Insect Mating Behaviors: A Review of the Regulatory Role of Neuropeptides
Simple Summary
Abstract
1. Introduction
2. How Insects Are Adapted to Mating Behavior
2.1. Attraction of Mates
2.1.1. Chemical Communication (Pheromones)
2.1.2. Acoustic Signals (Sounds)
2.1.3. Visual Displays
2.2. Courtship Rituals
2.2.1. Serenades
2.2.2. Dances
2.2.3. Physical Touch
2.2.4. Display of Gifts
2.2.5. Aphrodisiacs
2.3. Copulation
2.4. Post-Mating Behaviors
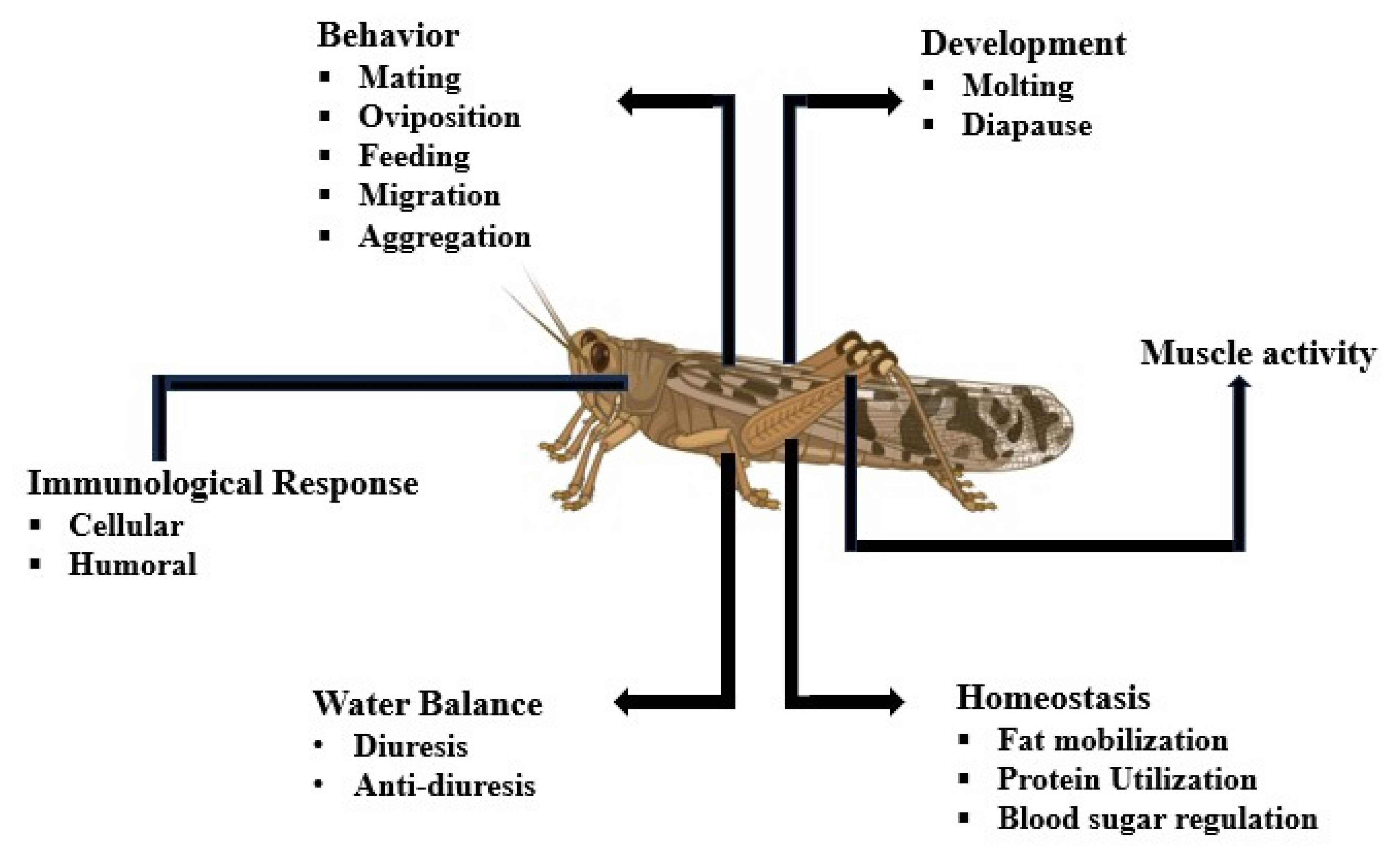
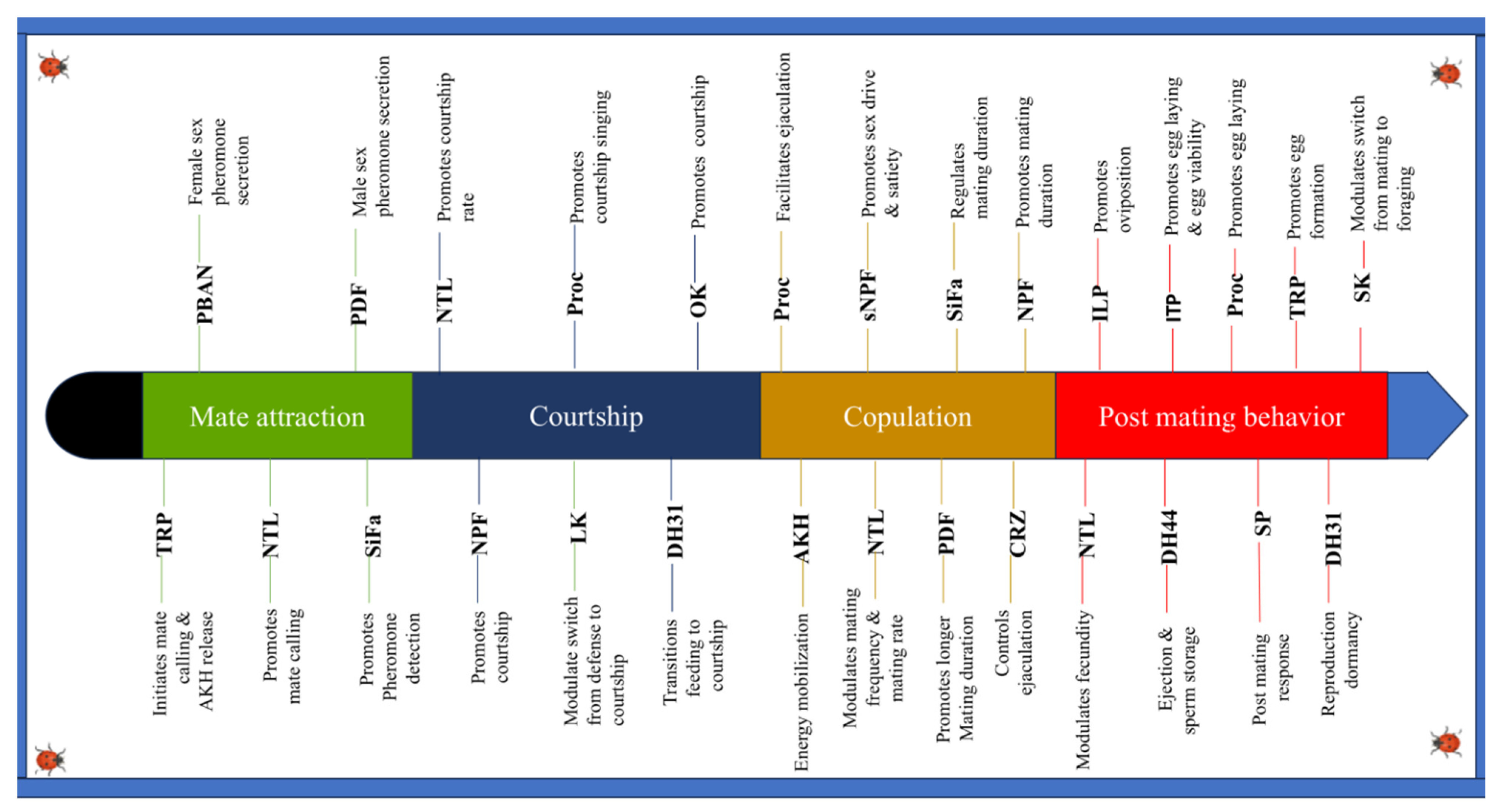
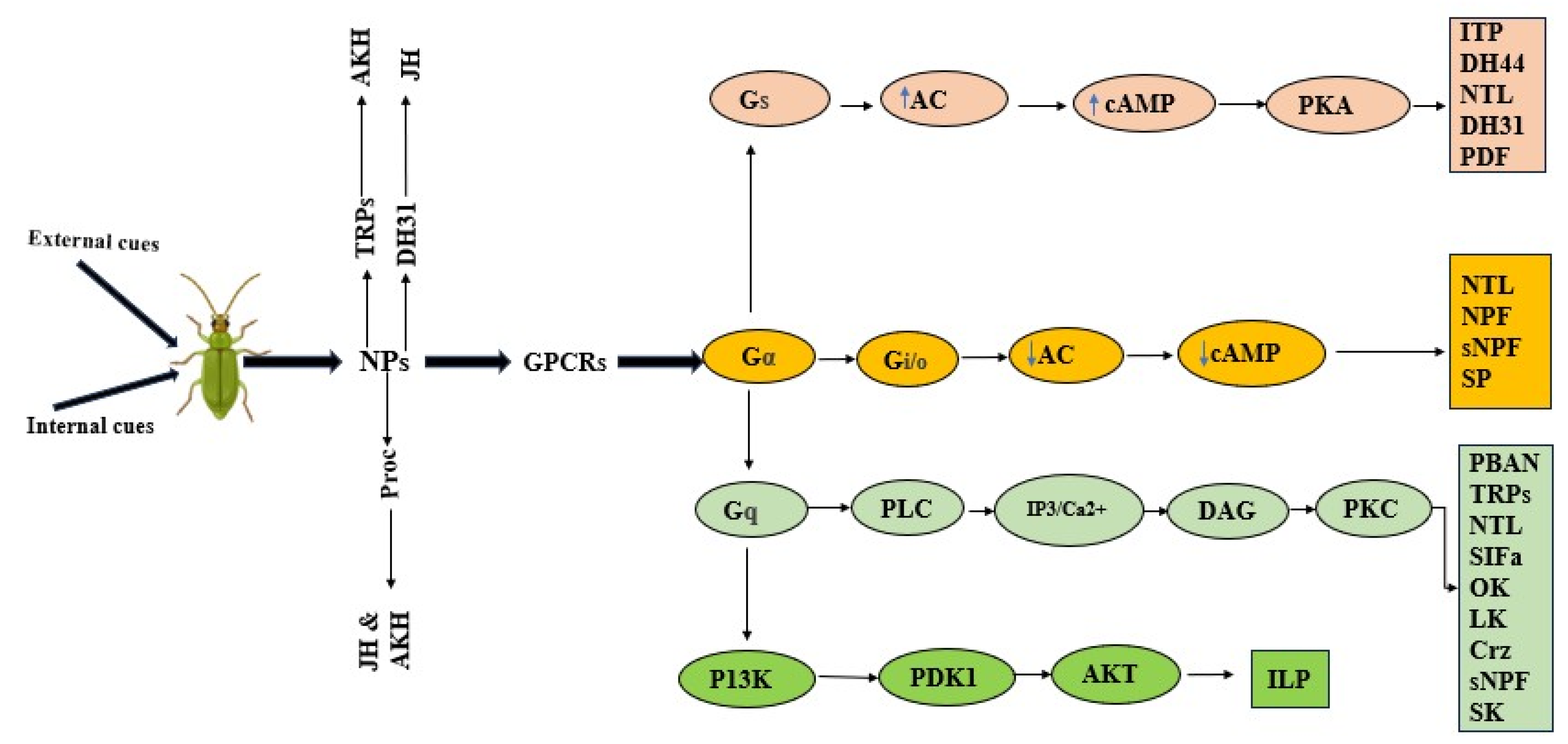
3. Role of Neuropeptides in the Mating Process
3.1. Role in Choice of Mates, Attraction, and Courtship
3.1.1. Effect on Receptivity
Pheromone Biosynthesis-Activating Neuropeptide (PBAN)
Tachykinin-Related Peptides (TRPs)
Natalisin (NTL)
3.1.2. Courtship Rituals
Neuropeptide F (NPF)
Diuretic Hormone 31 (DH 31)
Proctolin (Proc)
SIFamide (SIFa)
Orcokinin (OK)
Leucokinin (LK)
3.1.3. Mate Appeal
Pigment-Dispersing Factor (PDF)
3.2. Role in Copulation
3.2.1. Adipokinetic Hormone (AKH)
3.2.2. Corazonin (Crz)
3.2.3. Short Neuropeptide F (sNPF)
3.3. Role in Post-Mating Behaviors
3.3.1. Sex Peptide (SP)
3.3.2. Diuretic Hormones (DH44)
3.3.3. Insulin-like Peptides (ILPs)
3.3.4. Ion Transport Peptide (ITP)
3.3.5. Sulfakinin (SK)
4. Discussion of Findings
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Schoofs, L.; De Loof, A.; Van Hiel, M.B. Neuropeptides as regulators of behavior in insects. Annu. Rev. Entomol. 2017, 62, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Altstein, M.; Nässel, D.R. Neuropeptide signaling in insects. In Neuropeptide Systems as Targets for Parasite and Pest Control; Springer: Boston, MA, USA, 2010; pp. 155–165. [Google Scholar]
- Cui, H.-Y.; Zhao, Z.-W. Structure and function of neuropeptide F in insects. J. Integr. Agric. 2020, 19, 1429–1438. [Google Scholar] [CrossRef]
- Scherkenbeck, J.; Zdobinsky, T. Insect neuropeptides: Structures, chemical modifications and potential for insect control. Bioorg. Med. Chem. 2009, 17, 4071–4084. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, W.L. Chemistry of sex attraction. Proc. Natl. Acad. Sci. USA 1995, 92, 44–49. [Google Scholar] [CrossRef]
- Sandler, B.H.; Nikonova, L.; Leal, W.S.; Clardy, J. Sexual attraction in the silkworm moth: Structure of the pheromone-binding-protein–bombykol complex. Chem. Biol. 2000, 7, 143–151. [Google Scholar] [CrossRef]
