Conservation of Apis mellifera mellifera L. in the Middle Ural: A Review of Genetic Diversity, Ecological Adaptation, and Breeding Perspectives
Simple Summary
Abstract
1. Introduction
2. Ecological Conditions for Beekeeping in Perm Region
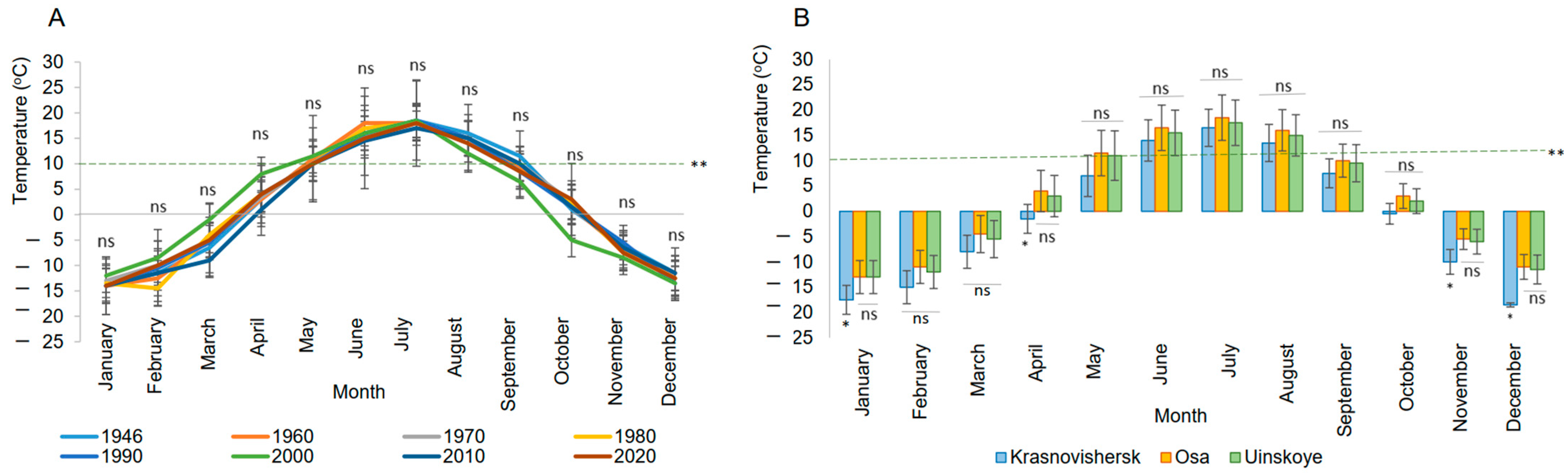
2.1. Climate Trends and Regional Temperature Variability
2.2. Forest Zones and Nectar Resources
2.3. Bee-Breeding Centers and Reserve for A. m. mellifera Honey Bees Prikamskaya Population
3. Characteristics of A. m. mellifera L. Honey Bees (Prikamskaya Population)
3.1. Ecologic Characteristics
3.2. Physiological Adaptations During Overwintering
3.3. Morphometric Characteristics of Northern and Southern Prikamskaya Population of Honey Bees
3.4. Genetic Characteristics of Northern and Southern Prikamskaya Population of Honey Bees
4. Challenges and Advances in Queen Rearing of A. m. mellifera in the Perm Region
5. Regulation in Beekeeping and Genetic Conservation: Challenges and Strategies in the Perm Region
6. Future Prospects for Conservation and Breeding in Russia
- Selective breeding and reproduction—Selection of queen bees with relevant traits, their reproduction, and testing to ensure high-quality genetic traits for commercial and conservation purposes.
- Controlled mating and genetic preservation—Establishment of mating stations to facilitate the controlled mating of virgin queens in isolation from other populations and breeds, preventing genetic dilution.
- Conservation of A. m. mellifera habitats—Expansion of protected natural areas (PNA) and state-designated protection zones around breeding apiaries to preserve the gene pool and prevent genetic contamination from non-native honey bee populations.
