Climate Change: A Major Factor in the Spread of Aedes aegypti (Diptera: Culicidae) and Its Associated Dengue Virus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
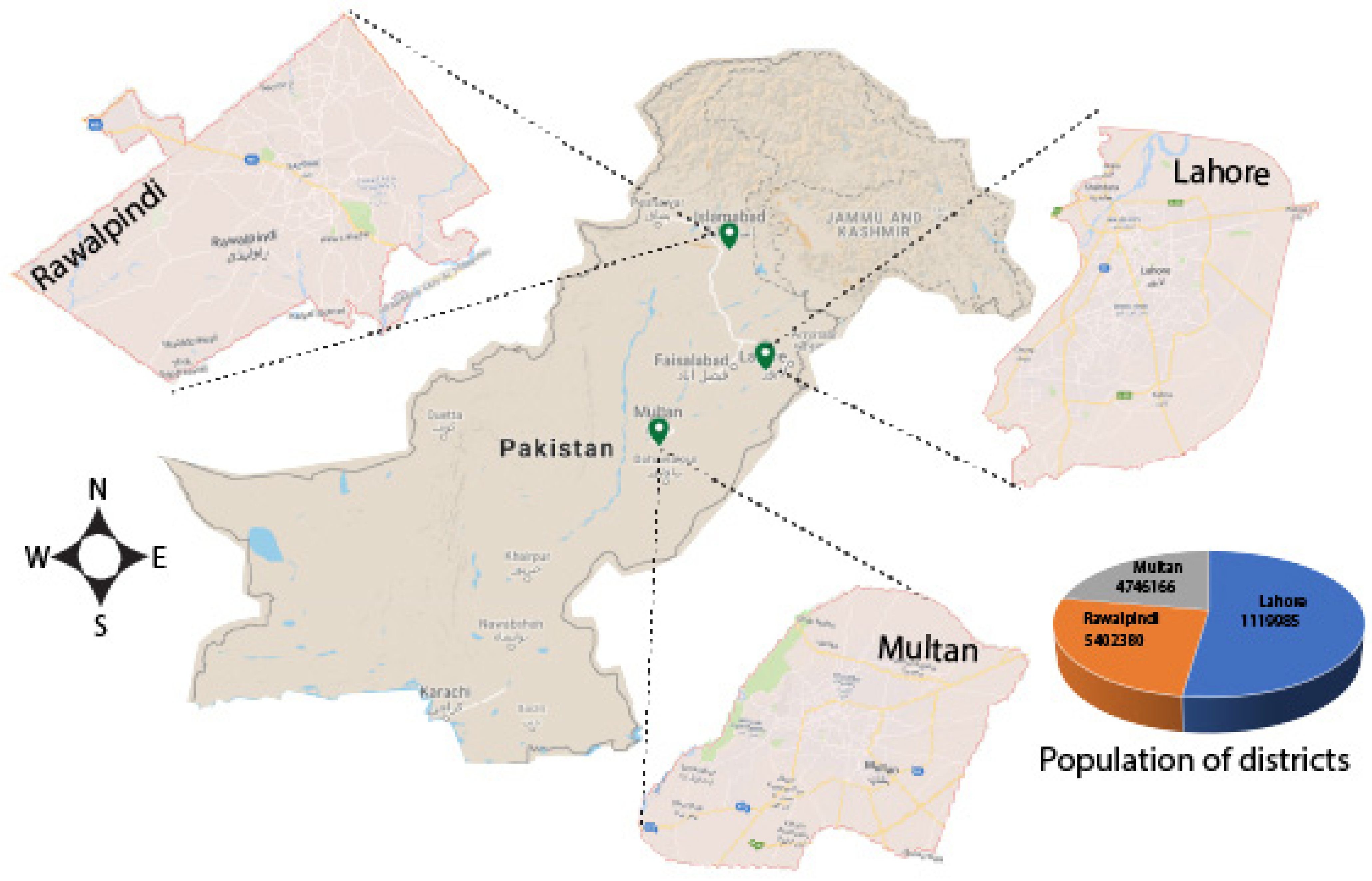
2.1. Meteorological and Epidemiological Data
2.2. Mosquito Culture and Maintenance
2.3. Effect of Temperature and Humidity
2.4. Density-Dependent Trials
2.5. Data Collection
2.6. Statistical Analyses
3. Results
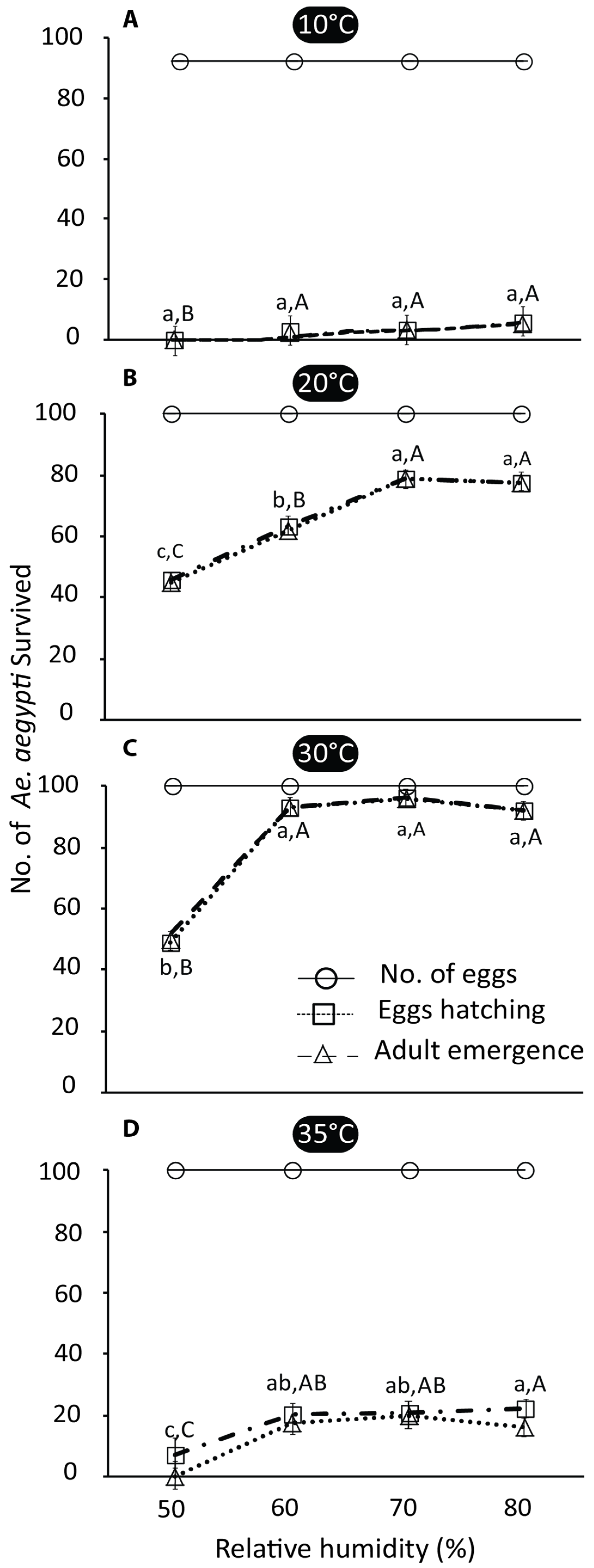
3.1. Egg Hatching and Adult Emergence
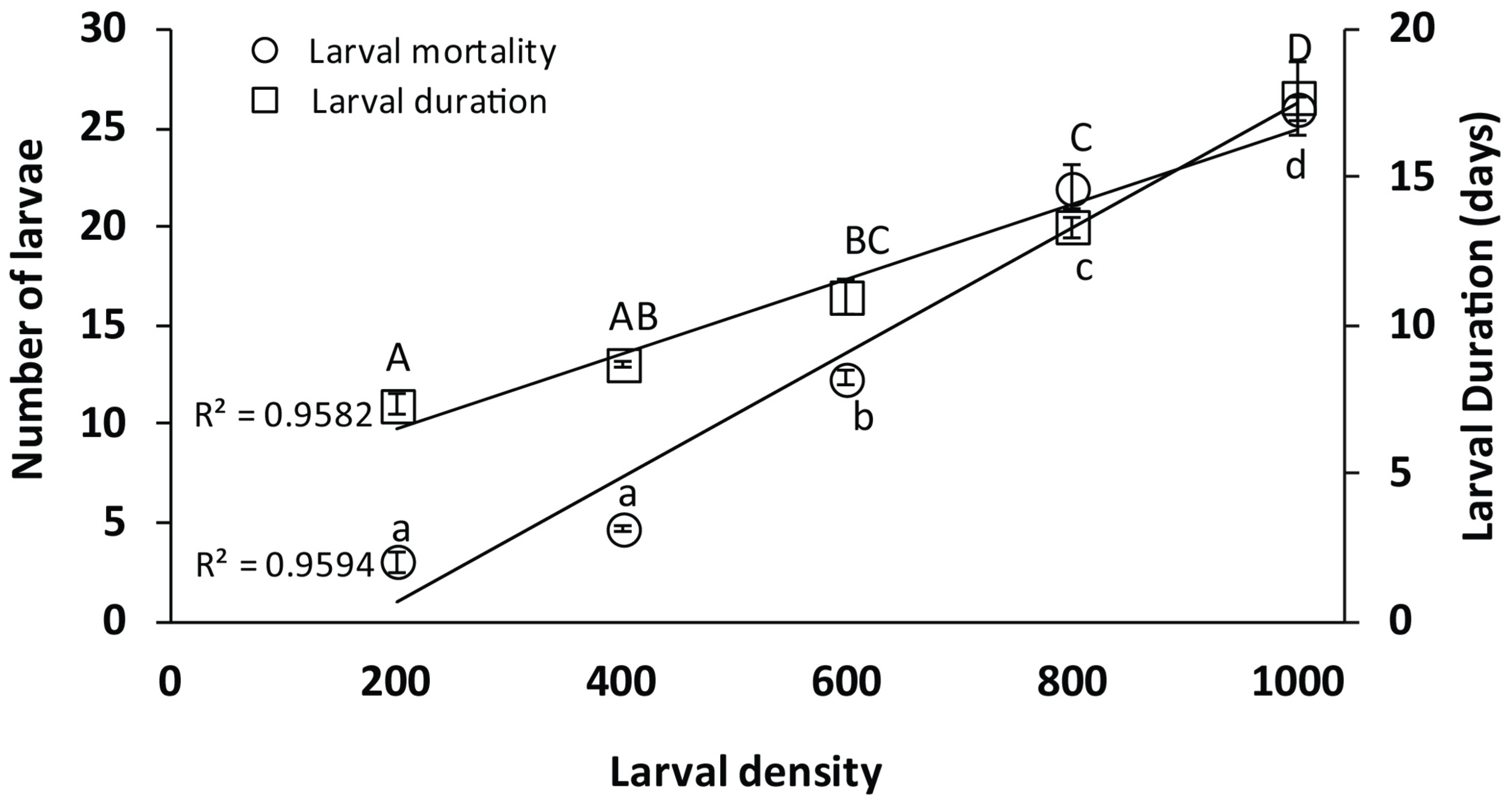
3.2. Larval Density
3.3. Meteorological Data
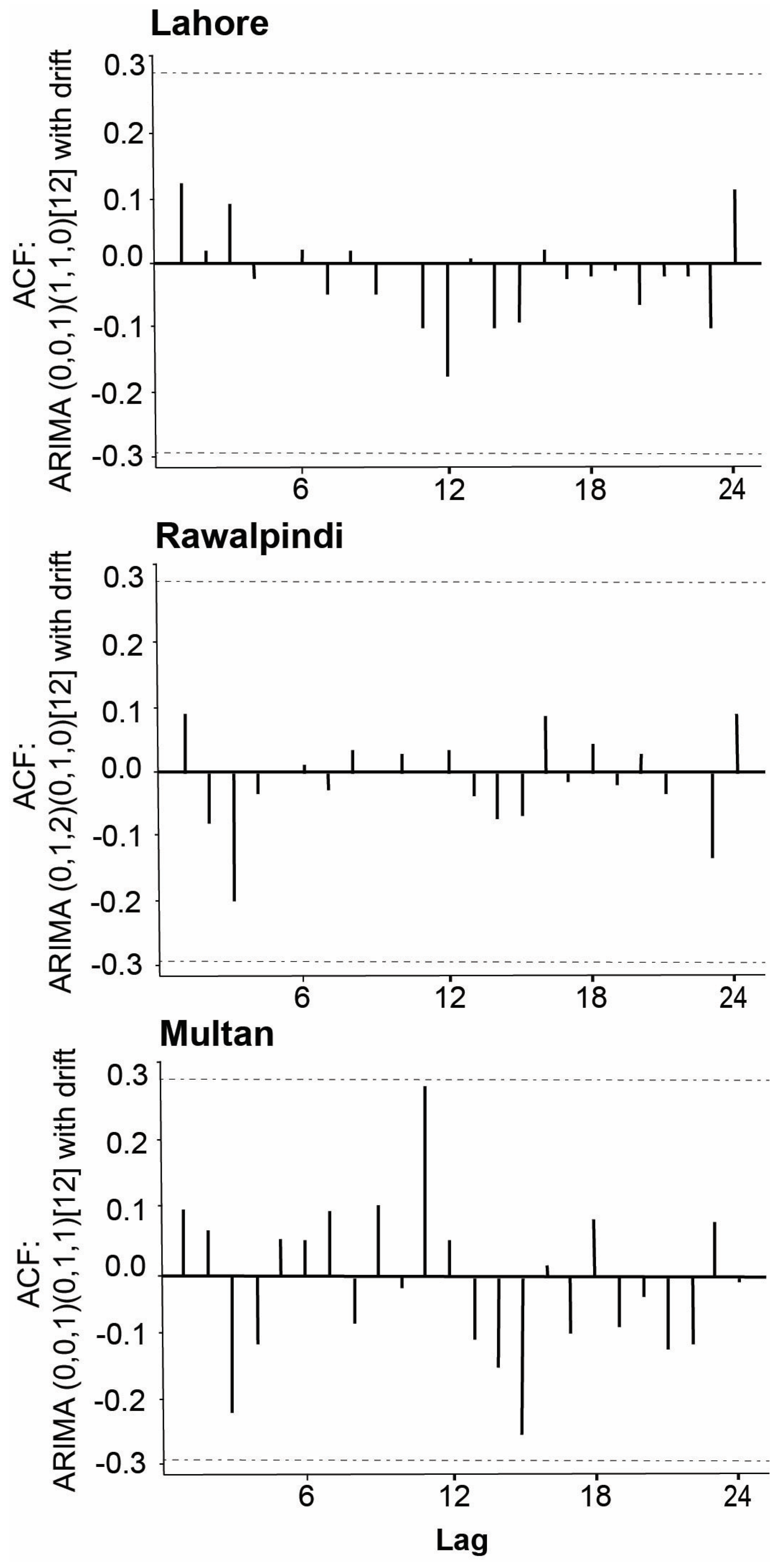
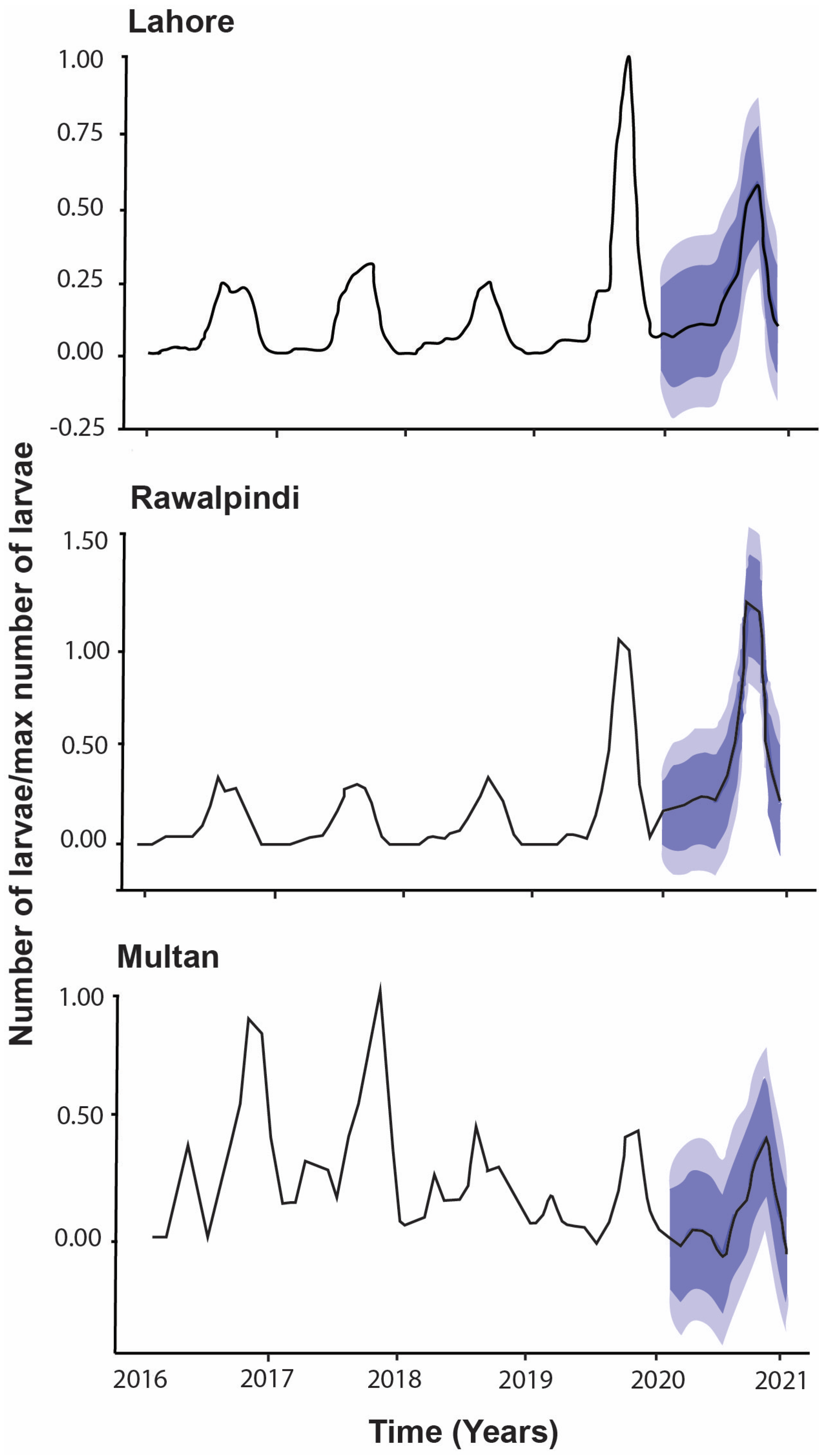
3.4. Temporal Occurrence of Vector Aedes Aegypti Larval Population
3.5. Pearson Correlation Analysis
4. Discussion
4.1. Effects of Temperature and Relative Humidity
4.2. Density-Dependent Trials
4.3. Temporal Occurrence of Vector Population
4.4. Population Density
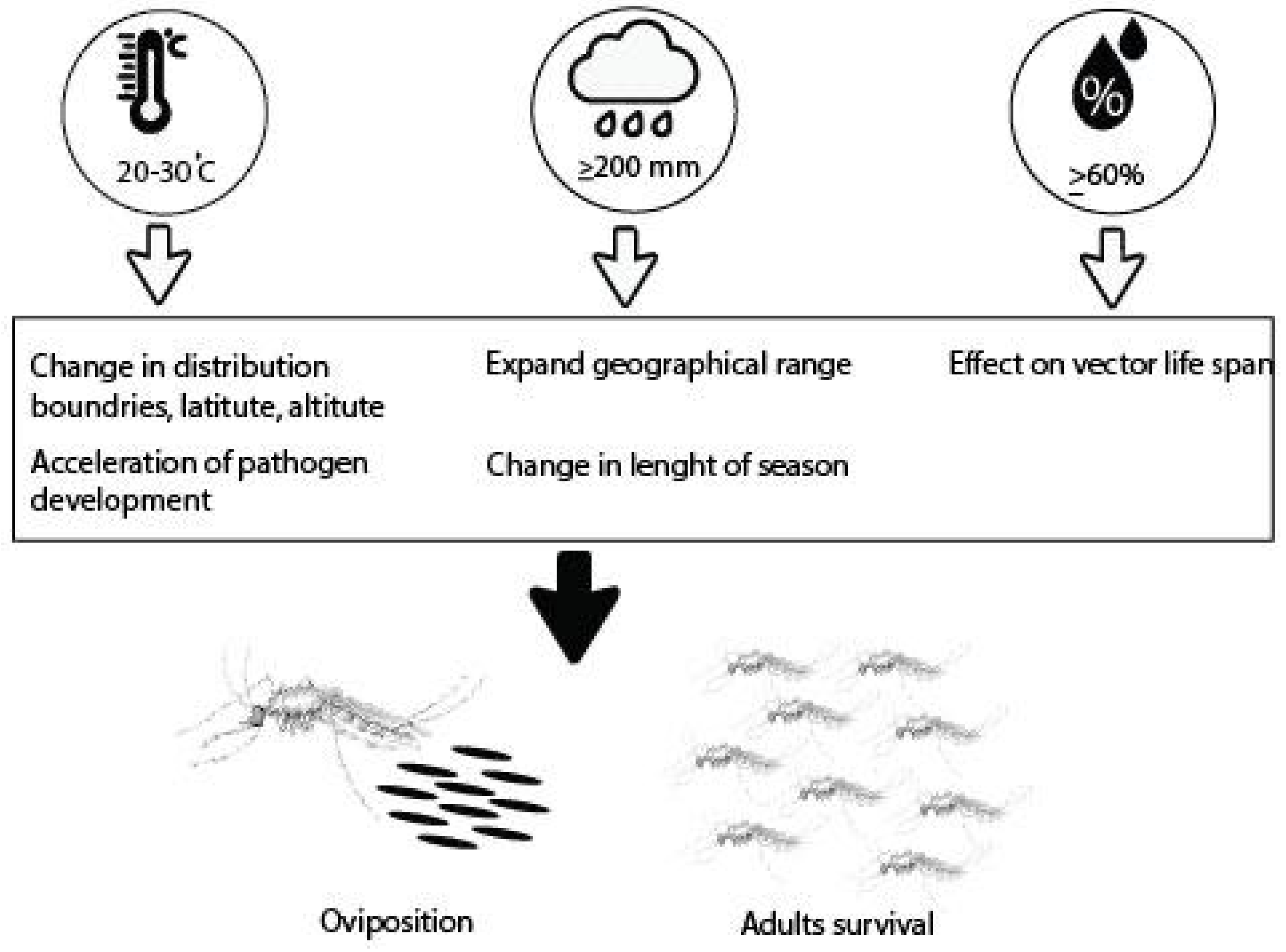
4.5. Climate Change
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- VijayaVenkataRaman, S.; Iniyan, S.; Goic, R. A review of climate change, mitigation and adaptation. Renew. Sustain. Energy Rev. 2012, 16, 878–897. [Google Scholar] [CrossRef]
- Blunden, J.; Hartfield, G.; Arndt, D.S.; Dunn, R.J.H.; Tye, M.R.; Blenkinsop, S.; Donat, M.; Durre, I.; Ziese, M.; Cooper, O.R.; et al. State of the Climate in 2017. Bull. Am. Meteorol. Soc. 2018, 99, Si-S332. [Google Scholar]
- Hansen, J.E.; Kharecha, P.; Sato, M.; Tselioudis, G.; Kelly, J.; Bauer, S.E.; Ruedy, R.; Jeong, E.; Jin, Q.; Rignot, E.; et al. Global warming has accelerated: Are the United Nations and the public well-informed? Environ. Sci. Policy Sustain. Dev. 2025, 67, 6–44. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Brisbois, B.W.; Ali, S.H. Climate change, vector-borne disease and interdisciplinary research: Social science perspectives on an environment and health controversy. EcoHealth 2010, 7, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Koenraadt, C.J. Climate change and highland malaria: Fresh air for a hot debate. Q. Rev. Biol. 2010, 85, 27–55. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Athni, T.S.; Shocket, M.S.; Couper, L.I.; Nova, N.; Caldwell, I.R.; Caldwell, J.M.; Childress, J.N.; Childs, M.L.; De Leo, G.A.; Kirk, D.G.; et al. The influence of vector-borne disease on human history: Socio-ecological mechanisms. Ecol. Lett. 2021, 24, 829–846. [Google Scholar] [CrossRef]