- Bierl, B.A.; Beroza, M.; Collier, C. Potent sex attractant of the gypsy moth: Its isolation, identification, and synthesis. Science 1970, 170, 87–89. [Google Scholar] [CrossRef]
- Pliske, T.E. Courtship behavior of the monarch butterfly, Danaus plexippus L. Ann. Entomol. Soc. Am. 1975, 68, 143–151. [Google Scholar] [CrossRef]
- Dickens, J.C.; Oliver, J.E.; Hollister, B.; Davis, J.C.; Klun, J.A. Breaking a paradigm: Male-produced aggregation pheromone for the Colorado potato beetle. J. Exp. Biol. 2002, 205, 1925–1933. [Google Scholar] [CrossRef]
- Sivinski, J.M.; Calkins, C. Pheromones and parapheromones in the control of tephritids. Fla. Entomol. 1986, 69, 157–168. [Google Scholar] [CrossRef]
- Everaerts, C.; Farine, J.-P.; Cobb, M.; Ferveur, J.-F. Drosophila cuticular hydrocarbons revisited: Mating status alters cuticular profiles. PLoS ONE 2010, 5, e9607. [Google Scholar] [CrossRef]
- Kocher, S.D.; Richard, F.-J.; Tarpy, D.R.; Grozinger, C.M. Queen reproductive state modulates pheromone production and queen-worker interactions in honeybees. Behav. Ecol. 2009, 20, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Tokro, P.; Brossut, R.; Sreng, L. Mise en evidence de la pheromone sexuelle chez les femelles de Blattella germanica L. Int. J. Trop. Insect Sci. 1993, 14, 115–126. [Google Scholar] [CrossRef]
- Nojima, S.; Schal, C.; Webster, F.X.; Santangelo, R.G.; Roelofs, W.L. Identification of the sex pheromone of the German cockroach, Blattella germanica. Science 2005, 307, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- LeMieux, J. Tsetse Fly Volatile Pheromones Could Treat Diseases They Spread. Genetic Engineering and Biotechnology News, 21 February 2023. [Google Scholar]
- Xu, H.; Turlings, T.C. Plant volatiles as mate-finding cues for insects. Trends Plant Sci. 2018, 23, 100–111. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Tittiger, C.; MacLean, M.; Keeling, C.I. Cytochromes P450: Terpene detoxification and pheromone production in bark beetles. Curr. Opin. Insect Sci. 2021, 43, 97–102. [Google Scholar] [CrossRef]
- Pitman, G.; Vité, J. Aggregation behavior of Dendroctonus ponderosae (coleoptera: Scolytidae) in response to chemical messengers1. Can. Entomol. 1969, 101, 143–149. [Google Scholar] [CrossRef]