- Collaborative research and expertise exchange—Strengthening national and international cooperation with honey bee breeders to integrate advanced queen-rearing methods and enhance genetic selection programs.
- Systematic queen distribution—Developing structured supply chains for A. m. mellifera queens to support conservation efforts, maintain genetic diversity, and promote sustainable beekeeping across Russia and internationally.
- Communication and knowledge transfer—Establishing direct communication channels between researchers and beekeepers to facilitate the exchange of scientific knowledge, improve colony management, and enhance breeding outcomes.
- Expansion of purebred colonies—Encouraging the increase in A. m. mellifera purebred colonies in private and commercial apiaries to safeguard genetic integrity and counteract uncontrolled hybridization with non-native bee populations.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilyasov, R.A.; Poskryakov, A.V.; Nikolenko, A.G. The genetic structure of dark European honey bee population in the Ural. In Honeybees: Biology, Behavior, and Benefits; Nova Science Publishers: Hauppauge, NY, USA, 2016; pp. 1–13. [Google Scholar]
- Ruttner, F. Biogeography and Taxonomy of Honeybees; Springer: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Ruttner, F.; Milner, E.; Dews, J.E. The Dark European Honey Bee: Apis mellifera mellifera Linnaeus 1758; British Isles Bee Breeders Association: Codnor, Derbyshire, UK, 2004. [Google Scholar]
- Alpatov, V.V. Biometrical studies on variation and races of the honeybee (Apis mellifera L.). Q. Rev. Biol. 1929, 4, 1–58. [Google Scholar] [CrossRef]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Osborne, J.L.; Settele, J. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Van Espen, M.; Williams, J.H.; Alves, F.; Hung, Y.; de Graaf, D.C.; Verbeke, W. Beekeeping in Europe facing climate change: A mixed methods study on perceived impacts and the need to adapt according to stakeholders and beekeepers. Sci. Total Environ. 2023, 888, 164255. [Google Scholar] [CrossRef]
- Jensen, A.B.; Palmer, K.A.; Boomsma, J.J.; Pedersen, B.V. Varying degrees of Apis mellifera ligustica introgression in protected populations of the black honeybee, Apis mellifera mellifera, in northwest Europe. Mol. Ecol. 2005, 14, 93–106. [Google Scholar] [CrossRef]
- Orlovskytė, S.; Budrys, E.; Skrodenytė-Arbačiauskienė, V.; Blažytė-Čereškienė, L. The dark European honey bee Apis mellifera mellifera in Lithuania: Data on mitotype diversity of native bee population. J. Apic. Res. 2024, 63, 1–4. [Google Scholar] [CrossRef]
- Mobus, B. Brood rearing in the winter cluster. Am. Bee J. 1998, 138, 511–514. [Google Scholar]
- Smoliński, S.; Glazaczow, A. Causal network linking honey bee (Apis mellifera) winter mortality to temperature variations and Varroa mite density. Sci. Total Environ. 2024, 954, 176245. [Google Scholar] [CrossRef] [PubMed]
- García, C.A.Y.; Rodrigues, P.J.; Tofilski, A.; Elen, D.; McCormak, G.P.; Oleksa, A.; Henriques, D.; Ilyasov, R.; Kartashev, A.; Bargain, C.; et al. Using the software DeepWings© to classify honey bees across Europe through wing geometric morphometrics. Insects 2022, 13, 1132. [Google Scholar] [CrossRef]
- Gray, A.; Adjlane, N.; Arab, A.; Ballis, A.; Brusbardis, V.; Bugeja, D.A.; Cadahia, L.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; et al. Honey bee colony loss rates in 37 countries using the COLOSS survey for winter 2019–2020: The combined effects of operation size, migration, and queen replacement. J. Apic. Res. 2022, 62, 204–210. [Google Scholar] [CrossRef]
