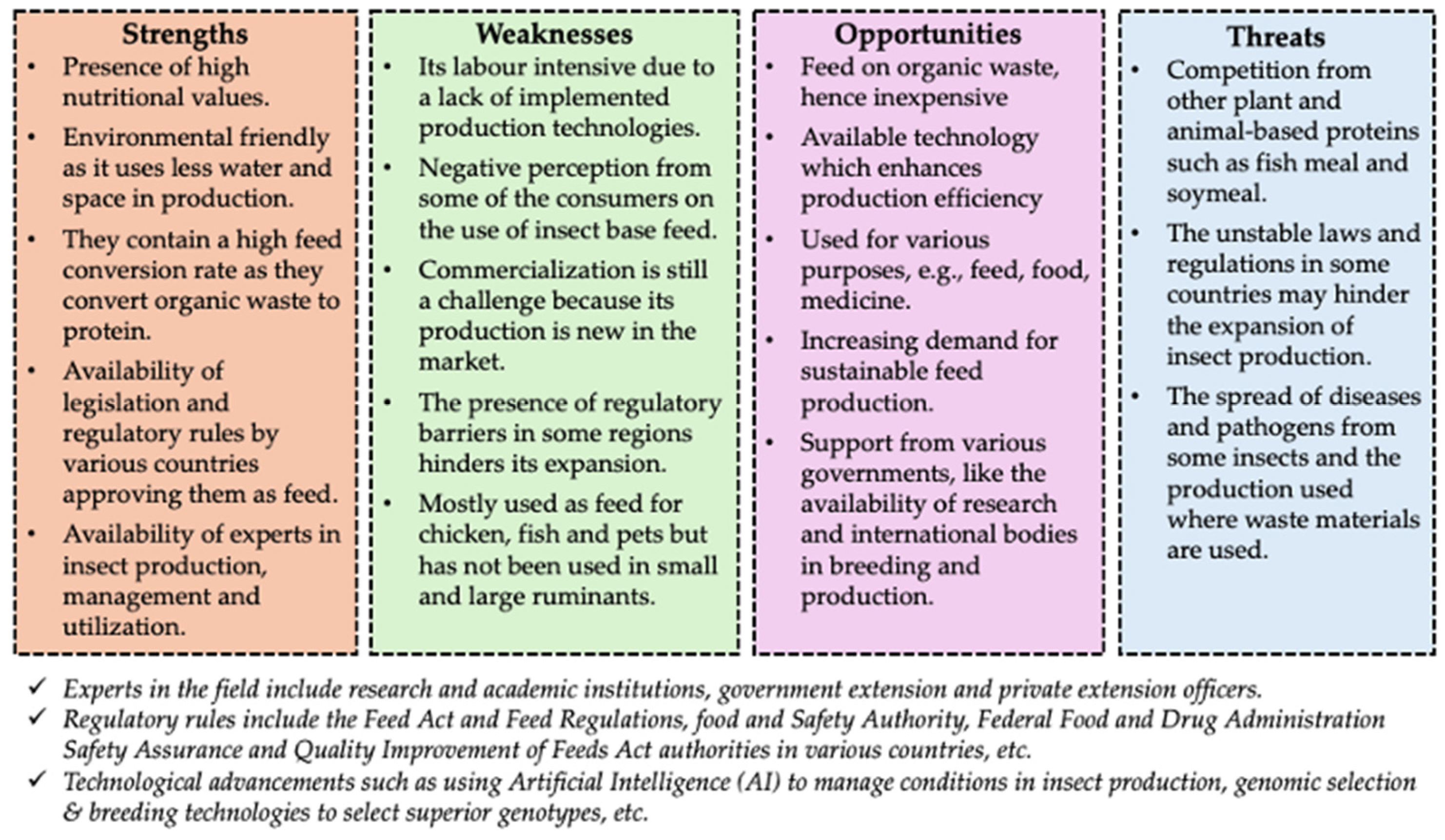
9. Challenges and Future Direction
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAFCO | Association of American Feed Control Officials |
| ADG | Average daily gain |
| APVMA | The Australian Pesticides and Veterinary Medicine Authority |
| As | Arsenic |
| BSF | Black soldier fly |
| DMI | Dry matter intake |
| EUR | Euros |
| BSFL | Black soldier fly larvae |
| FA | Fatty acids |
| GHC | Green gas emission |
| BUN | Blood urea nitrogen |
| Ca | Calcium |
| Cd | Cadmium |
| CFIA | Canadian Food Inspection Agency |
| CP | Crude protein |
| Cu | Copper |
| EFSA | The European Union food and Safety Authority |
| FAFR | The Feed Act and Feed Regulations |
| FAO | Food and Agricultural Organization |
| FDA | The Federal Food and Drug Administration |
| FFDCA | The Federal Food, Drug, and Cosmetic Act |
| FM | Fishmeal |
| Hg | Mercury |
| IBF | Insect-based feed |
| K | Potassium |
| MAFRA | The Ministry of Agriculture, Food, and Rural Affairs |
| MCP | Microbial crude protein |
| Mg | Magnesium |
| Mn | Manganese |
| Na | Sodium |
| P | Phosphate |
| Pb | Lead |
| SBM | Soybean meal |
| SFA | Saturated fatty acid |
| UFA | Unsaturated fatty acid |
| VFA | Volatile fatty acid |
| WHO | World Health Organization |
| Zn | Zinc |
References
- Chisoro, P.; Jaja, I.F.; Assan, N. Incorporation of local novel feed resources in livestock feed for sustainable food security and circular economy in Africa. Front. Sustain. 2023, 4, 1251179. [Google Scholar] [CrossRef]
- Tullo, E.; Finzi, A.; Guarino, M. Review: Environmental impact of livestock farming and Precision Livestock Farming as a mitigation strategy. Sci. Total Environ. 2019, 650, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Phesatcha, B.; Phesatcha, K.; Ampapon, T.; Wanapat, M. Using Azolla (Azolla microphylla) leaf meal and phytonutrient powder on rumen fermentation efficiency and nutrient degradability using in vitro technique. Anim. Biosci. 2024, 38, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Colin, R.L.; Sperber, J.L.; Buse, K.K.; Kononoff, P.J.; Watson, A.K.; Erickson, G.E. Effect of an algae feed additive on reducing enteric methane emissions from cattle. Transl. Anim. Sci. 2024, 8, txae109. [Google Scholar] [CrossRef]