- Montealegre-Z, F.; Jonsson, T.; Robert, D. Sound radiation and wing mechanics in stridulating field crickets (Orthoptera: Gryllidae). J. Exp. Biol. 2011, 214, 2105–2117. [Google Scholar] [CrossRef]
- Nahirney, P.C.; Forbes, J.G.; Douglas Morris, H.; Chock, S.C.; Wang, K. What the buzz was all about: Superfast song muscles rattle the tymbals of male periodical cicadas. FASEB J. 2006, 20, 2017–2026. [Google Scholar] [CrossRef]
- Oberdörster, U.; Grant, P.R. Acoustic adaptations of periodical cicadas (Hemiptera: Magicicada). Biol. J. Linn. Soc. 2007, 90, 15–24. [Google Scholar] [CrossRef]
- Wei, J.-Q.; Wang, X.-Y.; Zheng, X.-L.; Tong, X. Stridulatory Organs and Sound Recognition of Three Species of Longhorn Beetles (Coleoptera: Cerambycidae). Insects 2024, 15, 849. [Google Scholar] [CrossRef]
- Smithsonian Institution. Mating in Insects; Smithsonian Institution: Washington, DC, USA, 1996. [Google Scholar]
- Clemens, J.; Coen, P.; Roemschied, F.A.; Pereira, T.D.; Mazumder, D.; Aldarondo, D.E.; Pacheco, D.A.; Murthy, M. Discovery of a new song mode in Drosophila reveals hidden structure in the sensory and neural drivers of behavior. Curr. Biol. 2018, 28, 2400–2412.e6. [Google Scholar] [CrossRef] [PubMed]
- Fernández, Y.; Dowdy, N.J.; Conner, W.E. Extreme duty cycles in the acoustic signals of tiger moths: Sexual and natural selection operating in parallel. Integr. Org. Biol. 2020, 2, obaa046. [Google Scholar] [CrossRef] [PubMed]
- Larsen, O.N.; Gleffe, G.; Tengö, J. Vibration and sound communication in solitary bees and wasps. Physiol. Entomol. 1986, 11, 287–296. [Google Scholar] [CrossRef]
- Clark, D.C.; Moore, A.J. Social communication in the Madagascar hissing cockroach: Features of male courtship hisses and a comparison of courtship and agonistic hisses. Behaviour 1995, 132, 401–417. [Google Scholar] [CrossRef]
- National Geographic Society. Inside the Wild World of Bug Courtship and Mating Rituals; National Geographic: Washington, DC, USA, 2025. [Google Scholar]
- Horváth, G.; Malik, P.; Kriska, G.; Wildermuth, H. Ecological traps for dragonflies in a cemetery: The attraction of Sympetrum species (Odonata: Libellulidae) by horizontally polarizing black gravestones. Freshw. Biol. 2007, 52, 1700–1709. [Google Scholar] [CrossRef]
- Metters, D. Common Rhinoceros Beetle—Land for Wildlife. 2017. Available online: https://www.lfwseq.org.au/author/deborah-metters/page/22/ (accessed on 24 March 2025).