- Onen, H.; Luzala, M.M.; Kigozi, S.; Sikumbili, R.M.; Muanga, C.K.; Zola, E.N.; Wendji, S.N.; Buya, A.B.; Balciunaitiene, A.; Viškelis, J.; et al. Mosquito-borne diseases and their control strategies: An overview focused on green synthesized plant-based metallic nanoparticles. Insects 2023, 14, 221. [Google Scholar] [CrossRef]
- Reddy, P.P. Impact of climate change on insect pests, pathogens and nematodes. Pest Manag. Hortic. Ecosyst. 2013, 19, 225–233. [Google Scholar]
- Afrane, Y.A.; Zhou, G.; Lawson, B.W.; Githeko, A.K.; Yan, G. Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. Am. J. Trop. Med. Hyg. 2006, 74, 772–778. [Google Scholar] [CrossRef]
- Tabachnick, W. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J. Exp. Biol. 2010, 213, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.L.M.; Whitehead, S.A.; Thomas, M.B. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 2017, 15, e2003489. [Google Scholar] [CrossRef] [PubMed]
- Ezeakacha, N.F.; Yee, D.A. The role of temperature in affecting carry-over effects and larval competition in the globally invasive mosquito Aedes albopictus. Parasites Vectors 2019, 12, 123. [Google Scholar] [CrossRef]
- Baylis, M. Potential impact of climate change on emerging vector-borne and other infections in the UK. Environ. Health 2017, 16, 45–51. [Google Scholar] [CrossRef]
- Reiter, P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001, 109, 141–161. [Google Scholar] [PubMed]
- Hales, S.; De Wet, N.; Maindonald, J.; Woodward, A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet 2002, 360, 830–834. [Google Scholar] [CrossRef]
- Pedrosa de Almeida Costa, E.A.; de Mendonça Santos, E.M.; Cavalcanti Correia, J.; Ribeiro de Albuquerque, C.M. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae). Rev. Bras. Entomol. 2010, 54, 488–493. [Google Scholar] [CrossRef]