- Kolling, G.; Stivanin, S.; Gabbi, A.; Machado, F.; Ferreira, A.; Campos, M.; Tomich, T.; Cunha, C.; Dill, S.; Pereira, L.; et al. Performance and methane emissions in dairy cows fed oregano and green tea extracts as feed additives. J. Dairy Sci. 2018, 101, 4221–4234. [Google Scholar] [CrossRef]
- Akanmu, A.M.; Hassen, A.; Adejoro, F.A. Gas production, digestibility and efficacy of stored or fresh plant extracts to reduce methane production on different substrates. Animals 2020, 10, 146. [Google Scholar] [CrossRef]
- Bharanidharan, R.; Arokiyaraj, S.; Baik, M.; Ibidhi, R.; Lee, S.J.; Lee, Y.; Nam, I.S.; Kim, K.H. In vitro screening of East Asian plant extracts for potential use in reducing ruminal methane production. Animals 2021, 11, 1020. [Google Scholar] [CrossRef]
- Anankware, J.P.; Roberts, B.J.; Cheseto, X.; Osuga, I.; Savolainen, V.; Collins, C.M. The Nutritional profiles of five important edible insect species from West Africa—An analytical and literature Synthesis. Front. Nutr. 2021, 8, 792941. [Google Scholar] [CrossRef]
- Muya, G.M.N.; Mutiaka, B.K.; Bindelle, J.; Francis, F.; Megido, R.C. Human consumption of insects in Sub-Saharan Africa: Lepidoptera and potential species for breeding. Insects 2022, 13, 886. [Google Scholar] [CrossRef]
- Sabri, N.S.A.; Kamardan, M.I.F.; Wong, S.X.; Azman, N.F.; Akhir, F.N.M.; Othman, N.; Awang, N.; Kuroki, Y.; Hara, H. Future aspects of insects’ ingestion in Malaysia and Indonesia for human well-being and religion regulation. Future Foods 2023, 8, 100267. [Google Scholar] [CrossRef]
- Mei, Z.; Kuzhir, P.; Godeau, G. Update on Chitin and Chitosan from Insects: Sources, Production, Characterization, and Biomedical Applications. Biomimetics 2024, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Halloran, A.; Hansen, H.H.; Jensen, L.S.; Bruun, S. Comparing Environmental Impacts from Insects for Feed and Food as an Alternative to Animal Production. In Edible Insects in Sustainable Food Systems; Halloran, A., Flore, R., Vantomme, P., Roos, N., Eds.; Springer: Cham, Switzerland, 2018; pp. 163–180. [Google Scholar] [CrossRef]
- Okello, A.O.; Nzuma, J.M.; Otieno, D.J.; Kidoido, M.; Tanga, C.M. Farmers’ perceptions of commercial Insect-Based feed for sustainable livestock production in Kenya. Sustainability 2021, 13, 5359. [Google Scholar] [CrossRef]