- Brandorf, A.Z. The introduction of honey bees for reducing the biodiversity of pollinators. In Proceedings of the 3rd International Electronic Conference on Diversity: Biodiversity Conservation, Online, 15–17 October 2024; Cianfaglione, K., Ed.; MDPI: Basel, Switzerland, 2024; p. 08956. [Google Scholar]
- Krivtsov, N.I.; Goryacheva, I.I.; Borodachev, A.V. Investigation of honey bee (Apis mellifera L.) breeds and populations for developing criteria for genetic certification of bees. Russ. Agric. Sci. 2011, 37, 79–82. [Google Scholar] [CrossRef]
- Frunze, O.; Brandorf, A.; Kang, E.-J.; Choi, Y.-S. Beekeeping genetic resources and retrieval of honey bee Apis mellifera L. stock in the Russian Federation: A review. Insects 2021, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Lukic, B.; Raguz, N.; Kovačić, M.; Curik, I.; Obšteter, J.; Prešern, J.; Bubnič, J.; Lužaić, R.; Pihler, I.; Mirjanić, G.; et al. Genomic diversity and population structure of Carniolan honey bee in its native habitat. BMC Genom. 2024, 25, 849. [Google Scholar] [CrossRef]
- AgrowebCEE. Beekeeping Official Government Publications. Available online: http://www.agrowebcee.net/awru/beekeeping/ (accessed on 1 May 2025).
- Perm Krai Administration. Geographical Location and Boundaries of Perm Krai. Available online: http://en.psu.ru/about/city-region/ (accessed on 1 April 2025).
- Mellanby, K. Low temperature and insect activity. Proc. R. Soc. B Biol. Sci. 1939, 127, 473–487. [Google Scholar]
- Heinrich, B. Thermal relations of the honey bee, Apis mellifera L. Insectes Sociaux 1979, 26, 359–377. [Google Scholar]
- Vincze, C.; Leelőssy, Á.; Zajácz, E.; Tóth, J.; Pál, J.; Horváth, L. A review of short-term weather impacts on honey production. Int. J. Biometeorol. 2025, 69, 303–317. [Google Scholar] [CrossRef]
- GangMa, C.S.M.; Baaren, C.L. Effects of climate change on insect phenology. In Effects of Climate Change on Insects: Physiological, Evolutionary, and Ecological Responses; Oxford University Press: Oxford, UK, 2024; p. 89. [Google Scholar]
- Eremia, N.; Scripnic, E.; Modvala, S.; Chiriac, A. Influence of temperature on nectar collection and storage in the hive during honey harvest. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2017, 17, 31–34. [Google Scholar]
- Vesti Perm. Weather Trends in the Perm Region Based on Yandex Weather Data. Available online: https://vesti-perm.ru/pages/91799a97d6984dd3919c9032f7b2305c (accessed on 28 February 2025).
- Döke, M.A.; Frazier, M.; Grozinger, C.M. Overwintering honey bees: Biology and management. Curr. Opin. Insect Sci. 2015, 10, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Simankov, M.K.; Shurakov, S.A. Climatic chronicle of the summer seasons in the Perm region and productivity of bee colonies. Beekeeping 2020, 2, 10–12. (In Russian) [Google Scholar]
- Contosta, A.R.; Arndt, K.A.; Baulch, H.M.; Casson, N.J.; Harpold, A.; Morelli, T.L.; Sirén, A.P.K.; Templer, P.H. Threshold changes in winter temperature and precipitation drive threshold responses across nine global climate zones and associated biomes. Annu. Rev. Ecol. Evol. Syst. 2024, 55, 271–300. [Google Scholar] [CrossRef]
- Rundqvist, N.A.; Zadorina, O.V. Ural: Illustrated Encyclopedia of Local Places; Kvist: Ekaterinburg, Russia, 2013. (In Russian) [Google Scholar]
- Frunze, O.N. Forage Base and Biological Characteristics of Honey Bees (Apis mellifera L.) in the Perm Region. Ph.D. Thesis, Izhevsk State Agricultural Academy, Izhevsk, Russia, 2011. Available online: https://www.dissercat.com/content/kormovaya-baza-i-biologicheskie-osobennosti-medonosnykh-pchel-apis-mellifera-l-v-permskom-kr (accessed on 1 April 2025). (In Russian).
