An Optimized Bioassay System for the Striped Flea Beetle, Phyllotreta striolata
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungi, Plants, and Insects
2.2. Bioassay of EPF Bioactivity to SFB
2.3. Optimization of Bioassay System for SFB Larvae
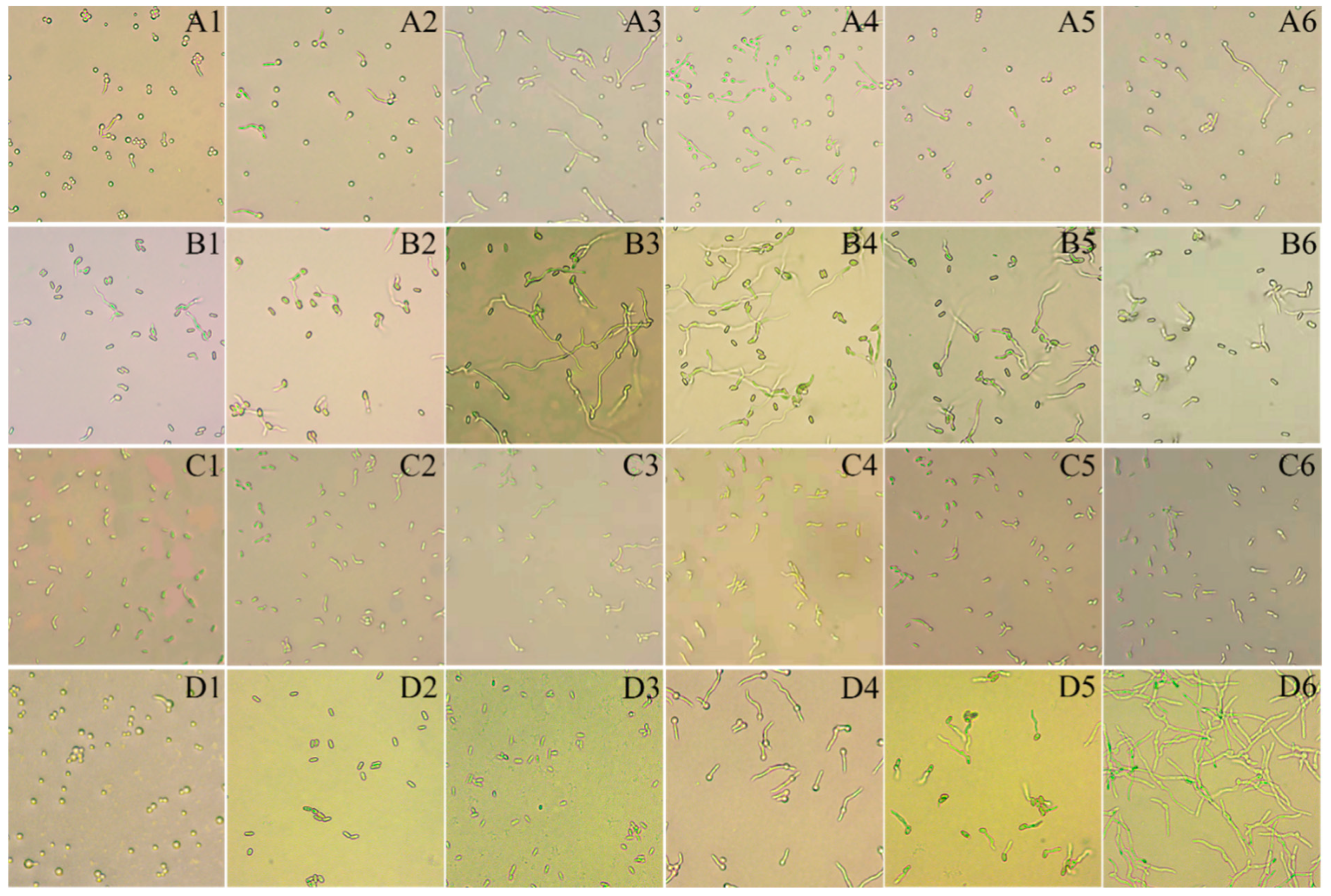
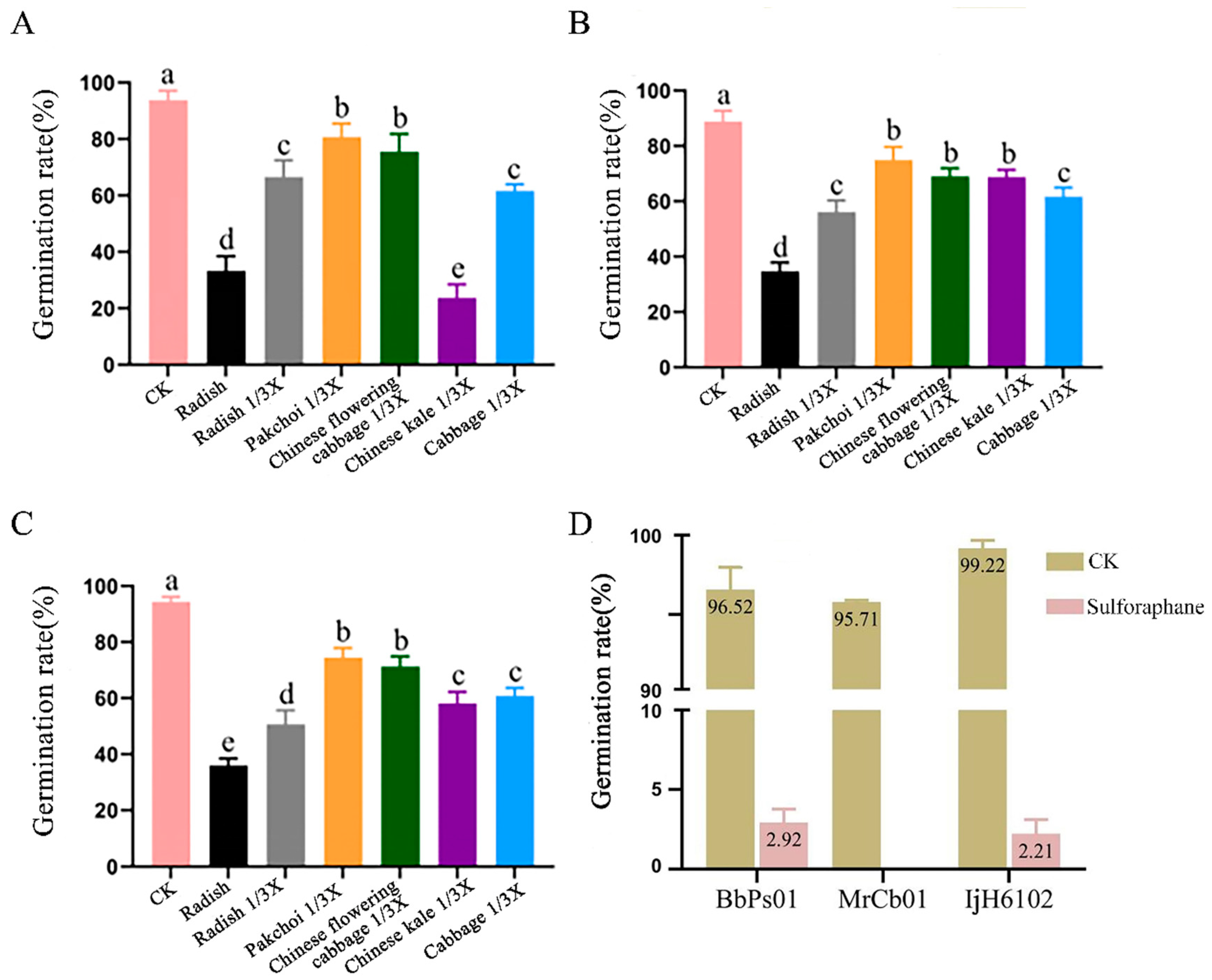
2.3.1. Effect of Plant Root Juice and Sulforaphane on EPF Conidia Germination
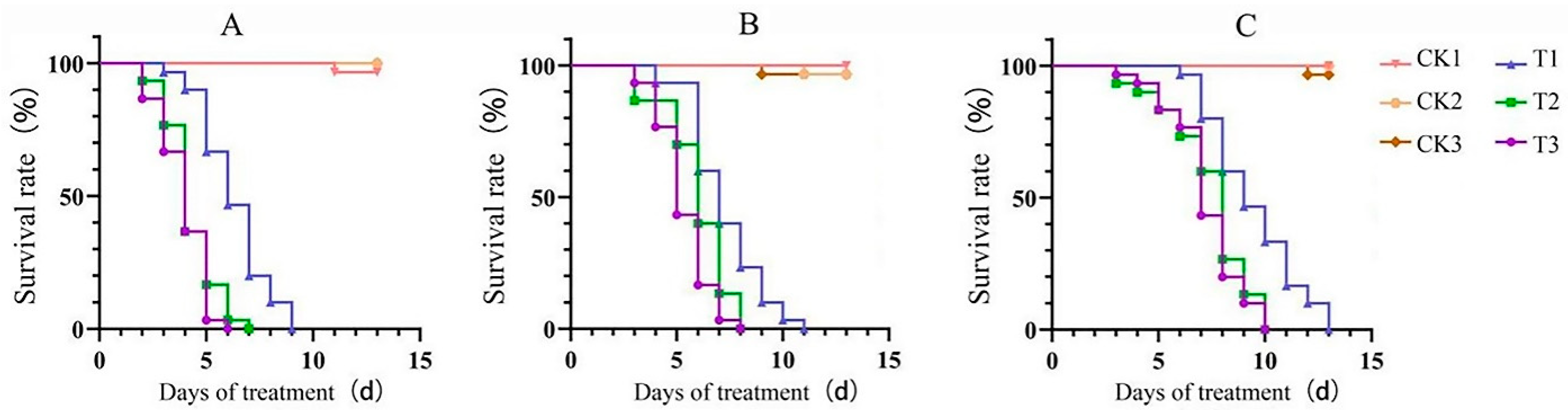
2.3.2. Bioassay of EPF on SFB Larvae Fed with Different Plant Roots
2.3.3. Data Analysis
3. Results
3.1. Bioactivity of EPF Strains to the SFB Adults and Larvae
3.2. Optimization of Bioassay System of EPF Larvae on P. Striolata
3.2.1. Effect of Root Juices and Sulforaphane on EPF Conidial Germination
3.2.2. Bioactivity of EPF on SFB Larvae Fed by Different Plant Roots
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pivnick, K.A.; Lamb, R.J.; Reed, D. Response of flea beetles, Phyllotreta spp., to mustard oils and nitriles in field trapping experiments. J. Chem. Ecol. 1992, 18, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Tansey, J.A.; Dosdall, L.M.; Keddie, B.A. Phyllotreta cruciferae and Phyllotreta striolata responses to insecticidal seed treatments with different modes of action. J. Appl. Entomol. 2009, 133, 201–209. [Google Scholar] [CrossRef]
- Guo, M.; Gao, R.; Nanda, S.; Li, Y.; Guo, C.; Zhou, X.; Zhang, Y.; Yang, C.; Pan, H. RNAi assays in the striped flea beetle (Phyllotreta striolata) suggest Psgamma-COPI and PsArf1COPI as potential molecular targets for pest control. Pestic. Biochem. Phys. 2023, 193, 105428. [Google Scholar] [CrossRef]
- Yong, X.; Yuhong, W.U.; Xiangfeng, J.; Jie, Z.; Zhenyu, L.I. Advances in integrated management of the striped flea beetle Phyllotreta striolata (Fabricius). Plant. Protection 2023, 49, 22–31. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, W.; He, R.; Pu, X.; Hu, Q.; Weng, Q. Screening of Fungal Strains and Formulations of Metarhizium anisopliae to Control Phyllotreta striolata in Chinese Flowering Cabbage. Insects 2023, 14, 567. [Google Scholar] [CrossRef] [PubMed]