- Van Huis, A.; Oonincx, D.G. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Fu, C.; Cheema, W.A.; Mobashar, M.; Shah, A.A.; Alqahtani, M.M. Insects as Sustainable feed: Enhancing animal nutrition and reducing livestock environmental impression. J. Anim. Physiol. Anim. Nutr. 2024, 109, 280–290. [Google Scholar] [CrossRef]
- Te Pas, M.F.W.; Veldkamp, T.; de Haas, Y.; Bannink, A.; Ellen, E.D. Adaptation of Livestock to New Diets Using Feed Components without Competition with Human Edible Protein Sources—A Review of the Possibilities and Recommendations. Animals 2021, 11, 2293. [Google Scholar] [CrossRef]
- Herrero, M.; Henderson, B.; Havlík, P.; Thornton, P.K.; Conant, R.T.; Smith, P.; Wirsenius, S.; Hristov, A.N.; Gerber, P.; Gill, M.; et al. Greenhouse gas mitigation potentials in the livestock sector. Nat. Clim. Change 2016, 6, 452–461. [Google Scholar] [CrossRef]
- Dopelt, K.; Radon, P.; Davidovitch, N. Environmental Effects of the Livestock Industry: The Relationship between Knowledge, Attitudes, and Behavior among Students in Israel. Int. J. Environ. Res. Public Health 2019, 16, 1359. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities. 2013, pp. xxi+–115. Available online: http://www.fao.org/docrep/018/i3437e/i3437e00.htm (accessed on 25 February 2025).
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151.
- Murta, D. The future of animal feeding. In Insects as Animal Feed: Novel Ingredients for Use in Pet, Aquaculture and Livestock Diets; CABI eBooks: Wallingford, UK, 2021; pp. 126–134. Available online: https://www.cabidigitallibrary.org/doi/10.1079/9781789245929.0016 (accessed on 25 February 2025). [CrossRef]
- McMichael, A.J.; Powles, J.W.; Butler, C.D.; Uauy, R. Food, livestock production, energy, climate change, and health. Lancet 2007, 370, 1253–1263. [Google Scholar] [CrossRef]
- Usman, H.S.; Yusuf, A.A. Legislation and legal frame work for sustainable edible insects use in Nigeria. Int. J. Trop. Insect Sci. 2021, 41, 2201–2209. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). The State of Food and Agriculture 2022: Climate Change and the Livestock Sector; FAO: Rome, Italy, 2022.