- Chan, Y.C.; Salahuddin, N.I.; Khan, J.; Tan, H.C.; Seah, C.L.K.; Li, J.; Chow, V.T.K. Dengue haemorrhagic fever outbreak in Karachi, Pakistan, 1994. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 619–620. [Google Scholar] [CrossRef]
- Siddiqui, F.J.; Haider, S.R.; Bhutta, Z.A. Endemic dengue fever: A seldom recognized hazard for Pakistani children. J. Infect. Dev. Ctries. 2009, 3, 306–312. [Google Scholar]
- Munir, M.A.; Alam, S.E.; Khan, Z.U.; Saeed, Q.; Arif, A.; Iqbal, R.; Nadeem Saqib, M.A.; Qureshi, H. Dengue fever in patients admitted in tertiary care hospitals in Pakistan. J. Pak. Med. Assoc. 2014, 64, 553. [Google Scholar]
- Khanani, R.M.; Arif, A.; Shaikh, R. Dengue in Pakistan: Journey from a disease-free to a hyper-endemic nation. J. Dow Univ. Health Sci. 2011, 5, 81–84. [Google Scholar]
- Chan, E.H.; Scales, D.A.; Brewer, T.F.; Madoff, L.C.; Pollack, M.P.; Hoen, A.G.; Choden, T.; Brownstein, J.S. Forecasting high-priority infectious disease surveillance regions: A socioeconomic model. Clin. Infect. Dis. 2013, 56, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Leach, S.A. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect. Dis. 2015, 15, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Menne, B. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 2009, 9, 365–375. [Google Scholar] [CrossRef]
- Majeed, S.; Sufyan, M.; Abbasi, A.; Binyameen, M.; Saleem, R.; Alkherb, W.A.H.; Faisal, S.; Ijaz, J.; Hameed, H. Efficacy of different blood meals for the mass rearing of Aedes aegypti (Diptera: Culicidae) population. Int. J. Trop. Insect Sci. 2024, 44, 1391–1398. [Google Scholar] [CrossRef]
- Couret, J.; Dotson, E.; Benedict, M.Q. Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera: Culicidae). PLoS ONE 2014, 9, e87468. [Google Scholar] [CrossRef]
- Custodio, J.M.O.; Nogueira, L.M.S.; Souza, D.A.; Fernandes, M.F.; Oshiro, E.T.; Oliveira, E.F.; Piranda, E.M.; Oliveira, A.G. Abiotic factors and population dynamic of Aedes aegypti and Aedes albopictus in an endemic area of dengue in Brazil. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, e18. [Google Scholar] [CrossRef]