- Perez, B. Calling behaviour in the female praying mantis, Hierodula patellifera. Physiol. Entomol. 2005, 30, 42–47. [Google Scholar] [CrossRef]
- Pervez, A. Courtship. In Reproductive Strategies in Insects; CRC Press: Boca Raton, FL, USA, 2022; pp. 119–142. [Google Scholar]
- Baker, C.A.; Clemens, J.; Murthy, M. Acoustic pattern recognition and courtship songs: Insights from insects. Annu. Rev. Neurosci. 2019, 42, 129–147. [Google Scholar] [CrossRef]
- O’woma, O.O.; Chigozirim, U.P.; Emmanuel, O.; Chukwuebuka, E.M. Reproductive and survival strategies utilized by insect. A review. Am. J. Zool. Res. 2016, 4, 1–6. [Google Scholar]
- Virant-Doberlet, M.; Cokl, A. Vibrational communication in insects. Neotrop. Entomol. 2004, 33, 121–134. [Google Scholar] [CrossRef]
- Xu, J.; Liu, W.; Yang, D.; Chen, S.; Chen, K.; Liu, Z.; Yang, X.; Meng, J.; Zhu, G.; Dong, S.; et al. Regulation of olfactory-based sex behaviors in the silkworm by genes in the sex-determination cascade. PLoS Genet. 2020, 16, e1008622. [Google Scholar] [CrossRef]
- Rebar, D.; Bailey, N.W.; Zuk, M. Courtship song’s role during female mate choice in the field cricket Teleogryllus oceanicus. Behav. Ecol. 2009, 20, 1307–1314. [Google Scholar] [CrossRef]
- Gray, D.A. Female house crickets, Acheta domesticus, prefer the chirps of large males. Anim. Behav. 1997, 54, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Cooley, J.R.; Marshall, D.C. Sexual signaling in periodical cicadas, Magicicada spp. (Hemiptera: Cicadidae). Behaviour 2001, 138, 827–855. [Google Scholar] [CrossRef]
- Ávila, G.R.; Deneubourg, J.-L.; Guisset, J.-L.; Wessel, N.; Kurths, J. Firefly courtship as the basis of the synchronization-response principle. Europhys. Lett. 2011, 94, 60007. [Google Scholar]
- Wise, T. Blue Dasher—Life on CSG Pond. 2021. Available online: https://www.lifeoncsgpond.com/blue-dasher (accessed on 24 March 2025).
- Dragonflywoman. Pachydiplax longipennis—The Dragonfly Woman. 2011. Available online: https://thedragonflywoman.com/tag/pachydiplax-longipennis/ (accessed on 24 March 2025).
- Heidinger, I.M.M.; Meixner, M.D.; Berg, S.; Büchler, R. Observation of the mating behavior of honey bee (Apis mellifera L.) queens using radio-frequency identification (RFID): Factors influencing the duration and frequency of nuptial flights. Insects 2014, 5, 513–527. [Google Scholar] [CrossRef]
- Uzsák, A.; Dieffenderfer, J.; Bozkurt, A.; Schal, C. Social facilitation of insect reproduction with motor-driven tactile stimuli. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140325. [Google Scholar] [CrossRef]
- Kruse, K.C.; Switzer, P.V. Physical contests for females in the Japanese beetle, Popillia japonica. J. Insect Sci. 2007, 7, 34. [Google Scholar] [CrossRef]
- Barrows, E.M.; Gordh, G. Sexual behavior in the Japanese beetle, Popillia japonica, and comparative notes on sexual behavior of other scarabs (Coleoptera: Scarabaeidae). Behav. Biol. 1978, 23, 341–354. [Google Scholar] [CrossRef]
- Wada-Katsumata, A.; Schal, C. Antennal grooming facilitates courtship performance in a group-living insect, the German cockroach Blattella germanica. Sci. Rep. 2019, 9, 2942. [Google Scholar] [CrossRef]
- Feng, H.Y.; Zhao, Y.Q.; Yang, T.; Zhou, Y.Y.; Gong, L.L.; Zhang, M.Q.; Ma, Y.F.; Hull, J.J.; Dewer, Y.; Zhang, F.; et al. Female contact sex pheromone recognition in the German cockroach (Blattella germanica) is mediated by two male antennae-enriched sensory neuron membrane proteins. Pest Manag. Sci. 2025, 81, 572–584. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, M.Q.; Ma, Y.F.; Feng, H.Y.; Zhao, Y.Q.; Zhou, Y.y.; He, M.; Smagghe, G.; He, P. RNAi of yellow-y, required for normal cuticle pigmentation, impairs courtship behavior and oviposition in the German cockroach (Blattella germanica). Arch. Insect Biochem. Physiol. 2024, 115, e22114. [Google Scholar] [CrossRef] [PubMed]
- Vahed, K. The function of nuptial feeding in insects: A review of empirical studies. Biol. Rev. 1998, 73, 43–78. [Google Scholar] [CrossRef]
- Vahed, K. The Evolution and Function of the Spermatophylax in Bushcrickets (Orthoptera: Tettigoniidae). Ph.D. Thesis, University of Nottingham, Nottingham, UK, 1994. [Google Scholar]
- Cumming, J.M. Sexual selection and the evolution of dance fly mating systems (Diptera: Empididae; Empidinae). Can. Entomol. 1994, 126, 907–920. [Google Scholar] [CrossRef]
- LeBas, N.R.; Hockham, L.R.; Ritchie, M.G. Sexual selection in the gift-giving dance fly, Rhamphomyia sulcata, favors small males carrying small gifts. Evolution 2004, 58, 1763–1772. [Google Scholar]
- Iftikhar, H.; Johnson, N.L.; Marlatt, M.L.; Carney, G.E. The role of miRNAs in Drosophila melanogaster male courtship behavior. Genetics 2019, 211, 925–942. [Google Scholar] [CrossRef]
- Gapon, D. An instance of intergeneric copulation in the family Rhopalidae (Heteroptera): Structure, functioning and congruence of the genitalia in two different species from the standpoint of the lock-and-key hypothesis. Zoosyst. Ross. 2019, 28, 3–18. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kovalev, A.E.; Gorb, S.N. Penetration mechanics of a beetle intromittent organ with bending stiffness gradient and a soft tip. Sci. Adv. 2017, 3, eaao5469. [Google Scholar] [CrossRef]
- Blomqvist, D.; Hoi, H.; Weinberger, I. S13-1 To see or not to see: The role of habitat density on the occurrence of extra-pair paternity and paternity assurance behaviors. Acta Zool. Sin. 2006, 52, 229–231. [Google Scholar]
- Benoit, J.B.; Jajack, A.J.; Yoder, J.A. Multiple traumatic insemination events reduce the ability of bed bug females to maintain water balance. J. Comp. Physiol. B 2012, 182, 189–198. [Google Scholar] [CrossRef]
- Wu, W.L.; Peng WenJun, P.W.; Hu FuLiang, H.F. Sperm storage of queen honeybee. Chin. Bull. Entomol. 2008, 45, 323–327. [Google Scholar]