- Hancz, C.; Sultana, S.; Nagy, Z.; Biró, J. The Role of Insects in Sustainable Animal Feed Production for Environmentally Friendly Agriculture: A Review. Animals 2024, 14, 1009. [Google Scholar] [CrossRef] [PubMed]
- Bailey, E. Mineral Supplements for Beef Cattle; University of Missouri Extension: Columbia, MO, USA, 2001; pp. 1–14. [Google Scholar]
- Zuidhof, M.J.; Molnar, C.L.; Morley, F.M.; Wray, T.L.; Robinson, F.E.; Khan, B.A.; Al-Ani, L.; Goonewardene, L.A. Nutritive value of house fly (Musca domestica) larvae as a feed supplement for turkey poults. Anim. Feed Sci. Technol. 2003, 105, 225–230. [Google Scholar] [CrossRef]
- Shah, A.A.; Totakul, P.; Matra, M.; Cherdthong, A.; Harnboonsong, Y.; Wanapat, M. Nutritional composition of various insects and potential uses as alternative protein sources in animal diets. Anim. Biosci. 2022, 35, 317–331. [Google Scholar] [CrossRef]
- Sampath, V.; Sureshkumar, S.; Seok, W.J.; Kim, I.H. Role and functions of micro and macro-minerals in swine nutrition: A short review. J. Anim. Sci. Technol. 2023, 65, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, H.M.; Nascimento, A.; Arruda, A.; Sarinho, A.; Lima, J.; Batista, L.; Dantas, M.F.; Andrade, R. Unlocking the Potential of Insect-Based Proteins: Sustainable Solutions for Global Food Security and Nutrition. Foods 2024, 13, 1846. [Google Scholar] [CrossRef] [PubMed]
- Selaledi, L.; Mbajiorgu, C.A.; Mabelebele, M. The use of yellow mealworm (T. molitor) as alternative source of protein in poultry diets: A review. Trop. Anim. Health Prod. 2020, 52, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Smetana, S.; Palanisamy, M.; Mathys, A.; Heinz, V. Sustainability of insect use for feed and food: Life Cycle Assessment perspective. J. Clean. Prod. 2016, 137, 741–751. [Google Scholar] [CrossRef]
- Ordoñez-Araque, R.; Quishpillo-Miranda, N.; Ramos-Guerrero, L. Edible insects for humans and Animals: Nutritional composition and an option for mitigating environmental damage. Insects 2022, 13, 944. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B. Environmental impact of insect production. In Insects as Food and Feed: From Production to Consumption; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017; pp. 79–93. Available online: https://research.wur.nl/en/publications/environmental-impact-of-insect-production (accessed on 23 February 2025).
- Lange, K.W.; Nakamura, Y. Potential contribution of edible insects to sustainable consumption and production. Front. Sustain. 2023, 4, 1112950. [Google Scholar] [CrossRef]
- Halloran, A.; Hanboonsong, Y.; Roos, N.; Bruun, S. Life cycle assessment of cricket farming in north-eastern Thailand. J. Clean. Prod. 2017, 156, 83–94. [Google Scholar] [CrossRef]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef]
- Cai, M.; Li, L.; Zhao, Z.; Zhang, K.; Li, F.; Yu, C.; Yuan, R.; Zhou, B.; Ren, Z.; Yu, Z.; et al. Morphometric Characteristic of Black Soldier Fly (Hermetia illucens)·Wuhan Strain and Its Egg Production Improved by Selectively Inbreeding. Life 2022, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.C.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Sumner, S.M. Rearing methods for the black soldier fly, Hermetia illucens (L) (Ddiptera: Stratomyidae). J. Med. Entomol. 2002, 15, 205–208. [Google Scholar] [CrossRef]
- Newton, L.; Sheppard, G.; Watson, D.W.; Burtle, G.; Dove, R. Using the Black Soldier Fly, Hermetia illucens, as a Value-Added Tool for the Management of Swine Manure; Animal and Poultry Waste Management Center, North Carolina State University: Raleigh, NC, USA, 2005; 17p. [Google Scholar]