- do Nascimento, J.F.; Palioto-Pescim, G.F.; Pescim, R.R.; Suganuma, M.S.; Zequi, J.A.C.; Golias, H.C. Influence of abiotic factors on the oviposition of Aedes (Stegomyia) aegypti (Diptera: Culicidae) in Northern Paraná, Brazil. Int. J. Trop. Insect Sci. 2022, 42, 2215–2220. [Google Scholar] [CrossRef]
- Santos, I.C.D.S.; Braga, C.; de Souza, W.V.; de Oliveira, A.L.S.; Regis, L.N. The influence of meteorological variables on the oviposition dynamics of Aedes aegypti (Diptera: Culicidae) in four environmentally distinct areas in northeast Brazil. Mem. Inst. Oswaldo Cruz 2020, 115, e200046. [Google Scholar] [CrossRef]
- Jong, Z.W.; Kassim, N.F.A.; Naziri, M.A.; Webb, C.E. The effect of inbreeding and larval feeding regime on immature development of Aedes albopictus. J. Vector Ecol. 2017, 42, 105–112. [Google Scholar] [CrossRef]
- Bayoh, M.N.; Lindsay, S.W. Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Med. Vet. Entomol. 2004, 18, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Chadee, D.D. Effects of different temperature regimens on the development of Aedes aegypti (L.) (Diptera: Culicidae) mosquitoes. Acta Trop. 2011, 119, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Sukiato, F.; Wasserman, R.J.; Foo, S.C.; Wilson, R.F.; Cuthbert, R.N. The effects of temperature and shading on mortality and development rates of Aedes aegypti (Diptera: Culicidae). J. Vector Ecol. 2019, 44, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Canyon, D.; Hii, J.; Müller, R. Adaptation of Aedes aegypti (Diptera: Culicidae) oviposition behavior in response to humidity and diet. J. Insect Physiol. 1999, 45, 959–964. [Google Scholar] [CrossRef]
- Eisen, L.; Monaghan, A.J.; Lozano-Fuentes, S.; Steinhoff, D.F.; Hayden, M.H.; Bieringer, P.E. The impact of temperature on the bionomics of Aedes (Stegomyia) aegypti, with special reference to the cool geographic range margins. J. Med. Entomol. 2014, 51, 496–516. [Google Scholar] [CrossRef]
- Mulla, M.S.; Chaudhury, M.F.B. Influence of some environmental factors on the viability and hatching of Aedes aegypti (L.) eggs. Mosq. News. 1968, 28, 217–221. [Google Scholar]
- Fischer, S.; De Majo, M.S.; Cristian Di Battista, C.; Campos, R.E. Effects of temperature and humidity on the survival and hatching response of diapausing and non-diapausing Aedes aegypti eggs. J. Insect Physiol. 2025, 161, 104726. [Google Scholar] [CrossRef]