- Mongue, A.J.; Hansen, M.E.; Gu, L.; Sorenson, C.E.; Walters, J.R. Nonfertilizing sperm in Lepidoptera show little evidence for recurrent positive selection. Mol. Ecol. 2019, 28, 2517–2530. [Google Scholar] [CrossRef] [PubMed]
- Buskirk, R.E.; Sherman, K.J. The influence of larval ecology on oviposition and mating strategies in dragonflies. Fla. Entomol. 1985, 68, 39–51. [Google Scholar] [CrossRef]
- McDonough-Goldstein, C.E.; Pitnick, S.; Dorus, S. Drosophila female reproductive glands contribute to mating plug composition and the timing of sperm ejection. Proc. R. Soc. B 2022, 289, 20212213. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Rivera, A. Sperm removal during copulation confirmed in the oldest extant damselfly, Hemiphlebia mirabilis. PeerJ 2016, 4, e2077. [Google Scholar] [CrossRef]
- Denis, B.; Claisse, G.; Le Rouzic, A.; Wicker-Thomas, C.; Lepennetier, G.; Joly, D. Male accessory gland proteins affect differentially female sexual receptivity and remating in closely related Drosophila species. J. Insect Physiol. 2017, 99, 67–77. [Google Scholar] [CrossRef]
- Wedell, N. Female receptivity in butterflies and moths. J. Exp. Biol. 2005, 208, 3433–3440. [Google Scholar] [CrossRef]
- Birkhead, T.; Lee, K.; Young, P. Sexual cannibalism in the praying mantis Hierodula membranacea. Behaviour 1988, 106, 112–118. [Google Scholar] [CrossRef]
- Burke, N.W. Sexual cannibalism as a female resistance trait: A new hypothesis. Evolution 2024, 78, 612–623. [Google Scholar] [CrossRef]
- Su, J.; Zhao, J.; Li, Y.; Lu, Y. Breeding sites, migration paths and phylogenetic relationships of mosquitoes in seven cities in northern and southern China. Trop. Biomed. 2024, 41, 471–480. [Google Scholar]
- Hanin, O.; Azrielli, A.; Applebaum, S.W.; Rafaeli, A. Functional impact of silencing the Helicoverpa armigera sex-peptide receptor on female reproductive behaviour. Insect Mol Biol. 2012, 21, 161–167. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Guan, R.; Du, M.; Yin, X.; Zhao, W.; An, S. Trehalase is required for sex pheromone biosynthesis in Helicoverpa armigera. Insect Mol Biol. 2022, 31, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Cha, W.H.; Kim, B.; Lee, D.-W. Functional Analysis of Pheromone Biosynthesis Activating Neuropeptide Receptor Isoforms in Maruca vitrata. Cells 2023, 12, 1410. [Google Scholar] [CrossRef] [PubMed]
- Rafaeli, A. Pheromone biosynthesis activating neuropeptide (PBAN): Regulatory role and mode of action. Gen. Comp. Endocrinol. 2009, 162, 69–78. [Google Scholar] [CrossRef]
- Shiomi, K. Pheromone biosynthesis activating neuropeptide. In Handbook of Hormones; Elsevier: Amsterdam, The Netherlands, 2021; pp. 725–727. [Google Scholar]
- Matsumoto, S.; Ohnishi, A.; Lee, J.M.; Hull, J.J. Unraveling the pheromone biosynthesis activating neuropeptide (PBAN) signal transduction cascade that regulates sex pheromone production in moths. Vitam. Horm. 2010, 83, 425–445. [Google Scholar]
- Bober, R.; Azrielli, A.; Rafaeli, A. Developmental regulation of the pheromone biosynthesis activating neuropeptide-receptor (PBAN-R): Re-evaluating the role of juvenile hormone. Insect Mol. Biol. 2010, 19, 77–86. [Google Scholar] [CrossRef]
- Jurenka, R.; Nusawardani, T. The pyrokinin/pheromone biosynthesis-activating neuropeptide (PBAN) family of peptides and their receptors in Insecta: Evolutionary trace indicates potential receptor ligand-binding domains. Insect Mol. Biol. 2011, 20, 323–334. [Google Scholar] [CrossRef]
- Lee, D.-W.; Shrestha, S.; Kim, A.Y.; Park, S.J.; Yang, C.Y.; Kim, Y.; Koh, Y.H. RNA interference of pheromone biosynthesis-activating neuropeptide receptor suppresses mating behavior by inhibiting sex pheromone production in Plutella xylostella (L.). Insect Biochem. Mol. Biol. 2011, 41, 236–243. [Google Scholar] [CrossRef]
- Ashok, K.; Bhargava, C.N.; Asokan, R.; Pradeep, C.; Pradhan, S.K.; Kennedy, J.S.; Balasubramani, V.; Murugan, M.; Jayakanthan, M.; Geethalakshmi, V.; et al. CRISPR/Cas9 mediated editing of pheromone biosynthesis activating neuropeptide (PBAN) gene disrupts mating in the Fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). 3 Biotech 2023, 13, 370. [Google Scholar] [CrossRef]
- Nässel, D.R. Tachykinin-related peptides in invertebrates: A review. Peptides 1999, 20, 141–158. [Google Scholar] [CrossRef]
- Van Loy, T.; Vandersmissen, H.P.; Poels, J.; Van Hiel, M.B.; Verlinden, H.; Vanden Broeck, J. Tachykinin-related peptides and their receptors in invertebrates: A current view. Peptides 2010, 31, 520–524. [Google Scholar] [CrossRef]
- Winther, Å.M.; Nässel, D.R. Intestinal peptides as circulating hormones: Release of tachykinin-related peptide from the locust and cockroach midgut. J. Exp. Biol. 2001, 204, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, A.; Adamo, S.A. Stayin’alive: Endocrinological stress responses in insects. In Advances in Invertebrate (Neuro) Endocrinology; Apple Academic Press: Palm Bay, FL, USA, 2020; pp. 283–323. [Google Scholar]
- Pascual, N.; Maestro, J.L.; Chiva, C.; Andreu, D.; Bellés, X. Identification of a tachykinin-related peptide with orexigenic properties in the German cockroach. Peptides 2008, 29, 386–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shankar, S.; Chua, J.Y.; Tan, K.J.; Calvert, M.E.; Weng, R.; Ng, W.C.; Mori, K.; Yew, J.Y. The neuropeptide tachykinin is essential for pheromone detection in a gustatory neural circuit. eLife 2015, 4, e06914. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, J.G.; Pandit, A.A.; Zandawala, M.; Nässel, D.R.; Davies, S.-A.; Dow, J.A. DINeR: Database for insect neuropeptide research. Insect Biochem. Mol. Biol. 2017, 86, 9–19. [Google Scholar] [CrossRef]
- Gui, S.H.; Jiang, H.B.; Liu, X.Q.; Xu, L.; Wang, J.J. Molecular characterizations of natalisin and its roles in modulating mating in the oriental fruit fly, Bactrocera dorsalis (Hendel). Insect Mol. Biol. 2017, 26, 103–112. [Google Scholar] [CrossRef]