- Webster, C.D.; Rawles, S.D.; Koch, J.F.; Thompson, K.R.; Kobayashi, Y.; Gannam, A.L.; Twibell, R.G.; Hyde, N.M. Bio-Aag reutilization of distiller’s dried grains with solubles (DDGS) as a substrate for black soldier fly larvae, Hermetia illucens, along with poultry by-product meal and soybean meal, as total replacement of fish meal in diets for nile tilapia, Oreochromis niloticus. Aquac. Nutr. 2015, 22, 976–988. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Sheppard, D.C. Factors influencing mating and oviposition of black soldier flies (Diptera: Stratiomyidae) in a colony. J. Entomol. Sci. 2002, 37, 345–352. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, X.; Luo, S.; Jiang, M.; Liu, Q.; Cao, Y. The Development of Yellow Mealworm (Tenebrio molitor) as a Cheap and Simple Model to Evaluate Acute Toxicity, Locomotor Activity Changes, and Metabolite Profile Alterations Induced by Nanoplastics of Different Sizes. J. Appl. Toxicol. 2025, 35, 994–1003. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Kołodziejski, P.; Pruszyńska-Oszmałek, E.; Rawski, M.; Józefiak, D.; Józefiak, A. Tenebrio molitor and Zophobas morio full-fat meals as functional feed additives affect broiler chickens’ growth performance and immune system traits. Poult. Sci. 2020, 99, 196–206. [Google Scholar] [CrossRef]
- Sankian, Z.; Khosravi, S.; Kim, Y.-O.; Lee, S.-M. Effects of dietary inclusion of yellow mealworm (Tenebrio molitor) meal on growth performance, feed utilization, body composition, plasma biochemical indices, selected immune parameters and antioxidant enzyme activities of mandarin fish (Siniperca scherzeri) juveniles. Aquaculture 2018, 496, 79–87. [Google Scholar] [CrossRef]
- Motte, C.; Rios, A.; Lefebvre, T.; Do, H.; Henry, M.; Jintasataporn, O. Replacing fish meal with defatted insect meal (Yellow Mealworm Tenebrio molitor) improves the growth and immunity of pacific white shrimp (Litopenaeus vannamei). Animals 2019, 9, 258. [Google Scholar] [CrossRef]
- Miller, B.F.; Teotia, J.S.; Thatcher, T.O. Digestion of poultry manure by Musca domestica. Br. Poult. Sci. 1974, 15, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, Q. The Evaluation of Larvae of Musca domestica (Common House Fly) as Protein Source for Broiler Production. Ph.D. Dissertation, Stellenbosch University, Stellenbosch, South Africa, 2011. Available online: http://hdl.handle.net/10019.1/46243 (accessed on 24 February 2025).
- Huis, A.V.; Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security. 2013. Available online: http://www.fao.org/docrep/018/i3253e/i3253e.pdf (accessed on 25 February 2025).
- Ramos-Elorduy, J. Threatened edible insects in Hidalgo, Mexico and some measures to preserve them. J. Ethnobiol. Ethnomed. 2006, 2, 51. [Google Scholar] [CrossRef]
- Cerritos, R.; Cano-Santana, Z. Harvesting grasshoppers Sphenarium purpurascens in Mexico for human consumption: A comparison with insecticidal control for managing pest outbreaks. Crop Prot. 2007, 27, 473–480. [Google Scholar] [CrossRef]
- Omuse, E.R.; Tonnang, H.E.; Yusuf, A.A.; Machekano, H.; Egonyu, J.P.; Kimathi, E.; Niassy, S. The Global Atlas of Edible Insects: Analysis of Diversity and Commonality Contributing to Food Systems and Sustainability. Sci. Rep. 2024, 14, 5045. [Google Scholar] [CrossRef] [PubMed]
- Peshuk, L.V.; Kyrylov, Y.E.; Ibatullin, I.I.; Marenkov, M. Entomophagy as a Promising and New Protein Source of the Future for Solving Food and Fodder Security Problems. J. Chem. Technol. 2022, 30, 627–638. [Google Scholar] [CrossRef]
- Makkar, H.P.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Li, M.; Mao, C.; Li, X.; Jiang, L.; Zhang, W.; Li, M.; Liu, H.; Fang, Y.; Liu, S.; Yang, G.; et al. Edible Insects: A new sustainable nutritional resource worth promoting. Foods 2023, 12, 4073. [Google Scholar] [CrossRef]