- Wu, W. Feeding Habits of Phyllotreta Striolata (fabricius). Chin. J. Ecol. 2002, 21, 32–34. [Google Scholar]
- Tengfei, X.; Nanda, S.; Fengliang, J.; Qingsheng, L.; Xia, F. Control efficiency and mechanism of spinetoram seed-pelleting against the striped flea beetle Phyllotreta striolata. Sci. Rep. 2022, 12, 9524. [Google Scholar] [CrossRef]
- Reddy, G.V.; Tangtrakulwanich, K.; Miller, J.H.; Ophus, V.L.; Prewett, J. Sustainable management tactics for control of Phyllotreta cruciferae (Coleoptera: Chrysomelidae) on canola in Montana. J. Econ. Entomol. 2014, 107, 661–666. [Google Scholar] [CrossRef]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the Art and Future Opportunities. J. Agr. Food. Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, D.; Shan, L.; Chen, G.; Zhang, B. Advances in the Mechanisms of Entomopathogenic Fungi Infecting Insect Hosts and the Defense Strategies of Insects. Biodivers. Sci. 2023, 31, 23273. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Ying, S.H.; Zheng, P.; Wang, Z.L.; Zhang, S.; Xie, X.Q.; Shang, Y.; St, L.R.; Zhao, G.P.; Wang, C.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Tu, C.; Han, R.; Luo, J.; Xu, L. Enhanced capacity of a leaf beetle to combat dual stress from entomopathogens and herbicides mediated by associated microbiota. Integr. Zool. 2024, 19, 1092–1104. [Google Scholar] [CrossRef]
- Sui, L.; Lu, Y.; Xu, M.; Liu, J.; Zhao, Y.; Li, Q.; Zhang, Z. Insect hypovirulence-associated mycovirus confers entomopathogenic fungi with enhanced resistance against phytopathogens. Virulence 2024, 15, 2401978. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, P.; Ali, A.; Zang, L.S. Molecular Identification and Virulence of Four Strains of Entomopathogenic Fungi Against the Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). J. Econ. Entomol. 2022, 115, 731–738. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect Pathogens As Biological Control Agents: Back to the Future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R.; Ownley, B.H. Can We Use Entomopathogenic Fungi As Endophytes for Dual Biological Control of Insect Pests and Plant Pathogens. Biol. Control 2017, 116, 36–45. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Garrido-Jurado, I.; Yousef-Yousef, M.; González-Mas, N. Multitrophic interactions of entomopathogenic fungi in BioControl. Biocontrol 2022, 67, 457–472. [Google Scholar] [CrossRef]