- Carrington, L.B.; Armijos, M.V.; Lambrechts, L.; Scott, T.W. Fluctuations at a low mean temperature accelerate dengue virus transmission by Aedes aegypti. PLoS Neglected Trop. Dis. 2013, 7, e2190. [Google Scholar] [CrossRef]
- Thomson, R.M. Studies on the behaviour of Anopheles minimus. J. Malar. Inst. India 1941, 4, 217–245. [Google Scholar]
- Dickerson, C.Z. The Effects of Temperature and Humidity on the Eggs of Aedes aegypti (L.) and Aedes albopictus (Skuse) in Texas. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, December 2007. [Google Scholar]
- Estallo, E.L.; Ludueña-Almeida, F.F.; Introini, M.V.; Zaidenberg, M.; Almirón, W.R. Weather variability associated with Aedes (Stegomyia) aegypti (Dengue vector) oviposition dynamics in Northwestern Argentina. PLoS ONE 2015, 10, e0127820. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.M.; Salinas, E.; Lopez, J.R.; Del Angel-Cabañas, G.; Martinez, L.; Bown, D.N. Laboratory oviposition, fecundity and egg hatching ability of colonized Anopheles albimanus from southwestern Mexico. J. Am. Mosq. Control Assoc. 1988, 4, 509–515. [Google Scholar]
- Awang, M.F.; Dom, N.C. The effect of temperature on the development of immature stages of Aedes spp. against breeding containers. Int. J. Glob. Warm. 2020, 21, 215–233. [Google Scholar] [CrossRef]
- Onyango, M.G.; Bialosuknia, S.M.; Payne, A.F.; Mathias, N.; Kuo, L.; Vigneron, A.; Kramer, L.D. Increased temperatures reduce the vectorial capacity of Aedes mosquitoes for Zika virus. Emerg. Microbes Infect. 2020, 9, 67–77. [Google Scholar] [CrossRef]
- Yoshioka, M.; Couret, J.; Kim, F.; McMillan, J.; Burkot, T.R.; Dotson, E.M.; Kitron, U.; Vazquez-Prokopec, G.M. Diet and density dependent competition affect larval performance and oviposition site selection in the mosquito species Aedes albopictus (Diptera: Culicidae). Parasites Vectors 2012, 5, 225. [Google Scholar] [CrossRef] [PubMed]
- Zapletal, J.; Erraguntla, M.; Adelman, Z.N.; Myles, K.M.; Lawley, M.A. Impacts of diurnal temperature and larval density on aquatic development of Aedes aegypti. PLoS ONE 2018, 13, e0194025. [Google Scholar] [CrossRef]