- Van Huis, A. Edible insects: Challenges and prospects. Entomol. Res. 2022, 52, 161–177. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Osei-Owusu, J.; Asante, K.; Dofuor, A.K.; Boateng, B.O.; Debrah, S.K.; Ninsin, K.D.; Siddiqui, S.A.; Chia, S.Y. Insects as food and medicine: A sustainable solution for global health and environmental challenges. Front. Nutr. 2023, 10, 1113219. [Google Scholar] [CrossRef]
- Hussein, M.; Pillai, V.V.; Goddard, J.M.; Park, H.G.; Kothapalli, K.S.; Ross, D.A.; Ketterings, Q.M.; Brenna, J.T.; Milstein, M.B.; Marquis, H.; et al. Sustainable production of housefly (Musca domestica) larvae as a protein-rich feed ingredient by utilizing cattle manure. PLoS ONE 2017, 12, e0171708. [Google Scholar] [CrossRef]
- Vandeweyer, D.; De Smet, J.; Van Looveren, N.; Van Campenhout, L. Biological contaminants in insects as food and feed. J. Insects Food Feed 2021, 7, 807–822. [Google Scholar] [CrossRef]
- De Marchi, L.; Wangorsch, A.; Zoccatelli, G. Allergens from Edible Insects: Cross-reactivity and Effects of Processing. Curr. Allergy Asthma Rep. 2021, 21, 35. [Google Scholar] [CrossRef]
- Halloran, A.; Ayieko, M.; Oloo, J.; Konyole, S.O.; Alemu, M.H.; Roos, N. What determines farmers’ awareness and interest in adopting cricket farming? A pilot study from Kenya. Int. J. Trop. Insect Sci. 2021, 41, 2149–2164. [Google Scholar] [CrossRef]
- Halloran, A.; Vantomme, P.; Hanboonsong, Y.; Ekesi, S. Regulating edible insects: The challenge of addressing food security, nature conservation, and the erosion of traditional food culture. Food Secur. 2015, 7, 739–746. [Google Scholar] [CrossRef]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Lähteenmäki-Uutela, A.; Marimuthu, S.B.; Meijer, N. Regulations on insects as food and feed: A global comparison. J. Insects Food Feed 2021, 7, 849–856. [Google Scholar] [CrossRef]
- Baigts-Allende, D.; Doost, A.S.; Ramírez-Rodrigues, M.; Dewettinck, K.; Van Der Meeren, P.; De Meulenaer, B.; Tzompa-Sosa, D. Insect protein concentrates from Mexican edible insects: Structural and functional characterization. LWT 2021, 152, 112267. [Google Scholar] [CrossRef]
- Abril, S.; Pinzón, M.; Hernández-Carrión, M.; Del Pilar Sánchez-Camargo, A. Edible Insects in Latin America: A sustainable alternative for our food security. Front. Nutr. 2022, 9, 904812. [Google Scholar] [CrossRef]
- Megido, R.C.; Francis, F.; Haubruge, E.; Le Gall, P.; Tomberlin, J.; Miranda, C.; Jordan, H.; Picard, C.; Pino, M.; Ramos-Elordy, J.; et al. A worldwide overview of the status and prospects of edible insect production. Entomol. Gen. 2024, 44, 3–27. [Google Scholar] [CrossRef]
- Kim, J.W. Insect industry for future super foods. Korean Soc. Food Sci. Anim. Resour. 2019, 8, 74–77. Available online: https://koreascience.kr/article/JAKO201919263351724.page (accessed on 23 February 2025).
- DiGiacomo, K.; Leury, B.J. Review: Insect meal: A future source of protein feed for pigs? Animal 2019, 13, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Seyedalmoosavi, M.M.; Mielenz, M.; Veldkamp, T.; Daş, G.; Metges, C.C. Growth efficiency, intestinal biology, and nutrient utilization and requirements of black soldier fly (Hermetia illucens) larvae compared to monogastric livestock species: A review. J. Anim. Sci. Biotechnol. 2022, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Derler, H.; Lienhard, A.; Berner, S.; Grasser, M.; Posch, A.; Rehorska, R. Use Them for What They Are Good at: Mealworms in Circular Food Systems. Insects 2021, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Weththasinghe, P.; Rocha, S.D.C.; Øyås, O.; Lagos, L.; Hansen, J.Ø.; Mydland, L.T.; Øverland, M. Modulation of Atlantic salmon (Salmo salar) gut microbiota composition and predicted metabolic capacity by feeding diets with processed black soldier fly (Hermetia illucens) larvae meals and fractions. Anim. Microbiome 2022, 4, 9. [Google Scholar] [CrossRef]