- Nagalingam, T.; Costamagna, A.C. Two Methods for Rearing the Striped Flea Beetle Phyllotreta Striolata (coleoptera: Chrysomelidae) under Laboratory Conditions. Can. Entomol. 2019, 151, 677–683. [Google Scholar] [CrossRef]
- Yang, Z.; Kunert, G.; Sporer, T.; Rnig, J.K.; Beran, F. Glucosinolate Abundance and Composition in Brassicaceae Influence Sequestration in a Specialist Flea Beetle. J. Chem. Ecol. 2020, 46, 186–197. [Google Scholar] [CrossRef]
- Sharma, S.; Rani, H.; Kaur, G.; Kumar, S.; Sheikh, S.; Samota, M.K. Comprehensive Overview of Glucosinolates in Crucifers: Occurrence, Roles, Metabolism, and Transport Mechanisms—A Review. Phytochem. Rev. 2024, 1–28. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, C. Data Processing System (DPS) Software with Experimental Design, Statistical Analysis and Data Mining Developed for Use in Entomological Research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira Virgílio, M.L.; Quintela, E.D.; Maciel, L.H.R.; Goulart, G.S.S.; Silva, J.F.A.E.; de Carvalho Barros Cortes, M.V. Metarhizium Anisopliae Engineering Mediated by a CRISPR/Cas9 Recyclable System. Folia. Microbiol. 2025. [Google Scholar] [CrossRef]
- Saberi, R.R.; Hassanisaadi, M.; Vatankhah, M.; Soroush, F.; Varma, R.S. Nano/microencapsulation of plant biocontrol agents by chitosan, alginate, and other important biopolymers as a novel strategy for alleviating plant biotic stresses. Int. J. Biol. Macromol. 2022, 222, 1589–1604. [Google Scholar] [CrossRef]
- Beran, F.; Pauchet, Y.; Kunert, G.; Reichelt, M.; Wielsch, N.; Vogel, H.; Reinecke, A.; Svatoš, A.; Mewis, I.; Schmid, D.; et al. Phyllotreta striolata flea beetles use host plant defense compounds to create their own glucosinolate-myrosinase system. Proc. Natl. Acad. Sci. USA 2014, 111, 7349–7354. [Google Scholar] [CrossRef]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou-Hashem, M.; El-Domiaty, M.; Chen, S. Glucosinolate Biosynthesis and the Glucosinolate–Myrosinase System in Plant Defense. Agronomy 2020, 10, 1786. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Q. The Response of Glucosinolate-myrosinase System in Plant to Environmental Stress. World Sci. Technol. Res. Dev. 2010, 32, 15–18+51. [Google Scholar] [CrossRef]
- Abdel-Massih, R.M.; Debs, E.; Othman, L.; Attieh, J.; Cabrerizo, F.M. Glucosinolates, a Natural Chemical Arsenal: More to Tell Than the Myrosinase Story. Front. Microbiol. 2023, 14, 1130208. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, H.; Li, J. Recent research advances on glucosinolate-myrosinase defense system. Plant Physiol. Commun. 2017, 53, 2069–2077. [Google Scholar] [CrossRef]
- Li, Z.; Costamagna, A.C.; Beran, F.; You, M. Biology, Ecology, and Management of Flea Beetles in Brassica Crops. Annu. Rev. Entomol. 2024, 69, 199–217. [Google Scholar] [CrossRef]
- Li, M.; Sack, F.D. Myrosin idioblast cell fate and development are regulated by the Arabidopsis transcription factor FAMA, the auxin pathway, and vesicular trafficking. Plant Cell 2014, 26, 4053–4066. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Gomez-Jodar, I.; Moreno, D.A.; Garcia-Viguera, C.; Periago, P.M. Broccoli and Radish Sprouts Are Safe and Rich in Bioactive Phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]



| EPF Strain | LT-P Equation (y = A + Bx) and Significant Test | LT50 (95% Confidence Interval, ×106 Spores/mL) | SFB | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept (A) | Slope (B) | SE | R | χ2 | DF | p * | ||||
| BbPs01 | −6.4358 | 19.9537 | 2.5585 | 0.9795 | 0.5304 | 1 | 0.4664 | 3.74 | (3.60–3.87) | adults |
| MrCb01 | 0.7881 | 5.6747 | 0.4247 | 0.9871 | 5.9961 | 7 | 0.5402 | 5.52 | (5.20–5.83) | |
| IjH6102 | −0.0238 | 5.1670 | 0.6452 | 0.9872 | 1.566 | 5 | 0.9053 | 9.38 | (8.78–10.27) | |
| BbPs01 | −1.6211 | 8.8061 | 0.7288 | 0.9175 | 5.8315 | 5 | 0.3230 | 5.65 | (5.35–5.91) | larvae |
| MrCb01 | −3.4999 | 9.6228 | 0.9387 | 0.9641 | 9.9295 | 4 | 0.0416 | 7.64 | (7.32–7.93) | |
| IjH6102 | −3.7913 | 9.3184 | 1.7005 | 0.9795 | 1.3739 | 2 | 0.5031 | 8.78 | (8.24–9.16) | |