- Jiang, H.; Lkhagva, A.; Daubnerová, I.; Chae, H.-S.; Šimo, L.; Jung, S.-H.; Yoon, Y.-K.; Lee, N.-R.; Seong, J.Y.; Žitňan, D.; et al. Natalisin, a tachykinin-like signaling system, regulates sexual activity and fecundity in insects. Proc. Natl. Acad. Sci. USA 2013, 110, E3526–E3534. [Google Scholar] [CrossRef]
- Lee, G.; Bahn, J.H.; Park, J.H. Sex-and clock-controlled expression of the neuropeptide F gene in Drosophila. Proc. Natl. Acad. Sci. USA 2006, 103, 12580–12585. [Google Scholar] [CrossRef]
- Huang, Y.C.; Shi, M.; Chen, X.X. Advances in research on the insect neuropeptide F. Chin. J. Appl. Entomol. 2015, 52, 1315–1325. [Google Scholar]
- Liu, W.; Ganguly, A.; Huang, J.; Wang, Y.; Ni, J.D.; Gurav, A.S.; Aguilar, M.A.; Montell, C. Neuropeptide F regulates courtship in Drosophila through a male-specific neuronal circuit. eLife 2019, 8, e49574. [Google Scholar] [CrossRef]
- Van Wielendaele, P.; Wynant, N.; Dillen, S.; Zels, S.; Badisco, L.; Broeck, J.V. Neuropeptide F regulates male reproductive processes in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2013, 43, 252–259. [Google Scholar] [CrossRef]
- Kim, W.J.; Jan, L.Y.; Jan, Y.N. A PDF/NPF neuropeptide signaling circuitry of male Drosophila melanogaster controls rival-induced prolonged mating. Neuron 2013, 80, 1190–1205. [Google Scholar] [CrossRef] [PubMed]
- Rohwedder, A.; Selcho, M.; Chassot, B.; Thum, A.S. Neuropeptide F neurons modulate sugar reward during associative olfactory learning of Drosophila larvae. J. Comp. Neurol. 2015, 523, 2637–2664. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Jang, H.; Oh, Y. The role of diuretic hormones (DHs) and their receptors in Drosophila. BMB Rep. 2023, 56, 209. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Kuang, M.C.; Hossain, I.; Xuan, Y.; Beebe, L.; Shepherd, A.K.; Rolandi, M.; Wang, J.W. A nutrient-specific gut hormone arbitrates between courtship and feeding. Nature 2022, 602, 632–638. [Google Scholar] [CrossRef]
- Kurogi, Y.; Imura, E.; Mizuno, Y.; Hoshino, R.; Nouzova, M.; Matsuyama, S.; Mizoguchi, A.; Kondo, S.; Tanimoto, H.; Noriega, F.G.; et al. Female reproductive dormancy in Drosophila is regulated by DH31-producing neurons projecting into the corpus allatum. Development 2023, 150, dev201186. [Google Scholar] [CrossRef]
- Zhou, Y.; Nagata, S. Proctolin. In Handbook of Hormones; Elsevier: Amsterdam, The Netherlands, 2021; pp. 789–790. [Google Scholar]
- Vezenkov, S.R.; Danalev, D.L. From molecule to sexual behavior: The role of the neuropentapeptide proctolin in acoustic communication in the male grasshopper Chorthippus biguttulus. Eur. J. Pharmacol. 2009, 619, 57–60. [Google Scholar] [CrossRef]
- Tanaka, Y. Proctolin. In Handbook of Hormones; Elsevier: Amsterdam, The Netherlands, 2016; pp. 438–439. [Google Scholar]
- Chiang, R.G.; Martens, J.D.; O’donnell, M.J. The vagina muscles of the blood-sucking insect Rhodnius prolixus as a model for exploring the physiological effects of proctolin. Physiol. Entomol. 2010, 35, 154–159. [Google Scholar] [CrossRef]
- Taylor, C.A.; Winther, Å.M.; Siviter, R.J.; Shirras, A.D.; Isaac, R.E.; Nässel, D.R. Identification of a proctolin preprohormone gene (Proct) of Drosophila melanogaster: Expression and predicted prohormone processing. J. Neurobiol. 2004, 58, 379–391. [Google Scholar] [CrossRef]
- Ormerod, K.G.; LePine, O.K.; Bhutta, M.S.; Jung, J.; Tattersall, G.J.; Mercier, A.J. Characterizing the physiological and behavioral roles of proctolin in Drosophila melanogaster. J. Neurophysiol. 2016, 115, 568–580. [Google Scholar] [CrossRef]
- Eaton, J.L. Morphology of abdominal segments eight and nine of the female tobacco hornworm, Manduca sexta (Lepidoptera: Sphingidae). Ann. Entomol. Soc. Am. 1986, 79, 629–635. [Google Scholar] [CrossRef]
- Belanger, J.H.; Orchard, I. The locust ovipositor opener muscle: Proctolinergic central and peripheral neuromodulation in a centrally driven motor system. J. Exp. Biol. 1993, 174, 343–362. [Google Scholar] [CrossRef]
- Nässel, D.R.; O’shea, M. Proctolin-like immunoreactive neurons in the blowfly central nervous system. J. Comp. Neurol. 1987, 265, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Orchard, I.; Lee, D.H.; Da Silva, R.; Lange, A.B. The proctolin gene and biological effects of proctolin in the blood-feeding bug, Rhodnius prolixus. Front. Endocrinol. 2011, 2, 59. [Google Scholar] [CrossRef] [PubMed]
- Orchard, I.; Belanger, J.H.; Lange, A.B. Proctolin: A review with emphasis on insects. J. Neurobiol. 1989, 20, 470–496. [Google Scholar] [CrossRef]
- Lange, A.B.; Kisana, A.; Leyria, J.; Orchard, I. The Male Reproductive System of the Kissing Bug, Rhodnius prolixus Stål, 1859 (Hemiptera: Reduviidae: Triatominae): Arrangements of the Muscles and the Myoactivity of the Selected Neuropeptides. Insects 2023, 14, 324. [Google Scholar] [CrossRef]
- Verleyen, P.; Huybrechts, J.; Schoofs, L. SIFamide illustrates the rapid evolution in Arthropod neuropeptide research. Gen. Comp. Endocrinol. 2009, 162, 27–35. [Google Scholar] [CrossRef]
- Lismont, E.; Mortelmans, N.; Verlinden, H.; Vanden Broeck, J. Molecular cloning and characterization of the SIFamide precursor and receptor in a hymenopteran insect, Bombus terrestris. Gen. Comp. Endocrinol. 2018, 258, 39–52. [Google Scholar] [CrossRef]
- Ayub, M.; Hermiz, M.; Lange, A.B.; Orchard, I. SIFamide influences feeding in the Chagas disease vector, Rhodnius prolixus. Front. Neurosci. 2020, 14, 134. [Google Scholar] [CrossRef]
- Arendt, A.; Neupert, S.; Schendzielorz, J.; Predel, R.; Stengl, M. The neuropeptide SIFamide in the brain of three cockroach species. J. Comp. Neurol. 2016, 524, 1337–1360. [Google Scholar] [CrossRef]
- Sellami, A.; Veenstra, J.A. SIFamide acts on fruitless neurons to modulate sexual behavior in Drosophila melanogaster. Peptides 2015, 74, 50–56. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, T.; Zhang, X.; Ryu, T.H.; Wong, K.; Wu, Z.; Wei, Y.; Schweizer, J.; Nguyen, K.-N.H.; Kwan, A.; et al. Peptidergic neurons with extensive branching orchestrate the internal states and energy balance of male Drosophila melanogaster. bioRxiv 2024. bioRxiv:2024.2006.2004.597277. [Google Scholar]