- Murylev, A.V. Honey resources of the southern and middle taiga of the Perm region. Vestn. KrasGAU 2015, 5, 170–173. (In Russian) [Google Scholar]
- Buszmakow, S.A.; Pietuchow, A.W.; Gatina, E.L. Pszczoła środkowoeuropejska (Apis mellifera mellifera) w ochranianym parku krajobrazowym «Malinowy Hutor» [The Central European honey bee (Apis mellifera mellifera) in the protected landscape park “Malinowy Hutor”]. In Proceedings of the Materials of the International Seminar “The Bee in the Natural Environment”, Hajnówka/Białowieża, Poland, 21–24 November 2013; pp. 58–59. (In Polish). [Google Scholar]
- Murylev, A.V.; Petukhov, A.V.; Lipatov, V.Y. Adaptations of honeybees Apis mellifera mellifera L. and Apis mellifera carpathica to low winter temperatures. Russ. J. Ecol. 2012, 5, 409–411. [Google Scholar] [CrossRef]
- Smith, J. Ecological Characteristics and Their Role in Environmental Interactions; Green Earth Publications: Delhi, India, 2019. [Google Scholar]
- Brandorf, A.Z.; Ivoilova, M.M. Methodological Guidelines for Selection and Breeding Evaluation of Central Russian Honey Bees; Research Institute of Agriculture of the North-East: Kirov, Russia, 2015. (In Russian) [Google Scholar]
- Korishev, V. The role of carbon dioxide in the life of bees. Beekeeping 2004, 7, 30–31. (In Russian) [Google Scholar]
- Eskov, E.K.; Eskova, M.D.; Spasik, S.E. Hypo- and hyperthermia of bees anesthetized with carbon dioxide. Russ. Agric. Sci. 2014, 40, 382–384. [Google Scholar] [CrossRef]
- Brandorf, A.Z.; Rychkov, I.N. Egg production of queen bees of the Central Russian breed depending on the method of obtaining queens. In Scientific Support for the Innovative Development of the Agro-Industrial Complex: Materials of the All-Russian Scientific and Practical Conference Dedicated to the 90th Anniversary of Udmurtia’s Statehood; Izhevsk State Agricultural Academy: Izhevsk, Russia, 2010; Volume 2, pp. 73–75. [Google Scholar]
- Savuskina, L.N.; Borodachev, A.V. Egg production and productivity of Apis mellifera mellifera L. In Apis mellifera mellifera L. in Development Strategies of World Beekeeping; Brandorf, A.Z., Ivoilova, M.M., Eds.; Agricultural Research Institute of the North-East: Kirov, Russia, 2019; pp. 78–81. [Google Scholar]
- Grankin, N.N.; Bakina, S.N.; Tyapkina, A.P.; Fomina, E.A. Some results of work on breeding Apis mellifera mellifera L. for aggressiveness. In Apis mellifera mellifera L. in Development Strategies of World Beekeeping; Brandorf, A.Z., Ivoilova, M.M., Eds.; Agricultural Research Institute of the North-East: Kirov, Russia, 2019; pp. 39–45. [Google Scholar]
- Murylev, A.V.; Petukhov, A.V. Quality characteristics of queen bees Apis mellifera mellifera L. and Apis mellifera carpatica under conditions of the Kama Uralvorland region. Biomics 2016, 2, 161–164. (In Russian) [Google Scholar]
- Murylev, A.V.; Petukhov, A.V. Seasonal changes in dry body weight in honey bees Apis mellifera mellifera L. and Apis mellifera carpathica in Perm region. Proc. Irkutsk. State Univ. 2012, 5, 57–60. (In Russian) [Google Scholar]