- Wada, Y. Effect of larval density on the development of Aedes aegypti (L.) and the size of adults. Quaest. Entomol. 1965, 1, 223–249. [Google Scholar]
- Ciota, A.T.; Matacchiero, A.C.; Kilpatrick, A.M.; Kramer, L.D. The effect of temperature on life history traits of Culex mosquitoes. J. Med. Entomol. 2014, 51, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Tong, S.; Donald, K.; Parton, K.A.; Ni, J. Climatic variables and transmission of malaria: A 12-year data analysis in Shuchen County, China. Public Health Rep. 2003, 118, 65. [Google Scholar] [CrossRef]
- Bouma, M.; Dye, C.; Van der Kaay, H. Plasmodium falciparum malaria and climate change in the Northwest Frontier Province of Pakistan. Am. J. Trop. Med. Hyg. 1996, 55, 131–137. [Google Scholar] [CrossRef]
- Chen, M.J.; Lin, C.Y.; Wu, Y.T.; Wu, P.C.; Lung, S.C.; Su, H.J. Effects of extreme precipitation on the distribution of infectious diseases in Taiwan, 1994–2008. PLoS ONE 2012, 7, e34651. [Google Scholar] [CrossRef]
- Sirisena, P.D.N.N.; Noordeen, F.; Kurukulasuriya, H.; Romesh, T.A.; Fernando, L. Effect of climatic factors and population density on the distribution of dengue in Sri Lanka: A GIS-based evaluation for prediction of outbreaks. PLoS ONE 2017, 12, e0166806. [Google Scholar] [CrossRef]
- Noureldin, E.; Shaffer, L. Role of climatic factors in the incidence of dengue in Port Sudan City, Sudan. East. Mediterr. Health J. 2019, 25, 852–860. [Google Scholar] [CrossRef]
- Pathirana, S.; Kawabata, M.; Goonatilake, R. Study of potential risk of dengue disease outbreak in Sri Lanka using GIS and statistical modelling. J. Rural Trop. Public Health 2009, 8, 8–17. [Google Scholar]
- Pinto, E.; Coelho, M.; Oliver, L.; Massad, E. The influence of climate variables on dengue in Singapore. Int. J. Environ. Health Res. 2011, 21, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.G.; Roy, P.; Das, S.; Mogha, N.S.; Bansal, A.K. Empirical model for estimating dengue incidence using temperature, rainfall, and relative humidity: A 19-year retrospective analysis in East Delhi. Epidemiol. Health 2016, 38, e2016052. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Suzilah, I.; Wan Najdah, W.M.A.; Topek, O.; Mustafakamal, I.; Lee, H.L. Factors determining dengue outbreak in Malaysia. PLoS ONE 2018, 13, e0193326. [Google Scholar] [CrossRef]