| Treatment | LT-P Equation (y = A + Bx) and Significant Test | LT50 (95% Confidence Interval, d) | ||||||
|---|---|---|---|---|---|---|---|---|
| Intercept (A) | Slope (B) | SE | R | χ2 | DF | p * | ||
| BbPs01 | ||||||||
| radish | −1.2612 | 8.3083 | 0.7686 | 0.9975 | 1.0016 | 4 | 0.9096 | 5.67 (5.42–5.96) |
| pakchoi | 1.1145 | 6.8293 | 0.6669 | 0.9800 | 5.2306 | 3 | 0.1557 | 3.71 (3.49–3.93) |
| Chinese flowering cabbage | 1.2657 | 7.7841 | 0.8072 | 0.9928 | 7.0994 | 3 | 0.0688 | 3.02 (2.84–3.25) |
| MrCb01 | ||||||||
| radish | −2.2915 | 8.9729 | 1.1702 | 0.9946 | 0.6289 | 3 | 0.8898 | 6.50 (5.98–6.86) |
| pakchoi | −4.9387 | 12.2585 | 1.1733 | 0.8976 | 5.8392 | 3 | 0.1197 | 6.47 (6.23–6.69) |
| Chinese flowering cabbage | −2.0057 | 10.6790 | 0.9995 | 0.9647 | 9.7197 | 4 | 0.0454 | 4.53 (4.34–4.75) |
| IjH6102 | ||||||||
| radish | −3.3354 | 8.8608 | 0.9731 | 0.9952 | 1.0100 | 4 | 0.9083 | 8.72 (8.34–9.06) |
| pakchoi | −2.0050 | 8.3098 | 0.9637 | 0.9708 | 5.4900 | 3 | 0.1392 | 6.97 (6.67–7.29) |
| Chinese flowering cabbage | −1.9607 | 8.4843 | 0.7950 | 0.9762 | 7.6721 | 4 | 0.1044 | 6.61 (6.34–6.92) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Pu, X.; Wu, Y.; Zhang, K.; Berestetskiy, A.; Hu, Q.; Weng, Q. An Optimized Bioassay System for the Striped Flea Beetle, Phyllotreta striolata. Insects 2025, 16, 510. https://doi.org/10.3390/insects16050510
Yao L, Pu X, Wu Y, Zhang K, Berestetskiy A, Hu Q, Weng Q. An Optimized Bioassay System for the Striped Flea Beetle, Phyllotreta striolata. Insects. 2025; 16(5):510. https://doi.org/10.3390/insects16050510
Chicago/Turabian StyleYao, Liyan, Xinhua Pu, Yuanlin Wu, Ke Zhang, Alexander Berestetskiy, Qiongbo Hu, and Qunfang Weng. 2025. "An Optimized Bioassay System for the Striped Flea Beetle, Phyllotreta striolata" Insects 16, no. 5: 510. https://doi.org/10.3390/insects16050510
APA StyleYao, L., Pu, X., Wu, Y., Zhang, K., Berestetskiy, A., Hu, Q., & Weng, Q. (2025). An Optimized Bioassay System for the Striped Flea Beetle, Phyllotreta striolata. Insects, 16(5), 510. https://doi.org/10.3390/insects16050510