- Wong, K.; Schweizer, J.; Nguyen, K.-N.H.; Atieh, S.; Kim, W.J. Neuropeptide relay between SIFa signaling controls the experience-dependent mating duration of male Drosophila. bioRxiv 2019. bioRxiv:819045. [Google Scholar]
- Tanaka, Y. Orcokinins. In Handbook of Hormones; Elsevier: Amsterdam, The Netherlands, 2021; pp. 791–793. [Google Scholar]
- Silva, V.; Palacios-Muñoz, A.; Volonté, M.; Frenkel, L.; Ewer, J.; Ons, S. Orcokinin neuropeptides regulate reproduction in the fruit fly, Drosophila melanogaster. Insect Biochem. Mol. Biol. 2021, 139, 103676. [Google Scholar] [CrossRef] [PubMed]
- Ons, S.; Bellés, X.; Maestro, J.L. Orcokinins contribute to the regulation of vitellogenin transcription in the cockroach Blattella germanica. J. Insect Physiol. 2015, 82, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R.; Wu, S.-F. Leucokinins: Multifunctional neuropeptides and hormones in insects and other invertebrates. Int. J. Mol. Sci. 2021, 22, 1531. [Google Scholar] [CrossRef]
- Zandawala, M.; Yurgel, M.E.; Texada, M.J.; Liao, S.; Rewitz, K.F.; Keene, A.C.; Nässel, D.R. Modulation of Drosophila post-feeding physiology and behavior by the neuropeptide leucokinin. PLoS Genet. 2018, 14, e1007767. [Google Scholar] [CrossRef]
- Nässel, D.R. Leucokinin and associated neuropeptides regulate multiple aspects of physiology and behavior in Drosophila. Int. J. Mol. Sci. 2021, 22, 1940. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, B.; Zhang, L.; Yang, T.; Zhang, Z.; Gao, Z.; Zhang, W. A neural circuit encoding mating states tunes defensive behavior in Drosophila. Nat. Commun. 2020, 11, 3962. [Google Scholar] [CrossRef]
- Nässel, D.R. A brief history of insect neuropeptide and peptide hormone research. Cell Tissue Res. 2024, 399, 129–159. [Google Scholar] [CrossRef]
- Shafer, O.T.; Yao, Z. Pigment-dispersing factor signaling and circadian rhythms in insect locomotor activity. Curr. Opin. Insect Sci. 2014, 1, 73–80. [Google Scholar] [CrossRef]
- Nagata, S. Pigment dispersing hormone. In Handbook of Hormones; Elsevier: Amsterdam, The Netherlands, 2021; pp. 861–862. [Google Scholar]
- Wei, H.; Yasar, H.; Funk, N.W.; Giese, M.; Baz, E.-S.; Stengl, M. Signaling of pigment-dispersing factor (PDF) in the Madeira cockroach Rhyparobia maderae. PLoS ONE 2014, 9, e108757. [Google Scholar] [CrossRef] [PubMed]
- Krupp, J.J.; Billeter, J.-C.; Wong, A.; Choi, C.; Nitabach, M.N.; Levine, J.D. Pigment-dispersing factor modulates pheromone production in clock cells that influence mating in Drosophila. Neuron 2013, 79, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Iga, M.; Nakaoka, T.; Suzuki, Y.; Kataoka, H. Pigment dispersing factor regulates ecdysone biosynthesis via Bombyx neuropeptide G protein coupled receptor-B2 in the prothoracic glands of Bombyx mori. PLoS ONE 2014, 9, e103239. [Google Scholar] [CrossRef]
- Lee, G.; Park, J.H. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 2004, 167, 311–323. [Google Scholar] [CrossRef]
- Wicher, D.; Agricola, H.-J.; Söhler, S.; Gundel, M.; Heinemann, S.H.; Wollweber, L.; Stengl, M.; Derst, C. Differential receptor activation by cockroach adipokinetic hormones produces differential effects on ion currents, neuronal activity, and locomotion. J. Neurophysiol. 2006, 95, 2314–2325. [Google Scholar] [CrossRef]
- Marco, H.G.; Gäde, G. Adipokinetic hormone: A hormone for all seasons? In Advances in Invertebrate (NEURO) Endocrinology; Apple Academic Press: Palm Bay, FL, USA, 2020; pp. 129–175. [Google Scholar]
- Khan, Z.; Tondravi, M.; Oliver, R.; Vonhoff, F.J. Drosophila corazonin neurons as a hub for regulating growth, stress responses, ethanol-related behaviors, copulation persistence and sexually dimorphic reward pathways. J. Dev. Biol. 2021, 9, 26. [Google Scholar] [CrossRef]
- Tayler, T.D.; Pacheco, D.A.; Hergarden, A.C.; Murthy, M.; Anderson, D.J. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 20697–20702. [Google Scholar] [CrossRef]
- Yang, J.; Huang, H.; Yang, H.; He, X.; Jiang, X.; Shi, Y.; Alatangaole, D.; Shi, L.; Zhou, N. Specific activation of the G protein-coupled receptor BNGR-A21 by the neuropeptide corazonin from the silkworm, Bombyx mori, dually couples to the Gq and Gs signaling cascades. J. Biol. Chem. 2013, 288, 11662–11675. [Google Scholar] [CrossRef]
- Cholewiński, M.; Chowański, S.; Lubawy, J.; Urbański, A.; Walkowiak-Nowicka, K.; Marciniak, P. Short neuropeptide F in integrated insect physiology. J. Zhejiang Univ. Sci. B 2024, 25, 389–409. [Google Scholar] [CrossRef]
- Chen, W.; Shi, W.; Li, L.; Zheng, Z.; Li, T.; Bai, W.; Zhao, Z. Regulation of sleep by the short neuropeptide F (sNPF) in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2013, 43, 809–819. [Google Scholar] [CrossRef]
- Nässel, D.R.; Wegener, C. A comparative review of short and long neuropeptide F signaling in invertebrates: Any similarities to vertebrate neuropeptide Y signaling? Peptides 2011, 32, 1335–1355. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Lee, S.G.; Auge, A.-C.; Jan, L.Y.; Jan, Y. Sexually satiated male uses gustatory-to-neuropeptide integrative circuits to reduce time investment for mating. bioRxiv 2016. bioRxiv:88724. [Google Scholar]
- Peng, J.; Chen, S.; Büsser, S.; Liu, H.; Honegger, T.; Kubli, E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr. Biol. 2005, 15, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Aigaki, T. Evolution of sex-peptide in Drosophila. Fly 2016, 10, 172–177. [Google Scholar] [CrossRef]
- Chen, P.S.; Stumm-Zollinger, E.; Aigaki, T.; Balmer, J.; Bienz, M.; Böhlen, P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell 1988, 54, 291–298. [Google Scholar] [CrossRef]
- Smith, D.; Hosken, D.; Ffrench-Constant, R.; Wedell, N. Variation in sex peptide expression in D. melanogaster. Genet. Res. 2009, 91, 237–242. [Google Scholar] [CrossRef]