- Krivtsov, N.I.; Grankin, N.N. Central Russian Bees and Their Selection; GNU NIIP of the Russian Academy of Agricultural Sciences: Rybnoe, Russia, 2004. (In Russian) [Google Scholar]
- Lipatov, V.Y.; Petukhov, A.V.; Murylev, A.V. Thermoregulation of the hive and swarm ball of honey bees Apis mellifera L. In Apidology and Beekeeping; IzhGTU: Izhevsk, Russia, 2012; pp. 62–66. (In Russian) [Google Scholar]
- Shurakov, A.I. Preservation of the Gene Pool of Central Russian Bees and the Main Directions of Beekeeping Development in Perm; Perm State Pedagogical University: Perm, Russia, 1999; p. 30. (In Russian) [Google Scholar]
- Mannapov, A.G.; Mamontova, Y.A. Influence of combs with naturally corresponding cell architecture on the biological and economically useful traits of honey bees. Izv. TSHA 2018, 3, 137–146. (In Russian) [Google Scholar] [CrossRef]
- Simankov, M.K. The relationship of weather conditions, nectar productivity of plants, productivity of bee colonies, workload of honey goiter, and concentration of sugars of its contents. Beekeeping 2024, 7, 19–23. (In Russian) [Google Scholar]
- Yaroshevich, G.S.; Mazina, G.S.; Kuzmin, A.A.; Vladimirova, S.V. Influence of regional weather conditions on the productivity of bee colonies under different management methods. Izv. Velikoluk. GSKHA 2019, 2, 26. [Google Scholar]
- Abdulgazina, N.M.; Yumaguzhin, F.G. Dependence of honey productivity of bees on their breed affiliation: Influence of honey bee enzymes on their economically useful traits. Fundam. Res. 2014, 9, 2177–2180. [Google Scholar]
- Gulov, A.; Sayfutdinova, Z.; Brandorf, A. The honey bee Apis mellifera L. biodiversity in Russia and its preservation. Genet. Breed. Anim. 2022, 4, 114–123. [Google Scholar] [CrossRef]
- Zinatullina, Z.Y.; Dolnikova, T.Y.; Domatskaya, T.F.; Domatsky, A.N. Monitoring diseases of honey bees (Apis mellifera) in Russia. Ukr. J. Ecol. 2018, 8, 106–112. [Google Scholar]
- Petukhov, A.V.; Popov, A.S.; Kazakova, A.N. Resistance to nosematosis of bees of different races. In Proceedings of the Materials of the First International Scientific and Practical Conference, Kirov, Russia, 10–12 March 2014; North-East Agricultural Research Institute: Kirov, Russia; pp. 65–68. (In Russian). [Google Scholar]
- McMullan, D.; Ilyasov, R.A. Formation of natural resistance to the Varroa destructor mite in the population of the dark forest bee in Ireland. Beekeeping 2022, 1, 60–62. (In Russian) [Google Scholar]
- Bennett, A.F.; Lenski, R.E. Evolution and physiological adaptations. In Physiological Ecology of Animals; Raven, P.H., Fox, S.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 105–126. [Google Scholar]
- Murylev, A.V.; Petukhov, A.V. To the method of studying the point of maximum hypothermia in honey bees. In Proceedings of the Problems of Modern Biology: III International Scientific and Practical Conference, Moscow, Russia, 15–17 March 2012; pp. 91–94. (In Russian). [Google Scholar]
- Lavrsky, A.Y.; Lebedinsky, I.A.; Petukhov, A.V. Changes in the epithelium of the middle gut of honey bees Apis mellifera mellifera L.; associated with the exit from the overwintering. Nat. Tech. Sci. 2011, 6, 165–169. [Google Scholar]