- Somboonsak, P. Forecasting dengue fever epidemics using ARIMA model. In Proceedings of the 2019 2nd Artificial Intelligence and Cloud Computing Conference, Kobe, Japan, 21–23 December 2019; Association for Computing Machinery: New York, NY, USA, 2020; pp. 144–150. [Google Scholar] [CrossRef]
- Murray, N.E.A.; Quam, M.B.; Wilder-Smith, A. Epidemiology of dengue: Past, present and future prospects. Clin. Epidemiol. 2013, 5, 299–309. [Google Scholar]
- Rey, J.R.; Walton, W.E.; Wolfe, R.J.; Connelly, C.R.; O’Connell, S.M.; Berg, J.; Sakolsky-Hoopes, G.E.; Laderman, A.D. North American wetlands and mosquito control. Int. J. Environ. Res. Public Health 2012, 9, 4537–4605. [Google Scholar] [CrossRef]
- Sirisena, P.; Noordeen, F. Evolution of dengue in Sri Lanka—Changes in the virus, vector, and climate. Int. J. Infect. Dis. 2014, 19, 6–12. [Google Scholar] [CrossRef]
- Tun-Lin, W.; Burkot, T.; Kay, B. Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, Australia. Med. Vet. Entomol. 2000, 14, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ebi, K.L.; Nealon, J. Dengue in a changing climate. Environ. Res. 2016, 151, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Rohani, A.; Wong, Y.C.; Zamre, I.; Lee, H.L.; Zurainee, M.N. The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in Aedes aegypti (L.). Southeast Asian J. Trop. Med. Public Health 2009, 40, 942–950. [Google Scholar]
- Wu, P.C.; Lay, J.G.; Guo, H.R.; Lin, C.Y.; Lung, S.C.; Su, H.J. Higher temperature and urbanization affect the spatial patterns of dengue fever transmission in subtropical Taiwan. Sci. Total Environ. 2009, 407, 2224–2233. [Google Scholar] [CrossRef] [PubMed]



| Lahore | |||||
|---|---|---|---|---|---|
| Scale Value | No. of Patients | Tmax | RH | Precipitation | No. of Larva |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 0.2 | 95 | 8.12 | 17.4 | 115.5 | 17,881 |
| 0.4 | 190 | 16.24 | 34.8 | 231 | 35,762 |
| 0.6 | 285 | 24.36 | 52.2 | 346.5 | 53,643 |