- Carvalho, G.B.; Kapahi, P.; Anderson, D.J.; Benzer, S. Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Curr. Biol. 2006, 16, 692–696. [Google Scholar] [CrossRef]
- Yapici, N.; Kim, Y.-J.; Ribeiro, C.; Dickson, B.J. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 2008, 451, 33–37. [Google Scholar] [CrossRef]
- Johnson, E.C.; Bohn, L.M.; Taghert, P.H. Drosophila CG8422 encodes a functional diuretic hormone receptor. J. Exp. Biol. 2004, 207, 743–748. [Google Scholar] [CrossRef]
- Lee, K.-M.; Daubnerová, I.; Isaac, R.E.; Zhang, C.; Choi, S.; Chung, J.; Kim, Y.-J. A neuronal pathway that controls sperm ejection and storage in female Drosophila. Curr. Biol. 2015, 25, 790–797. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, G.; Zeng, F.; Liu, C.; Mao, J. Insulin-like peptides regulate vitellogenesis and oviposition in the green lacewing, Chrysopa septempunctata. Bull. Entomol. Res. 2017, 107, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Toullec, J.-Y.; Lee, C.-Y. The crustacean hyperglycemic hormone superfamily: Progress made in the past decade. Front. Endocrinol. 2020, 11, 578958. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.B.; Leyria, J.; Orchard, I. The hormonal and neural control of egg production in the historically important model insect, Rhodnius prolixus: A review, with new insights in this post-genomic era. Gen. Comp. Endocrinol. 2022, 321, 114030. [Google Scholar] [CrossRef] [PubMed]
- Leyria, J. Endocrine factors modulating vitellogenesis and oogenesis in insects: An update. Mol. Cell. Endocrinol. 2024, 587, 112211. [Google Scholar] [CrossRef]
- Weger, A.A.; Rittschof, C.C. The diverse roles of insulin signaling in insect behavior. Front. Insect Sci. 2024, 4, 1360320. [Google Scholar] [CrossRef]
- Nässel, D.R.; Broeck, J.V. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 2016, 73, 271–290. [Google Scholar] [CrossRef]
- Chen, K.; Dou, X.; Eum, J.H.; Harrison, R.E.; Brown, M.R.; Strand, M.R. Insulin-like peptides and ovary ecdysteroidogenic hormone differentially stimulate physiological processes regulating egg formation in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2023, 163, 104028. [Google Scholar] [CrossRef]
- Yu, B.; Li, D.T.; Wang, S.L.; Xu, H.J.; Bao, Y.Y.; Zhang, C.X. Ion transport peptide (ITP) regulates wing expansion and cuticle melanism in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2016, 25, 778–787. [Google Scholar] [CrossRef]
- Gáliková, M.; Klepsatel, P. Ion transport peptide regulates energy intake, expenditure, and metabolic homeostasis in Drosophila. Genetics 2022, 222, iyac150. [Google Scholar] [CrossRef]
- Sajadi, F.; Paluzzi, J.-P.V. Molecular characterization, localization, and physiological roles of ITP and ITP-L in the mosquito, Aedes aegypti. Front. Insect Sci. 2024, 4, 1374325. [Google Scholar] [CrossRef]
- Begum, K.; Li, B.; Beeman, R.W.; Park, Y. Functions of ion transport peptide and ion transport peptide-like in the red flour beetle Tribolium castaneum. Insect Biochem. Mol. Biol. 2009, 39, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Audsley, N.; Jensen, D.; Schooley, D.A. Signal transduction for Schistocerca gregaria ion transport peptide is mediated via both cyclic AMP and cyclic GMP. Peptides 2013, 41, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R.; Wu, S.-F. Cholecystokinin/sulfakinin peptide signaling: Conserved roles at the intersection between feeding, mating and aggression. Cell. Mol. Life Sci. 2022, 79, 188. [Google Scholar] [CrossRef] [PubMed]
- Zels, S.; Verlinden, H.; Dillen, S.; Vleugels, R.; Nachman, R.J.; Broeck, J.V. Signaling properties and pharmacological analysis of two sulfakinin receptors from the red flour beetle, Tribolium castaneum. PLoS ONE 2014, 9, e94502. [Google Scholar] [CrossRef]
- Li, H.-F.; Dong, B.; Peng, Y.-Y.; Luo, H.-Y.; Ou, X.-L.; Ren, Z.-L.; Park, Y.; Wang, J.-J.; Jiang, H.-B. The Neuropeptide Sulfakinin, a peripheral regulator of insect behavioral switch between mating and foraging. bioRxiv 2024. bioRxiv:2024.2007.2030.605941. [Google Scholar]
- Wu, S.; Guo, C.; Zhao, H.; Sun, M.; Chen, J.; Han, C.; Peng, Q.; Qiao, H.; Peng, P.; Liu, Y.; et al. Drosulfakinin signaling in fruitless circuitry antagonizes P1 neurons to regulate sexual arousal in Drosophila. Nat. Commun. 2019, 10, 4770. [Google Scholar] [CrossRef]
- Gui, S.-H.; Pei, Y.-X.; Xu, L.; Wang, W.-P.; Jiang, H.-B.; Nachman, R.J.; Kaczmarek, K.; Zabrocki, J.; Wang, J.-J. Function of the natalisin receptor in mating of the oriental fruit fly, Bactrocera dorsalis (Hendel) and testing of peptidomimetics. PLoS ONE 2018, 13, e0193058. [Google Scholar] [CrossRef]
- Bloch, G.; Hazan, E.; Rafaeli, A. Circadian rhythms and endocrine functions in adult insects. J. Insect Physiol. 2013, 59, 56–69. [Google Scholar] [CrossRef]
- Hammock, E.A.; Young, L.J. Oxytocin, vasopressin and pair bonding: Implications for autism. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 2187–2198. [Google Scholar] [CrossRef]
- Takemura, S.-Y. Connectome of the fly visual circuitry. Microscopy 2015, 64, 37–44. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ombuya, A.; Guo, J.; Liu, W. Insect Mating Behaviors: A Review of the Regulatory Role of Neuropeptides. Insects 2025, 16, 506. https://doi.org/10.3390/insects16050506
Ombuya A, Guo J, Liu W. Insect Mating Behaviors: A Review of the Regulatory Role of Neuropeptides. Insects. 2025; 16(5):506. https://doi.org/10.3390/insects16050506
Chicago/Turabian StyleOmbuya, Alfayo, Jianyang Guo, and Wanxue Liu. 2025. "Insect Mating Behaviors: A Review of the Regulatory Role of Neuropeptides" Insects 16, no. 5: 506. https://doi.org/10.3390/insects16050506
APA StyleOmbuya, A., Guo, J., & Liu, W. (2025). Insect Mating Behaviors: A Review of the Regulatory Role of Neuropeptides. Insects, 16(5), 506. https://doi.org/10.3390/insects16050506