- Abro, Z.; Macharia, I.; Mulungu, K.; Subramanian, S.; Tanga, C.M.; Kassie, M. The potential economic benefits of insect-based feed in Uganda. Front. Insect Sci. 2022, 2, 968042. [Google Scholar] [CrossRef]
- Safavi, A.; Thrastardottir, R.; Thorarinsdottir, R.I.; Unnthorsson, R. Insect Production: A Circular Economy Strategy in Iceland. Sustainability 2024, 16, 9063. [Google Scholar] [CrossRef]
- Malematja, E.; Sebola, N.A.; Manyelo, T.G.; Kolobe, S.D.; Mabelebele, M. Copping out of novel feeds: HOW climate change pledgers and food summits overlooked insect protein. Heliyon 2023, 9, e22773. [Google Scholar] [CrossRef]
- Ahmed, E.; Fukuma, N.; Hanada, M.; Nishida, T. Insects as novel ruminant feed and a potential mitigation strategy for methane emissions. Animals 2021, 11, 2648. [Google Scholar] [CrossRef]
- Jimenez, L.E.R.; Ghavipanje, N.; Renna, M.; Ortega, O.A.C.; Vargas-Bello-Pérez, E.; Gonzalez-Ronquillo, M. Dietary water fly (Notonecta sp.) meal as an alternative protein source for sheep: Effects on performance, nutrient intake, nitrogen balance, and fermentation parameters. Anim. Sci. J. 2024, 95, e70006. [Google Scholar]
- Jayanegara, A.; Novandri, B.; Yantina, N.; Ridla, M. Use of black soldier fly larvae (Hermetia illucens) to substitute soybean meal in ruminant diet: An in vitro rumen fermentation study. Vet. World 2017, 10, 1436–1446. [Google Scholar] [CrossRef]
- Martinson, G.O.; Müller, A.K.; Matson, A.L.; Corre, M.D.; Veldkamp, E. Nitrogen and phosphorus control soil methane uptake in tropical montane forests. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG005970. [Google Scholar] [CrossRef]
- Phesatcha, B.; Phesatcha, K.; Matra, M.; Wanapat, M. Cricket (Gryllus bimaculatus) meal pellets as a protein supplement to improve feed efficiency, ruminal fermentation and microbial protein synthesis in Thai native beef cattle. Anim. Biosci. 2023, 36, 1384–1392. [Google Scholar] [CrossRef]
- Nekrasov, R.V.; Ivanov, G.A.; Chabaev, M.G.; Zelenchenkova, A.A.; Bogolyubova, N.V.; Nikanova, D.A.; Sermyagin, A.A.; Bibikov, S.O.; Shapovalov, S.O. Effect of Black Soldier Fly (Hermetia illucens L.) Fat on Health and Productivity Performance of Dairy Cows. Animals 2022, 12, 2118. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, O.; Gülşen, N.; İnal, F.; Alataş, M.S.; İnanç, Z.S.; Ahmed, I.; Şişman, D.; Küçük, A.E. Comparative Analysis of In Vitro Fermentation Parameters in Total Mixed Rations of Dairy Cows with Varied Levels of Defatted Black Soldier Fly Larvae (Hermetia illucens) as a Substitute for Soybean Meal. Fermentation 2023, 9, 652. [Google Scholar] [CrossRef]
- Hervás, G.; Boussalia, Y.; Labbouz, Y.; Della Badia, A.; Toral, P.; Frutos, P. Insect oils and chitosan in sheep feeding: Effects on in vitro ruminal biohydrogenation and fermentation. Anim. Feed Sci. Technol. 2022, 285, 115222. [Google Scholar] [CrossRef]
- Thirumalaisamy, G.; Malik, P.K.; Trivedi, S.; Kolte, A.P.; Bhatta, R. Effect of Long-Term supplementation with silkworm pupae oil on the methane yield, ruminal protozoa, and archaea community in sheep. Front. Microbiol. 2022, 13, 780073. [Google Scholar] [CrossRef]
- Lu, S.; Chen, S.; Paengkoum, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Wang, Q.; Thongpea, S.; Paengkoum, P. Effects of Black Soldier Fly (Hermetia illucens L., BSF) Larvae Addition on In Vitro Fermentation Parameters of Goat Diets. Insects 2024, 15, 343. [Google Scholar] [CrossRef]