- Lavrsky, A.Y.; Petukhov, A.V.; Lebedinsky, I.A. Annual development of the ventricular epithelium in Central Russian and Carpathian honey bees. In Apidology and Beekeeping; IzhGTU: Izhevsk, Russia, 2012; pp. 56–61. (In Russian) [Google Scholar]
- Lebedinsky, I.A.; Lavrsky, A.Y.; Petukhov, A.V. Changes in the parameters of rectal glands as a manifestation of an adaptive mechanism in the life cycle of an Apis mellifera L. colony. Izv. Samara Sci. Cent. Russ. Acad. Sci. 2014, 16, 594–598. (In Russian) [Google Scholar]
- Frunze, O.N.; Petukhov, A.B.; Maksimov, A.Y.; Alakina, S.N. Seasonal dynamics in the activity of certain enzymes of the honey bees of the Central Russian race in the Perm region. In Proceedings of the Beekeeping-XXI Century Dark Bee (Apis mellifera mellifera L.), International Conference, Moscow, Russia, 15–17 September 2008; pp. 360–364. (In Russian). [Google Scholar]
- Frunze, O.N.; Petukhov, A.B.; Maksimov, A.Y. Phosphatase activity in bees of the Central Russian and the Carpathian breeds. Beekeeping 2009, 5, 18–19. (In Russian) [Google Scholar]
- Petukhov, A.V.; Frunze, O.N.; Bessonova, E.N. Dynamics of enzyme activity in the ontogenesis of worker honey bees (Apis mellifera mellifera) and drone honey bees (Apis mellifera mellifera). In Collected Papers. Regional Component in Teaching Biology; Perm State Pedagogical University: Perm, Russia, 2003. (In Russian) [Google Scholar]
- Frunze, O.N.; Petukhov, A.B.; Maksimov, A.Y. Catalase activity in bees of the Central Russian and the Carpathian breeds. Beekeeping 2009, 4, 15–16. (In Russian) [Google Scholar]
- Kekeçoğlu, M.; Bouga, M.; Soysal, M.İ.; Harizanis, P. Morphometrics as a tool for the study of genetic variability of honey bees. J. Tekirdag Agric. Fac. 2007, 4, 7–15. [Google Scholar]
- Carreck, N.L.; Andree, M.; Brent, C.; Cox-Foster, D.; Dade, H.; Ellis, J.D.; Hatjina, F.; VanEngelsdorp, D. Standard methods for Apis mellifera anatomy and dissection. J. Apic. Res. 2013, 52, 1–40. [Google Scholar] [CrossRef]
- Krivtsov, N.I.; Lebedev, V.I.; Tunikov, G.M. Apiculture; Moscow: Koloss, Russia, 2000. [Google Scholar]
- Bilash, G.D.; Krivtsov, N.I. Bee Breeding; Agropromizdat: Moscow, Russia, 1991. [Google Scholar]
- Simankov, M.K.; Kolbina, L.M. Morpho-ethological characteristics of honey bees Apis mellifera L. of Perm region. Agrar. Bull. Ural. 2021, 205, 91–100. [Google Scholar] [CrossRef]
- Simankov, M.K. Morphological characteristics of honey bees in the Perm region. Beekeeping 2020, 3, 14–16. (In Russian) [Google Scholar]
- Il’yasov, R.A.; Petukhov, A.V.; Poskryakov, A.V.; Nikolenko, A.G. Local honeybee (Apis mellifera mellifera L.) populations in the Urals. Russ. J. Genet. 2007, 43, 709–711. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Lee, M.; Takahashi, J.; Kwon, H.W.; Nikolenko, A.G. A revision of subspecies structure of western honey bee Apis mellifera. Saudi J. Biol. Sci. 2020, 27, 3615–3621. [Google Scholar] [CrossRef]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Simankov, M.K. From the experience of artificial reproduction of infertile Central Russian queens in the Perm region. Beekeeping 2025, 2, 10–13. (In Russian) [Google Scholar]