| 0.8 | 380 | 32.48 | 69.6 | 462 | 71,524 |
| 1 | 475 | 40.6 | 87 | 577.5 | 89,405 |
| Rawalpindi | |||||
| Scale Value | No. of Patients | Tmax | RH | Precipitation | No. of Larva |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 0.2 | 577.6 | 7.74 | 19 | 107.1 | 11,869 |
| 0.4 | 1155.2 | 15.48 | 38 | 214.2 | 23,738 |
| 0.6 | 1732.8 | 23.22 | 57 | 321.3 | 35,607 |
| 0.8 | 2310.4 | 30.96 | 76 | 428.4 | 47,476 |
| 1 | 2888 | 38.7 | 95 | 535.5 | 59,345 |
| Multan | |||||
| Scale Value | No. of Patients | Tmax | RH | Precipitation | No. of Larva |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 0.2 | 5.2 | 8.42 | 18.2 | 16.34 | 224.6 |
| 0.4 | 10.4 | 16.84 | 36.4 | 32.68 | 449.2 |
| 0.6 | 15.6 | 25.26 | 54.6 | 49.02 | 673.8 |
| 0.8 | 20.8 | 33.68 | 72.8 | 65.36 | 898.4 |
| 1 | 26 | 42.1 | 91 | 81.7 | 1123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, S.; Akram, W.; Sufyan, M.; Abbasi, A.; Riaz, S.; Faisal, S.; Binyameen, M.; Bashir, M.I.; Hassan, S.; Zafar, S.; et al. Climate Change: A Major Factor in the Spread of Aedes aegypti (Diptera: Culicidae) and Its Associated Dengue Virus. Insects 2025, 16, 513. https://doi.org/10.3390/insects16050513
Majeed S, Akram W, Sufyan M, Abbasi A, Riaz S, Faisal S, Binyameen M, Bashir MI, Hassan S, Zafar S, et al. Climate Change: A Major Factor in the Spread of Aedes aegypti (Diptera: Culicidae) and Its Associated Dengue Virus. Insects. 2025; 16(5):513. https://doi.org/10.3390/insects16050513
Chicago/Turabian StyleMajeed, Shahid, Waseem Akram, Muhammad Sufyan, Asim Abbasi, Sidra Riaz, Shahla Faisal, Muhammad Binyameen, Muhammad I. Bashir, Shahzad Hassan, Saba Zafar, and et al. 2025. "Climate Change: A Major Factor in the Spread of Aedes aegypti (Diptera: Culicidae) and Its Associated Dengue Virus" Insects 16, no. 5: 513. https://doi.org/10.3390/insects16050513
APA StyleMajeed, S., Akram, W., Sufyan, M., Abbasi, A., Riaz, S., Faisal, S., Binyameen, M., Bashir, M. I., Hassan, S., Zafar, S., Kucher, O., Piven, E. A., & Kucher, O. D. (2025). Climate Change: A Major Factor in the Spread of Aedes aegypti (Diptera: Culicidae) and Its Associated Dengue Virus. Insects, 16(5), 513. https://doi.org/10.3390/insects16050513