- Lu, S.; Paengkoum, S.; Chen, S.; Long, Y.; Niu, X.; Thongpea, S.; Taethaisong, N.; Meethip, W.; Paengkoum, P. Effects of black soldier fly larvae (Hermetia illucens L.) as feed supplements on muscle nutrient composition, meat quality, and antioxidant capacity in Qianbei goat. Anim. Biosci. 2024, 37, 2167–2177. [Google Scholar] [CrossRef]
- Astuti, D.; Anggraeny, A.; Khotijah, L.; Suharti, S.; Jayanegara, A. Performance, physiological status, and rumen fermentation profiles of pre-and post-weaning goat kids fed cricket meal as a protein source. Trop. Anim. Sci. J. 2019, 42, 145–151. [Google Scholar] [CrossRef]
- Dewi Apri, A.; Komalasari, K. Feed and animal nutrition: Insect as animal feed. IOP Conf. Ser. Earth Environ. Sci. 2020, 465, 012002. [Google Scholar] [CrossRef]
- Hassanien, H.; Abou El-Fadel, M.; El-Badawy, M.; Phillip, Y.; Hussein, A.; Khayyal, A.; Elmenniawy, M.; El-Sanafawy, H.A.; Radwan, M.A.; Rodríguez, G.B.; et al. Dietary inclusion of Tenebrio molitor L. frass affects nutrient digestibility, ruminal fermentation activities, blood metabolites, and milk performance in goats. J. Agric. Food Res. 2025, 19, 101727. [Google Scholar] [CrossRef]
- Odinya, D.W.; Ateka, J.M.; Mbeche, R.M.; Gicheha, M.G. Smallholder farmers’ intention to use insect-based feed in dairy cattle diet in Kenya. Int. J. Trop. Insect Sci. 2022, 42, 3695–3711. [Google Scholar] [CrossRef]
- Ranga, L.; Noci, F.; Vale, A.P.; Dermiki, M. Insect-Based Feed Acceptance amongst Consumers and Farmers in Ireland: A Pilot Study. Sustainability 2023, 15, 11006. [Google Scholar] [CrossRef]
- Roccatello, R.; Cerroni, S.; Dabbou, S. Sustainability of insect-based feed and consumer willingness to pay for novel food: A stated preference study. Future Foods 2024, 9, 100336. [Google Scholar] [CrossRef]
- Jamaludin, M.A.; Amid, A.; Puat, N.S.M.; Saffine, S.S. BSFL as alternative halal animal feed. J. Halal Ind. Serv. 2024, 7, 1. [Google Scholar] [CrossRef]
- Eggink, K.M.; Lund, I.; Pedersen, P.B.; Hansen, B.W.; Dalsgaard, J. Biowaste and by-products as rearing substrates for black soldier fly (Hermetia illucens) larvae: Effects on larval body composition and performance. PLoS ONE 2022, 17, e0275213. [Google Scholar] [CrossRef]
- Salahuddin, M.; Abdel-Wareth, A.A.A.; Hiramatsu, K.; Tomberlin, J.K.; Luza, D.; Lohakare, J. Flight toward Sustainability in Poultry Nutrition with Black Soldier Fly Larvae. Animals 2024, 14, 510. [Google Scholar] [CrossRef]
- Da-Silva, W.C.; Da Silva, É.B.R.; Da Silva, J.A.R.; Martorano, L.G.; Belo, T.S.; Sousa, C.E.L.; Camargo-Júnior, R.N.C.; Andrade, R.L.; De Souza Santos, A.G.; De Carvalho, K.C.; et al. Nutritional Value of the Larvae of the Black Soldier Fly (Hermetia illucens) and the House Fly (Musca domestica) as a Food Alternative for Farm Animals—A Systematic Review. Insects 2024, 15, 619. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kichamu, N.; Astuti, P.K.; Kusza, S. The Role of Insect-Based Feed in Mitigating Climate Change: Sustainable Solutions for Ruminant Farming. Insects 2025, 16, 516. https://doi.org/10.3390/insects16050516
Kichamu N, Astuti PK, Kusza S. The Role of Insect-Based Feed in Mitigating Climate Change: Sustainable Solutions for Ruminant Farming. Insects. 2025; 16(5):516. https://doi.org/10.3390/insects16050516
Chicago/Turabian StyleKichamu, Nelly, Putri Kusuma Astuti, and Szilvia Kusza. 2025. "The Role of Insect-Based Feed in Mitigating Climate Change: Sustainable Solutions for Ruminant Farming" Insects 16, no. 5: 516. https://doi.org/10.3390/insects16050516
APA StyleKichamu, N., Astuti, P. K., & Kusza, S. (2025). The Role of Insect-Based Feed in Mitigating Climate Change: Sustainable Solutions for Ruminant Farming. Insects, 16(5), 516. https://doi.org/10.3390/insects16050516