- Simankov, M.K. Artificial reproduction of virgin queens in the Perm region. Beekeeping 2005, 2, 10–13. (In Russian) [Google Scholar]
- Büchler, R.; Andonov, S.; Bernstein, R.; Bienefeld, K.; Costa, C.; Du, M.; Gabel, M.; Given, K.; Hatjina, F.; Harpur, B.A.; et al. Standard methods for rearing and selection of Apis mellifera queens 2.0. J. Apic. Res. 2024, 64, 555–611. [Google Scholar] [CrossRef]
- Krivtsov, N.I. Breed zoning and the best bees for Russia. Beekeeping 2003, 3, 10–13. (In Russian) [Google Scholar]
- Goncharov, S.M. Saving the gene pool. Beekeeping 2013, 6, 10–14. (In Russian) [Google Scholar]


| Est. | Length of Proboscis (mm) | Length of Tergite 3 (mm) | Width of Tergite 3 (mm) | Length of Forewing (mm) | Width of Forewing (mm) | Cubital Index (%) | Tarsal Index (%) | Length of Sternite 4 (mm) |
|---|---|---|---|---|---|---|---|---|
| Standard A. m. mellifera L. | ||||||||
| Min-max | 6.0–6.4 * | − | − | 9.3–9.6 * | 2.9–3.3 * | 60–65 * | 52–58 * | − |
| Cv, % | 5.9–6.4 ** | 2.4 ** | 5.0 ** | − | − | − | 54.0–55.5 * | 3.0 ** |
| Northern (Krasnovisherskaya) | ||||||||
| M ± SEM | 6.00 ± 0.007 ***** | 2.30 ± 0.002 ***** | 4.80 ± 0.005 ***** | 9.30 ± 0.008 ***** | 3.10 ± 0.004 ***** | 61.6 ± 0.34 ***** | n.a | 3.05 ± 0.003 **** |
| Cv, % | 1.7 | 3.0 | 2.6 | 1.9 | 3.6 | 10.2 | n.a | 2.6 |
| n = 240 | n = 240 | n = 240 | n = 240 | n = 240 | n = 240 | n.a | n = 590 | |
| Southern (Osinskaya, Uinskaya) | ||||||||
| M ± SEM | 6.05 ± 0.008 *** | 2.41 ± 0.004 *** | 5.00 ± 0.008 *** | 9.20 ± 0.008 ***** | 3.20 ± 0.004 ***** | 64.1 ± 0.65 *** | n.a | 2.99 ± 0.002 **** |
| Cv, % | 2.5 | 2.5 | 2.3 | 2.9 | 3.6 | 14.9 | n.a | 3.9 |
| n = 530 | n = 950 | n = 676 | n = 870 | n = 640 | n = 530 | n.a | n = 755 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frunze, O.; Petukhov, A.V.; Brandorf, A.Z.; Simankov, M.K.; Kim, H.; Kwon, H.-W. Conservation of Apis mellifera mellifera L. in the Middle Ural: A Review of Genetic Diversity, Ecological Adaptation, and Breeding Perspectives. Insects 2025, 16, 512. https://doi.org/10.3390/insects16050512
Frunze O, Petukhov AV, Brandorf AZ, Simankov MK, Kim H, Kwon H-W. Conservation of Apis mellifera mellifera L. in the Middle Ural: A Review of Genetic Diversity, Ecological Adaptation, and Breeding Perspectives. Insects. 2025; 16(5):512. https://doi.org/10.3390/insects16050512
Chicago/Turabian StyleFrunze, Olga, Alexander V. Petukhov, Anna Z. Brandorf, Mikhail K. Simankov, Hyunjee Kim, and Hyung-Wook Kwon. 2025. "Conservation of Apis mellifera mellifera L. in the Middle Ural: A Review of Genetic Diversity, Ecological Adaptation, and Breeding Perspectives" Insects 16, no. 5: 512. https://doi.org/10.3390/insects16050512
APA StyleFrunze, O., Petukhov, A. V., Brandorf, A. Z., Simankov, M. K., Kim, H., & Kwon, H.-W. (2025). Conservation of Apis mellifera mellifera L. in the Middle Ural: A Review of Genetic Diversity, Ecological Adaptation, and Breeding Perspectives. Insects, 16(5), 512. https://doi.org/10.3390/insects16050512








